Abstract
Immunoglobulins from individuals with immunity to malaria have a strong antiparasitic effect when transferred to Plasmodium falciparum malaria infected patients. One prominent target of antiparasitic antibodies is the merozoite surface antigen 3 (MSP-3). We have investigated the antibody response against MSP-3 residues 194 to 257 (MSP-3194-257) on the molecular level. mRNA from peripheral blood leukocytes from clinically immune individuals was used as a source of Fab (fragment antibody) genes. A Fab-phage display library was made, and three distinct antibodies designated RAM1, RAM2, and RAM3 were isolated by panning. Immunoglobulin G1 (IgG1) and IgG3 full-length antibodies have been produced in CHO cells. Reactivity with the native parasite protein was demonstrated by immunofluorescence microscopy, flow cytometry, and immunoblotting. Furthermore, the antiparasitic effect of RAM1 has been tested in vitro in an antibody-dependent cellular inhibition (ADCI) assay. Both the IgG1 and the IgG3 versions of the antibody show an inhibitory effect on parasite growth.
Clinical immunity to Plasmodium falciparum malaria is gradually acquired over a dozen years of intense exposure to the parasite (12). Acquired immunity to malaria has been termed premunition and is characterized as being nonsterile and incomplete (43). The exact mechanism responsible for premunition is not known with certainty. However, a number of clinical studies carried out in the early sixties (9, 16, 23)—and subsequently confirmed and extended in the nineties (2, 33)—showed an unambiguous antiparasitic effect of antibodies transferred from adults with immunity to malaria to malaria-infected infants. Clinical effects observed in one of these studies correlated with the effect measured in the in vitro assay termed antibody-dependent cellular inhibition (ADCI) (2, 4). In the ADCI assay, immune antibody cooperates with monocytes in an in vitro malaria culture, and the antiparasitic effect is demonstrated by parasite growth inhibition. It has been shown that the antibody-merozoite complex by a contact-dependent mechanism stimulates the monocyte to secrete substances toxic to the asexual blood stages. The specific substances responsible for the subsequent, non-contact-dependent parasite growth inhibition include tumor necrosis factor alpha together with other molecules that are yet to be identified (4). The ADCI assay has been used for identification and characterization of the merozoite surface protein 3 (MSP-3) (27).
An invariable structural feature of all reported MSP-3 sequences is the presence of three regions each of which contains three, four, or five conserved heptad repeat units. Previously published structural analyses suggest that the heptad repeat regions have an amphipathic alpha-helical secondary structure. A coiled-coil bundle conformation including these regions is a theoretical possibility supported by experimental data (24). The C-terminal part of MSP-3 contains a leucine zipper-like domain possibly implicated in dimerization and the formation of tetramers in vivo (5). MSP-3 contains a 96-amino-acid predicted globular region of high amino acid complexity. The region comprises amino acid residues 166 to 261 numbered according to the D10 sequence (GenBank accession number L07944) positioned C-terminal to the second putative α-helix (18). Naturally occurring antibodies affinity purified on the C-terminal part of this globular region (MSP-3 amino acid residues 194 to 257 [MSP-3194-257]) have been shown to exert a strong inhibition in ADCI assays (27). A recent phase I vaccine trial using a long synthetic peptide spanning this region of MSP-3 has shown promising results in terms of raising both humoral and cellular responses (1). Functional studies of the long synthetic peptide-induced responses have shown encouraging results in vitro in ADCI assays and in vivo in an immunocompromised BXN mouse model (13). Furthermore, an exceptional degree of conservation in this region makes it a prominent vaccine candidate (39).
Additionally, this region shows complete homology with a sequence of 11 amino acids (MSP-3220-230) from the MSP-6 antigen (MSP-6182-192), except for a valine-to-alanine substitution at position 229 of the MSP-3 sequence (49) as well as high homology with two additional antigens. The latter antigens and their resemblance to MSP-3 and MSP-6 have been described and designated H101 and H103 recently (31). It has been shown that naturally occurring antibodies affinity purified on MSP-6-derived peptides cross-react with MSP-3-derived homologous peptides and exert an ADCI effect in vitro, thereby confirming the biological relevance of these homologies (40).
Recombinant antibodies would be excellent tools to elucidate the role of isotype and fine specificity of anti-MSP-3 antibodies. All previous work has relied on either polyclonal bulk antibody or affinity-purified antiparasitic antibody. In contrast to the polyclonal antibody preparations used so far, specificity and isotype of recombinant antibodies can be controlled at will by use of DNA techniques.
To clarify the role of anti-MSP-3 antibodies, cross-reactivity with MSP-6, and functional properties in naturally occurring malaria immunity, we decided to clone antibodies directed against the target antigen fragment MSP-3194-257 (27). The present paper describes the isolation and characterization of three distinct antibodies directed to MSP-3194-257 as well as functional in vitro studies in the ADCI assay of one of these antibodies. The amino acid residues of the antigen fragments used in this study are numbered according to their position in the P. falciparum D10 clone of isolate FC27/PNG amino acid sequence (GenBank accession numbers L07944 [MSP-3] and AY518888[MSP-6]).
MATERIALS AND METHODS
Escherichia coli strains, helper phage, parasites, antigens, and control antibody.
E. coli strain Top10/F′Tetr (15) and kanamycin resistant helper phage VCSM13 from Stratagene (cat. no. 200251) were used.
The 3D7 clone (NF54 strain) of P. falciparum was propagated in human red blood cells (10). Lars Hviid, University Hospital, Copenhagen, Denmark, kindly provided parasites for specificity studies.
Recombinant MSP-3194-257 (MSP-3 DG210) antigen (27) was produced as a six-His-tagged protein in E. coli and purified by immobilized metal affinity chromatography. Recombinant antigen was kindly provided by Michael Theisen, Statens Serum Institute, Copenhagen, Denmark.
The peptides MSP-3190-217 (H-VEKDYERAKNAYQKANQAVLKAKEASSY-OH), MSP-3211-237 (biotin-AKEASSYDYILGWEFGGGVPEHKKEEN-OH), MSP-3230-257 (biotin-PEHKKEENMLSHLYVSSKDKENISKENE-OH), and MSP-6182-192 (biotin-ILGWEFGGGAP-OH) were chemically synthesized (Schafer-N, Denmark).
Negative control recombinant antibody.
Recombinant Fab to the Haemophilus influenzae type b capsular polysaccharide (19) (HibCP) was used as a negative control (a kind gift from Lotte Hougs, University Hospital Copenhagen). Vector, host, and production conditions for the control antibody were identical to the antimalarial antibodies (26).
Purified recombinant anti-rhesus D (RhD) (15) of the immunoglobulin G1 (IgG1) and IgG3 isotypes was also used as a negative control. Mammalian cloning vectors were kindly provided by Lars Norderhaug (26). Allotypes of heavy chain constant regions, hosts, and production conditions for the control recombinant antibodies (rAbs) were identical to those of the antimalarial Abs.
PIAG.
Positive control IgG (PIAG) was purified from a serum pool obtained from 30 adult Africans living permanently in Garitenga, Burkina Faso, where malaria is holoendemic. Donors were free of clinical symptoms and of heavy parasitemia and thus regarded as immune individuals (2). The IgG was extracted by ion-exchange chromatography on DEAE-Sephadex (Amersham Biosciences, Buckinghamshire, United Kingdom); protein concentration was determined to be 20 mg/ml with the bicinchoninic acid protein determination reagents (Sigma, St. Louis, MO), and the level of malaria-specific Ab was determined by immunofluorescence assay (2) with an endpoint titer of 1:52,000.
NIG.
Negative control IgG (NIG)was prepared as described above for PIAG from a commercially available pool from more than 1,000 healthy French blood donors (Biotransfusion CRTS, Lille, France). The immunofluorescence test was negative at a dilution of 1:200.
Sampling of peripheral blood leukocytes followed by PCR amplification and cloning of antibody genes.
A total of 100 ml of peripheral blood was collected with informed consent from each of 13 adults living in the village of Toubakouta, near Dielmo in an area of Senegal where malaria is holoendemic, with approximately 200 infectious bites per year (41). Donors were aged 19 to 27 years and were selected on the basis of permanent residency in the area and acquisition of immunity based on the absence of recorded malaria attacks for several years. Total leukocytes were separated by simple centrifugation, resuspended in 6 M guanidinium HCl, and stored in liquid N2 (17). RNA was isolated from the samples by acid phenol extraction according to the procedure of Chirgwin et al. (7). mRNA was converted to cDNA as described by Ørum et al. (29).
Amplification of antibody genes was carried out as separate primary and secondary reactions for variable heavy (VH) chain region genes and for entire light chain genes (Vκ-Cκ and Vλ-Cλ), respectively, as described in detail previously (15). Amplification products from the secondary extension PCR were separated on low-melting-point agarose followed by digestion with agarase. The extended VH amplificates were digested with restriction enzymes NheI and ApaI in two separate reactions.
The phagemid used in this work was pFAB73HHUI developed from pFAB4H (15) and described by Engberg et al. (17). Briefly, the vector harbors a truncated version of the M13 phage envelope protein pIII coding gene gIII. By cloning Fab encoding genes into pFAB73HHUI, the plasmid will encode a Fab-ΔpIII protein. pFAB73HHUI was digested with restriction enzymes NheI and ApaI. The VH amplificates were ligated into the plasmid and electroporated into E. coli Top10 Tet using an E. coli pulser set (Bio-Rad). Transformed bacteria were plated on LB agar (34) containing 50 mg/liter carbenicillin, 12.5 mg/liter tetracycline, and 2% glucose. Plates were incubated overnight at 37°C. Colonies were washed off the plates and used to start a liquid culture, which was eventually used for preparation of vector DNA with QIAGEN columns (QIAGEN GmbH, Hilden, Germany). The extended Vκ-Cκ and Vλ-Cλ products were digested with restriction enzymes AscI and SfiI. The Vκ-Cκ and Vλ-Cλ genes were subsequently cloned into SfiI- and AscI-digested pFAB73HHUI harboring VH genes.
Production of phage and panning procedure.
A 50-ml culture of the library in LB medium with 50 mg/liter carbenicillin, 12.5 mg/liter tetracycline, and 2% glucose was superinfected with VCSM13 helper phage (Stratagene) at an optical density at 600 nm (OD600) of 0.8. A multiplicity of infection of 100 was used and the mixture was incubated at 37°C with gentle shaking (50 rpm) for 1 h. Then the culture was diluted into 950 ml of medium as above but without glucose and incubated at 30°C overnight. After a 15-min spin at 10,000 × g, phage in the supernatant was precipitated with polyethylene glycol 6000 and sodium chloride at final concentrations of 4% and 0.5 M, respectively. The supernatant was incubated for 1 h on ice and centrifuged for 30 min at 12,000 × g. Precipitated phage was resuspended in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA; Sigma) and used immediately. The total number of phage (34) was 1.3 × 1013.
Biotinylated MSP-3194-257 was coupled to Dynabeads M-280 streptavidin (cat. no. 112.05; Dynal, Norway) as described by the manufacturer. After the antigen-coated beads were incubated with phage in an end-over-end mixer for 1 h, beads were captured with a magnetic particle concentrator (MPC-6; Dynal cat. no. 120.02) for 5 min and then washed six times in 10 ml of PBS with 0.05% Tween 20 for 2 min. After the last wash beads were resuspended in 1 ml of PBS with 1 mg of trypsin (Worthington), and phage was eluted during a 1-h incubation at 37°C. Then a volume of 3 ml of exponentially growing Top10 with an OD600 of 1 was added, and the mixture was incubated at 37°C for 30 min to allow eluted phage to attach to E. coli. The bacteria were finally plated on LB agar (34) containing 50 mg/liter carbenicillin, 12.5 mg/liter tetracycline, and 2% glucose and incubated overnight at 37°C. Colonies were washed off the plates with LB medium and stored as a glycerol stock at −80°C or grown for production of phage or DNA as described.
Subsequent to the first panning round, three series of pannings were carried out in parallel, to a total of four rounds. In the first series, the number of beads was reduced by a factor of 5 for each subsequent panning round. In the second and the third series, the number of beads was reduced by a factor of 10 and 20, respectively. After the fourth panning round, single colonies from all three series were grown in sterile microtiter plates (NUNC cat. no. 163320) for production of Fab-ΔpIII.
Nucleotide sequence analysis.
DNA sequencing of selected colonies was performed with the Sanger dideoxy method (35) using PRISM AmpliTaq FS and a Big Dye Terminator Cycle sequencing kit (cat. no. 4303152) from PE Biosystems according to the manufacturers' instructions. Reactions were analyzed on an ABI Prism 310 genetic analyzer (PE Applied Biosystems). The following primers were used: (forward) 5′-CTTGGAGGAGGGTGCC-3′ and (reverse) 5′-CTCGAGAAGGAGACAGTC-3′ for the variable heavy chain and (forward) 5′-TGGCGGGAAGATGAAGAC-3′ and (reverse) 5′-CACACAGGAAACAGCTATGA-3′ for the variable light chain. Sequences were aligned with Ig germ line genes by the ImMunoGeneTics database (http://imgt.cines.fr; Marie-Paule Lefranc, initiator and coordinator, Montpellier, France).
Production, detection, and semiquantitation of Fab.
E. coli cells infected with phagemid harboring heavy and light chain genes were grown in LB medium supplemented with 1% glucose, 50 μg/ml carbenicillin, and 12.5 μg/ml tetracycline overnight. A new culture was started in LB medium with 50 μg/ml carbenicillin and 12.5 μg/ml tetracycline. Isopropyl-β-d-thiogalactopyranoside was added at an OD600 of approximately 0.5 to a concentration of 50 μM, and the culture was grown overnight. Bacteria were spun down, and supernatants were used for further study.
Detection and semiquantitation were carried out with a sandwich enzyme-linked immunosorbent assay (ELISA) by use of a capture antibody (goat antihuman Fab; Sigma I5260) and a detection antibody (goat anti-human Fab conjugated to alkaline phosphatase; Sigma A8542). For the present purposes, dilutions of Fab-containing supernatants yielding the same level of reactivity were assumed to contain the same amounts of Fab.
In order to obtain purified and concentrated Fab, we modified the pFab73H vector harboring the antibody genes by cutting out the ΔpIII-coding gene with the EagI restriction enzyme. This leads to the production of Fab molecules with a six-His tag. Fab with a six-His tag was purified by chromatography with Ni-nitrilotriacetic acid-coupled agarose (cat. no. 30210; QIAGEN) as described previously (19).
Production of intact recombinant IgG1.
Intact RAM1-IgG1, RAM2-IgG1, RAM3-IgG1, and RAM1-IgG3 were produced in Chinese hamster ovary cells (CHO) essentially as previously described (26). In brief, VH and Vκ genes were amplified by PCR. Primers were designed to introduce restriction enzyme sites making amplificates compatible with the vector system for eukaryotic expression of intact IgG1 or IgG3 as previously described (26). CHO cells were transfected with a mixture of two plasmids encoding each of the immunoglobulin chain genes, and limiting dilution was used for isolation of good producers.
Protein was precipitated with 70% ammonium sulfate from cleared CHO culture supernatant, and the pelleted protein was resuspended in water. Subsequently, the buffer was changed to 70 mM sodium acetate, pH 5.0, by dialysis. Recombinant IgG was purified using DEAE Sepharose FF (Amersham Biosciences), the flowthrough was applied to an ABx column (J. T. Baker, Phillipsburg, NJ), the column was washed with 50 mM bicine, pH 8.5, and the IgG was finally eluted with a gradient of 50 mM bicine supplemented with 0.5 M NaCl, pH 8.5. Fractions containing Ab were pooled and precipitated with 70% ammonium sulfate, resuspended in water, and purified on a Superdex 200 column (Amersham Biosciences). Purity was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with silver staining, and protein concentration was determined spectrophotometrically.
ELISA.
ELISA plates (Maxisorb; NUNC 4-39454; Denmark) were coated with 200 ng per well of purified recombinant MSP-3194-257 in PBS, blocked with PBS-1% BSA, and used for standard ELISA with undiluted supernatant or supernatant diluted in PBS-1% BSA as previously described (14, 15). After a washing step, goat anti-human Fab conjugated to alkaline phosphatase (Sigma cat. no. A8542) was applied as a detection antibody. Finally, p-nitrophenyl phosphate (Sigma phosphatase substrate tablets, cat. no. 104-105) was used as a substrate. Color development was measured as the OD405 minus the OD490.
Similar experiments were performed using intact IgGs in ELISA wells coated with the synthetic peptides MSP-3190-217, MSP-3211-237, MSP-3230-257, or MSP-6182-192. In this ELISA goat anti-human IgG (Fc-specific) conjugated to alkaline phosphatase (Sigma cat. no. A9544) was used as a secondary antibody.
All ELISAs were done in triplicates.
Competition studies.
To wells coated with MSP-3194-257 and containing 50 μl of a fixed quantity of Fab-ΔpIII was added 50 μl of various dilutions of competition antigen, MSP-3194-257. Coating of wells with MSP-3194-257 and detection of bound antibody were carried out as above. RAM1, RAM2, or RAM3 Fab-ΔpIII were used in amounts yielding OD405 minus OD490 values of approximately 1 in ELISA on a coating of MSP-3194-257. The concentration of competition antigen ranged from 2 nM to 200 mM. Each dilution of competition antigen was tested in triplicate.
Using the same setup, dilutions of RAM3 Fab without ΔpIII were used to compete the binding of RAM1, RAM2, and RAM3 Fab-ΔpIII. In this experiment, bound Fab-ΔpIII was specifically detected with a mouse anti-pIII (cat. no. PSKAN 3; MoBiTech, Germany) followed by an anti-mouse alkaline phosphatase conjugate (cat. no. D314; Dako, Denmark).
Immunofluorescence microscopy.
Parasitized red blood cells enriched for late developmental stage parasites were obtained by magnet-activated cell sorting of an in vitro culture of P. falciparum clone 3D7 as previously described (45). Cells were washed twice in PBS and sedimented on a Superfrost Plus slide (cat. no. J1800AMNZ; Menzel GmbH & Co. KG), washed once in PBS, and fixed in 1% formaldehyde (Sigma cat. no. F1635) in PBS for 10 min, followed by methanol for 10 min. All handling was performed at room temperature. The slides were blocked for 2 h with PBS-3% BSA, incubated with RAM1, RAM2, or RAM3 IgG1 overnight, washed three times in PBS-0.05% Tween 20, and subsequently incubated with a goat anti-human Fc (cat. no. F9544; Sigma) at a 1/50 dilution in PBS-3% BSA for 30 min. The slides were washed three times as described above and incubated with an Alexa Fluor 488 chicken anti-goat IgG (Molecular Probes cat. no. A21467) at a 1/100 dilution in PBS-3% BSA containing propidium iodide (20 mg/liter) for 30 min. Slides were examined with a Zeiss LSM 510 confocal laser scanning microscope. To confirm the binding of RAM1-3 to the late-stage parasites only, similar and other fixing methods have been used with nonenriched parasite cultures including all blood cycle parasite stages.
Affinity studies by surface plasmon resonance.
The affinities of the antibodies were determined by surface plasmon resonance on a Biacore X instrument (Biacore AB, Uppsala, Sweden). MSP-3194-257 in 50 mM NaAc, pH 5.5, was immobilized on an EDC/NHS [N-ethyl-N′-(3-dimethyl aminopropyl)-carbodiimide hydrochloride/ (N-hydroxysuccinimide)]-activated CM5 sensor chip as described by the manufacturer (Biacore AB), yielding a surface density of approximately 50 resonance units. Purified Fab fragments were diluted in the recommended HBS buffer (0.01 M HEPES, 0.15 M NaCl, 3.4 mM EDTA, 0.05% surfactant P-20, pH 7.4; Biacore AB) and analyzed at 25°C using a flow rate of 10 μl per minute. Bound Fabs were dissociated using HBS buffer at a flow rate of 10 μl per minute. Association and dissociation rate constants were calculated from a minimum of four sensorgrams generated by the Biacore control software using the curve-fitting BIAevaluation software, version 3.0 (Biacore AB) and the 1:1 Langmuir model.
Flow cytometry.
P. falciparum culture enriched for late developmental stage parasites by magnet-activated cell sorting as described previously (45) was permeabilized and fixed by incubation in 32% ethanol for 30 min on ice. Parasites were then washed in PBS with 1% BSA, incubated overnight with Fab, washed twice with PBS-1% BSA, and incubated for 30 min with FITC-conjugated goat anti-human Fab (Sigma F5512) diluted 1:25. Cells were analyzed in a Coulter EPICS-2 flow cytometer. HibCP-specific Fab was used as control in identical concentrations. Fixed infected and fixed noninfected red cells were stained with propidium iodide (20 mg/liter) and compared to enable gating of infected red cells.
Immunoblotting.
Parasitized red blood cells, at late trophozoite and schizont stage, were obtained by magnet-activated cell sorting as previously described (45). The purified cells containing late-stage parasites were solubilized on ice in 2% Triton X-100 by ultrasound four times for 15 s each time and incubated for 2 h on ice before being mixed with a reducing sample buffer, heated to 100°C for 10 min, and subjected to SDS-PAGE in a morpholinepropanesulfonic acid-buffered 4 to 12% gradient gel (NOVEX, San Diego, CA). Proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (Immobilon, Millipore, Molsheim, France). Strips were incubated for 3 h with RAM1-IgG1, RAM2-IgG1, RAM3-IgG1, or anti-HibCP-IgG1 as a negative control. Protein G conjugated to alkaline phosphatase (cat no. 32391; Pierce Chemical Co., Rockford, IL) was used as a detecting conjugate. Finally, CSPD [disodium 3-(4-methospiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenylphosphate] (cat. no. CD100R; Tropix Inc., Bedford, MA,) chemiluminescence substrate was added, and light emission was detected with an Alpha Innotech FluorChem 8000 system (San Leandro, CA).
Parasite culture for ADCI.
The P. falciparum 3D7 clone derived from the NF54 strain, the NF54 strain, and the Uganda Palo Alto strain were cultured in AB+Rh+ erythrocytes in RPMI 1640 medium supplemented with hypoxanthine, 0.5% albumax (Gibco, Invitrogen Corporation, Carlsbad, CA), sodium bicarbonate, HEPES, penicillin, and streptomycin. Parasites were synchronized by alternate sorbitol treatment and plasmagel flotation, and mature parasites were used in the ADCI assay.
ADCI assay.
The ADCI assay was done essentially as described using previously published techniques (2, 20). A synchronized P. falciparum culture containing mature schizonts adjusted to 0.5% parasitemia with a final hematocrit of 2% in RPMI medium was added to wells containing a suspension of 2 × 105 monocytes. In addition to test Ab, the following controls were used simultaneously in each plate: (i) parasite culture without monocytes, (ii) culture with monocytes, (iii) culture with NIG, (iv) culture with monocytes and NIG, (v) culture with PIAG, (vi) culture with monocytes and PIAG, (vii) culture with anti-RhD-IgG1, (viii) culture with monocytes and anti-RhD-IgG1, (ix) culture with anti-RhD-IgG3, and (x) culture with monocytes and anti-RhD-IgG3. At 48 h and 72 h an additional 50 μl of RPMI medium containing 0.5% albumax, penicillin, and streptomycin was added to each well. At the end of the assay (96 h), the parasitemia in each well was determined, both by counting more than 50,000 erythrocytes on Giemsa-stained film under the microscope and by flow cytometry analysis after staining with hydroethidine. Determination of parasitemia by flow cytometry with a FACSCalibur (Becton-Dickinson, San Jose, CA) was done as previously reported (50). The CellQuest program (Becton-Dickinson) was used to determine the percentage of parasitemia. Flow cytometry and microscopy gave similar results.
The specific growth inhibition index (SGI) was calculated as 100 × {1 − [(% parasitemia with monocytes and test Ab/% parasitemia with test Ab)/(% parasitemia with monocytes and NIG/% parasitemia with NIG)]}.
Nucleotide accession numbers.
DNA sequences for all of the antibodies have been submitted to the GenBank database with the following accession numbers:AY543586 (RAM1 VH), AY543587 (RAM1 VL), AY882577 (RAM2 VH), AY882578 (RAM2 VL), AY882579 (RAM3 VH), and AY882580 (RAM3 VL) (15a).
RESULTS
Library.
The library contained 5 × 107 heavy chains, 1 × 108 kappa light chains, and 1 × 108 lambda light chains. Investigation of VH genes from 66 clones by PCR and BstNI digestion demonstrated no identical digestion patterns (data not shown), which suggests acceptable diversity.
Selection of antigen binding Fabs.
To obtain specific and preferably high-affinity antibodies, an antigen-reducing panning strategy was used. The phage stock produced from the first panning eluate was used in three separate panning series (series a to c). Each series had its own reduction factor for the number of beads used in subsequent pannings. The three different factors (5, 10, and 20) of antigen reduction were employed to cover the range of antigen reduction likely to be productive (21). The eluate from the third and fourth panning steps of each series was screened for the presence of Fab producers and antigen binders in ELISA. After the third panning there were no Fab producers among the 93 colonies screened (31 from each series). After the fourth panning, the eluate from the series employing the highest antigen reduction factor (20) was nonproductive, whereas reduction factors of 5 and 10 resulted in nine and eight binders, respectively (Table 1). Seven clones in each of these two series were identical and designated RAM1 (recombinant anti-MSP-3 no. 1). A reduction factor of 5 yielded two identical clones designated RAM2, and one clone designated RAM3 was recovered from the series with reduction factor 10. Antigen binding Fabs were analyzed by DNA sequencing of the genes encoding the VL and VH regions. Three distinct sets of antibody genes were found, of which all light chains were kappa light chains.
TABLE 1.
Colony screening results
| Panning no. and series | Bead reduction factor | No. of clones tested | Fab producers | MSP-3194-257 binders | Clones (no.) |
|---|---|---|---|---|---|
| 4.a | 20 | 118 | 2 | 0 | |
| 4.b | 10 | 132 | 9 | 8 | RAM1 (7) |
| RAM3 (1) | |||||
| 4.c | 5 | 126 | 12 | 9 | RAM1 (7) |
| RAM2 (2) | |||||
| Total | 376 | 23 | 17 |
To verify the origin of the Ab genes and to estimate the amount of somatic mutation, the amino acid sequence homology with human germ line V genes was determined by alignment using the ImMunoGeneTics database. RAM1 had amino acid substitutions at 10 out of 113 (8.8%) positions in the VH region and 8 out of 107 (7.5%) in the VL region. RAM3 had 5/121 (4.1%) and 7/103 (6.8%) in the VH and VL regions, respectively. In addition the heavy chain of RAM1 did not contain any D gene-derived sequence, leading to a short heavy chain CDR 3 region. In contrast, the RAM2 clone showed lower resemblance with published human germ line V genes, 22/119 (18%) and 23/107 (21%) for VH and VL, respectively.
Competition ELISA.
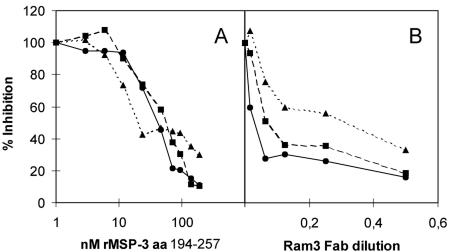
Competition of Fab-ΔpIII reactivity with the coating antigen MSP-3194-257 was done with soluble MSP-3194-257 in an ELISA. Reactivity of all three clones could be competed with soluble protein, thus confirming that the clones reacted with MSP-3194-257 in solution and not with a plastic or denaturation-dependent conformation (Fig. 1A).
FIG. 1.
Competition studies. (A) Fab-ΔpIII proteins from the three clones were competed with soluble panning antigen in ELISA. All three clones were susceptible to competition with soluble MSP-3194-257. (B) Fab-ΔpIII proteins from the three clones were competed with RAM3 Fab fragments without ΔpIII. Fab-ΔpIII was specifically detected with a mouse anti-pIII (MoBiTech, Germany). The ability of RAM3 to compete all three clones demonstrates that the epitopes of the clones are closely related. ▪, RAM1; •, RAM2; and ▴, RAM3.
Furthermore, we competed the three clones with RAM3 Fab. All three clones could be competed from binding to the panning antigen, thus demonstrating an overlap of the epitopes targeted or alternatively steric hindrance (Fig. 1B).
Affinity determination of Fabs by Biacore.
The Biacore results confirmed the binding of Fabs to the MSP-3194-257 antigen. The on and off rates of the three Fabs were determined by the curve fitting BIAevaluation software, version 3.0. On this basis the affinity constant (KD) of the three clones was estimated to be approximately 35, 20, and 46 nM for RAM1, RAM2, and RAM3, respectively. Despite the similarities in affinity, RAM1 had faster kinetics than RAM2 and RAM3. RAM1 had a higher on rate (kon = 1.5 × 105 M−1s−1) than RAM2 and RAM3 (kon = 3.3 × 104 M−1s−1 and 2.1 × 104 M−1s−1, respectively). Also, RAM1 had a higher off rate (koff = 5.2 × 10−3 s−1) compared to the off rates of RAM2 and RAM3 (koff = 6.6 × 10−4 s−1 and 9.6 × 10−4 s−1, respectively).
Immunofluorescence microscopy.
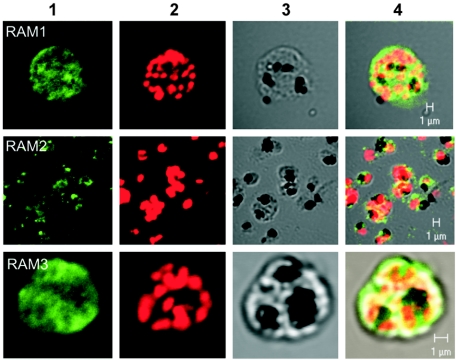
Specificity for the parasite was demonstrated by immunofluorescence and confocal microscopy (Fig. 2). By fixing 3D7 parasites to microscope slides by formaldehyde and methanol treatment, we were able to specifically detect red blood cells containing parasites, as demonstrated by propidium iodide staining of DNA. Staining with RAM1, RAM2, or RAM3 IgG using an anti-human-Fc fluorescein isothiocyanate (FITC) conjugate revealed that red blood cells containing parasites were recognized by the cloned antibodies (Fig. 2); extensive studies have shown no reaction with an isotype control using the same labeling technique (data not shown). Furthermore the RAM1, RAM2, or RAM3 reacted only with late stages of the parasite cycle (schizont) showing “grape-like” structures indicative of merozoite surface labeling and free merozoites (Fig. 2). In numerous immunofluorescence microscopy assays, using several fixing methods and unpurified malaria cultures, early stages (ring and trophozoite) did not react with the Ab (data not shown). These findings are in accordance with previous findings locating MSP-3 to the late schizont stage and the merozoite surface (22, 27).
FIG. 2.
Immunofluorescence microscopy. P. falciparum 3D7 parasites were fixed on slides. Individual slides were stained with RAM1, RAM2, or RAM3 IgG1 conjugated with FITC (column 1, green) and with propidium iodide (column 2, red). Column 4 shows the DNA and RAM reactivity added on a white-light photo (column 3) of the same cells. RAM1, RAM2, and RAM3 reacted with late-stage and burst (RAM2) schizont. Bar, 1 μm.
Flow cytometry.
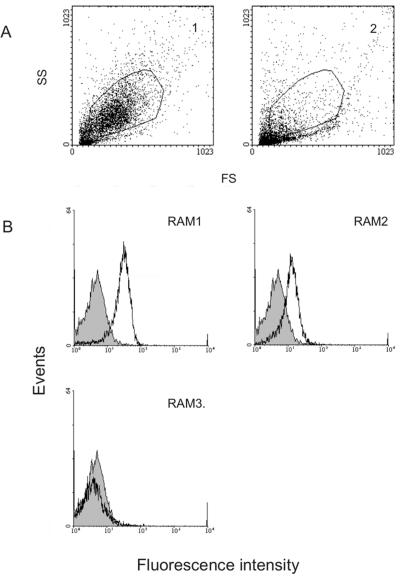
The specificity was further tested by flow cytometry. P. falciparum cultures were enriched for late stages and permeabilized with 32% ethanol. Propidium iodide-stained schizont-infected red cells were analyzed with a flow cytometer to enable identification of and gating on schizonts (Fig. 3). Schizont-enriched cells were incubated with Fab, and binding was subsequently detected with an anti-Fab FITC-conjugate. The mean channel fluorescence (MCF) of cells incubated with Fab was used as a measure of the reactivity of each antibody. The control anti-HibCP Fab gave an MCF of 3.5, whereas RAM1 and RAM2 gave MCF values of 19.6 and 9.5, respectively. In contrast, the RAM3 Fab (MCF of 3.2) did not react significantly with the parasite preparation used in this assay (Fig. 3).
FIG. 3.
Flow cytometry analyses of the reactivity of recombinant antibodies with schizonts. (A) Forward and side scatter for ethanol-fixed and permeabilized infected red blood cells (frame 1) and the same parameters for similarly treated noninfected red blood cells (frame 2). The gating was placed to include the majority of the infected cells. The gating was used for the histograms described below. Panel B illustrates histograms of various recombinant antimalaria antibodies compared with the control antibody directed against HibCP antigen (gray area). The anti-HibCP control Fab has an MCF of 3.5. RAM1, RAM2, and RAM3 have MCF values of 19.6, 9.5, and 3.2, respectively.
Western blotting.
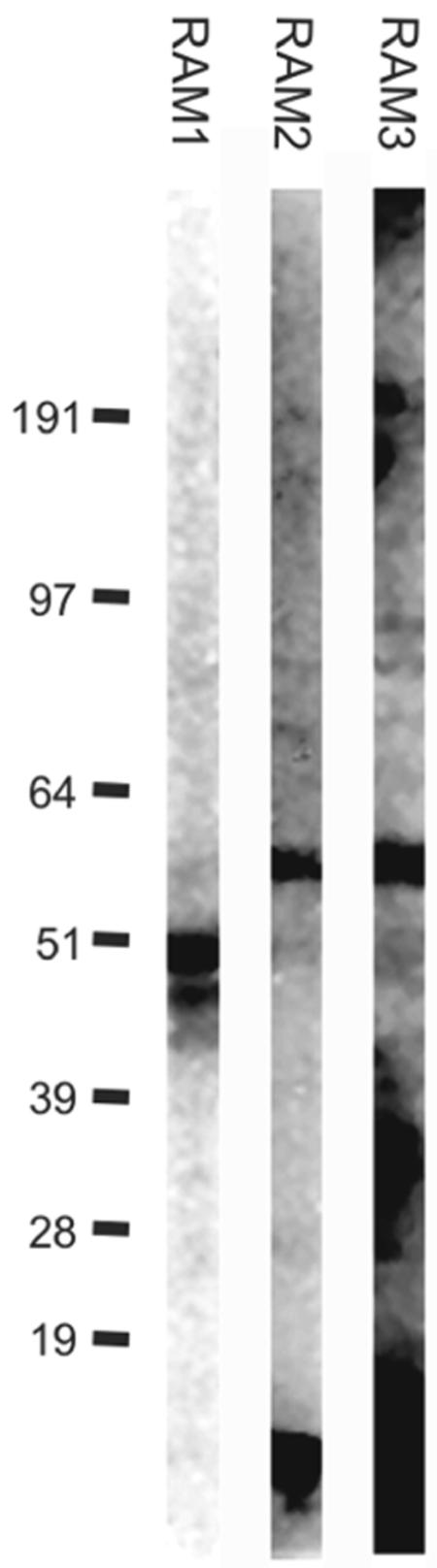
The molecular masses of reduced antigen were approximately 48 and 50 kDa in immunoblotting reactions with RAM1 and 62 kDa in reactions with RAM2 and RAM3 (Fig. 4). The 48- to 50- and 62-kDa bands are in agreement with molecular sizes previously published for MSP-3 (22, 27, 30). In addition, the RAM2 and RAM3 antibodies seemed to react with bands at lower molecular sizes: RAM2 with an approximately 14-kDa band and RAM3 apparently with a smear of bands in the same size as well as band sizes at about 35 kDa. These bands have not been seen with isotype controls (data not shown).
FIG. 4.
Immunoblot of RAM1-IgG1, RAM2-IgG1, and RAM3-IgG1 on P. falciparum material. RAM1-IgG1 reacted with two bands at 48 and 50 kDa; RAM2-IgG1 and RAM3-IgG1 reacted mainly with a band of approximately 62 kDa.
Epitope mapping.
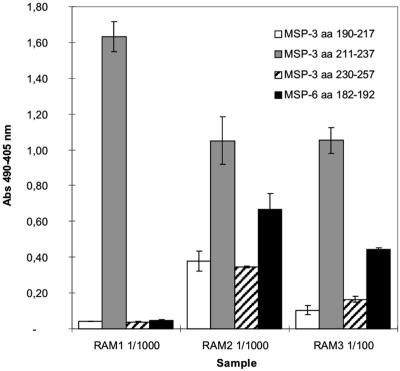
The three clones were examined by ELISA for reactivity with three peptides representing the N-terminal, the middle, and the C-terminal parts of the panning antigen. MSP-3 190-217 corresponds to the entire N-terminal helix region 3 with four heptad repeat units (22, 24). MSP-3211-237 includes one heptad repeat unit, and MSP-3230-257 consists of the non-heptad repeat C-terminal part of the panning antigen. Additionally, the three clones were tested for reactivity with an MSP-6-derived 11-mer peptide, MSP-6182-192. The MSP-6182-192 peptide is identical to the middle part of the MSP-3211-237 peptide (MSP-3220-230) with the exception of a valine-to-alanine substitution at position 191 (49). ELISA results are depicted in Fig. 5. RAM1 reacted strongly with MSP-3211-237 and did not react with MSP-M3190-217, MSP-3230-257, or MSP-6182-192 in ELISA. In contrast RAM2 reacted predominantly with the MSP-3211-237 and its MSP-6182-192 homolog; however, there was some cross-reactivity with the other two peptides tested (four times above background levels). RAM3 reacted significantly with only the MSP-3211-237 and MSP-6182-192 peptides.
FIG. 5.
ELISA reactivity of RAM1-IgG1, RAM2-IgG1, and RAM3-IgG1 with peptides. The RAM1, RAM2, and RAM3 clones had different reaction patterns on peptides derived from MSP-3 and MSP-6. Concentrations of the various antibodies were determined by spectrophotometry, and comparable amounts of RAM1 and RAM2 were used. The concentration of RAM3 used was 10 times higher. Reactivity is indicated as the OD405 minus the OD490. Bars indicate mean values, and error bars indicate two standard deviations of results obtained in triplicates. aa, amino acids.
Characterization of antiparasitic properties.
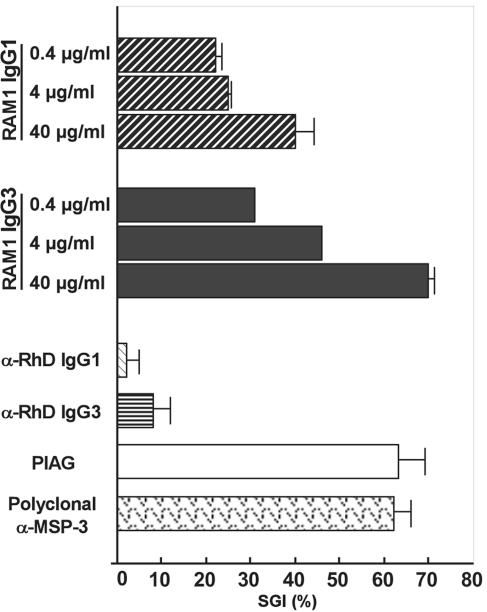
Based on the resemblance of the reactivity pattern of the RAM1 antibodies with the antiparasitic antibodies of immune individuals, we chose to assay the ability of RAM1-IgG1 and RAM1-IgG3 to inhibit the growth of P. falciparum parasites (1, 13). ADCI assays using the 3D7 clone derived from the NF54 strain, the NF54 strain itself, or the Uganda Palo Alto strain all showed a potent monocyte-dependent antiparasitic effect mediated by recombinant RAM1 of IgG1 and IgG3 subclasses. Results obtained with 3D7 are presented in Fig. 6. The SGI obtained with our monoclonal Ab was as high as that obtained with polyclonal African IgG, which has been shown to be clinically effective in passive transfer to humans (33). As was the case with total African adult IgG, no direct inhibition of parasite growth was observed when monocytes were omitted (data not shown). All observations previously made with natural human plasma-derived polyclonal Abs were thus reproduced with RAM1-IgG1 and RAM1-IgG3. However, the SGI exerted by RAM1-IgG1 and RAM1-IgG3 in ADCI was reached at concentrations of less than 1%—as estimated by protein concentration—of the needed concentration of polyclonal Abs in the pooled immune African IgG. The SGI obtained in ADCI with plasma-derived affinity-purified anti-MSP-3217-237 was of the same magnitude as that obtained with RAM1-IgG3.
FIG. 6.
SGI of IgG1 and IgG3 versions of RAM1 determined in ADCI. Recombinant human anti-RhD-IgG1 and anti-RhD-IgG3 were used as negative controls and tested, respectively, at 110 μg/ml and 10 μg/ml. Pooled IgG from immune Africans (PIAG) (2 mg/ml) and polyclonal affinity-purified anti-MSP-3211-237 Ab were used as positive controls. RAM1 IgG1 and IgG3 were tested in ADCI at three different concentrations (0.4 μg/ml, 4 μg/ml, and 40 μg/ml). SGI was calculated as the mean of three independent assays using the P. falciparum clone 3D7. Error bars represent two standard deviations.
The antiparasitic potency of RAM1-IgG1 and RAM1-IgG3 was compared in ADCI assays over a range of 10-fold Ab concentrations from 0.4 to 40 μg per ml. There was a clear dose-dependent effect, and the SGI induced by IgG3 was consistently 1.6 times greater than the inhibition induced by IgG1 (Fig. 6). Even at the lowest concentration of RAM1-IgG1 and RAM1-IgG3 (0.4 μg per ml), the SGI was higher than for the corresponding controls, 110 μg per ml anti-RhD-IgG1 and 10 μg per ml anti-RhD-IgG3.
DISCUSSION
We report the cloning of three distinct human recombinant antibodies against the P. falciparum antigen MSP-3. The objective of the study was to explore the antibody response against MSP-3 on a molecular level and to obtain highly defined antibodies for functional analysis. The phagemid library used in this study harbored cDNA-derived genes encoding Fab. The genes were obtained from peripheral blood leukocytes of African adults clinically immune to malaria and therefore potentially include genes encoding protective antibody specificities. The genes isolated in this study are thus encoding human antibodies directed against the native protein and are produced as part of an antiparasitic response during natural infection.
Roeffen et al. (32) and Sowa et al. (44) have previously published the isolation by phage display of human recombinant ScFv fragments against the gametocyte stage antigen Pfs48/45 and a variant of PfMSP-1, respectively. The ScFv libraries used in those studies were made from the peripheral blood lymphocytes of patients with acute malaria, i.e., not from individuals with immunity to malaria, as in the present study. Previous attempts to produce human anti-MSP-3 antibodies using Hu-SPL-SCID mice have not been successful in obtaining stable production of such antibodies (3).
Our panning procedure was designed to enable isolation preferentially of high-affinity binders by gradually reducing the amount of antigen used in subsequent rounds of panning. The panning strategy was established through preliminary optimizations and was inspired by previously published theoretical work (21). The basic notion was that good binders are present in very low frequency in the initial stock. To recover a minimum number of rare binders, a high initial concentration of antigen is needed to ensure Fab-antigen interaction. In subsequent rounds of panning, binders are assumed to be present with increasing frequency. Accordingly, the amount of antigen should be reduced for each panning, thereby ensuring competition between binders and consequently avoiding the survival of mediocre binders. Antigen concentration was decreased by reducing the number of antigen-coated magnetic beads. This procedure concomitantly reduced the matrix area for each panning, thus reducing transfer of deletion mutants by nonspecific adherence to matrix. During the panning procedure deletion mutants are believed to have a selective advantage due to a shorter replication time, and, if transferred through the washing steps, they will dominate the eluate (36). Our results indicate that deletion mutants, which are not being strained by Fab production, have a tendency to overgrow the phage population if there is not a strong selective advantage of Fab production during the panning procedures (Table 1). Our strategy of reducing the panning matrix area and the amount of antigen successfully introduced an advantage to the antigen-binding clones.
Surface plasmon resonance measurements showed that all three antibodies had affinities (KD) in the nanomolar range. Previous work on an invasion-inhibiting anti-circumsporozoite antibody has reported a KD of approximately 300 nM (kon, ≈4 × 103; koff, ≈1.2 × 10−3) (51), and HIV neutralizing antibodies have been shown to have a KD of 4.6 nM (kon, ≈8.4 × 104; koff, ≈3.9 × 10−4) (8), showing that the RAM antibodies described here indeed have affinities within a biologically relevant range. RAM1 had the fastest kinetics of the three; i.e., RAM1 was rapidly binding to the antigen and was also released from the antigen more quickly than the other two clones. The overrepresentation of RAM1 could point to either the on rate as being an important factor for selection in this specific panning procedure or the possibility that the productivity and subsequent display of Fabs by this clone were superior. The fact that RAM1 has been isolated from two parallel panning series indicates that this clone was either one in a few with the properties necessary to be selected in this particular setup or that it was overrepresented in the initial donor-derived mRNA and thereby in the constructed library. Antigen-coated magnetic beads of 2.8 μm were chosen due to practical considerations, but in fact they closely mimicked the natural presentation of MSP-3 on the merozoite surface. The fast reaction kinetics of RAM1 could be the optimal kinetics for a fast interaction of the immune system with the released merozoite being accessible for only a few minutes.
Sequencing of the three clones and the alignment studies showed that the antibodies isolated in this study contain variable regions of human origin and that for at least RAM1 and RAM3 little somatic hypermutation has taken place. In addition, it revealed that RAM2 was unusual compared to known humane germ line genes. We cannot at this time sort out whether the sequence differences found represent somatic mutation, undescribed germ line genes present in African populations, or artifacts introduced during library construction.
The flow cytometry technique used in this study is novel in the context of malaria parasites. Untreated late-stage parasite culture did not react with the antibodies, probably due to lack of access to the parasite residing inside the schizonts. Flow cytometry was therefore done with ethanol-permeabilized and fixated schizont-infected red cells. RAM1 and RAM2 demonstrated specific binding to this parasite preparation. In contrast, no reactivity of RAM3 was detectable. One reason for the lack of binding of RAM3 could be that its epitope is not conserved after the fixation and permeabilization procedure used.
The three clones had different patterns of reactivity with MSP-3 peptides in Western blotting and in flow cytometry, despite the fact that they were all selected on the same panning antigen, MSP-3194-257. Differences in SDS-PAGE mobility of MSP-3 full-length recombinants have been assigned to major conformational differences including complex tertiary structures (18). Recent work on the conformation and polymerization of MSP-3 molecules has suggested an elongated form of the molecule and the generation of intramolecular cross-linking, making the molecules more compact and faster migrating on SDS-PAGE gels (5). Such intramolecular cross-linking could be the basis for the diverse mobilities of MSP-3 in gels. In an initial work on MSP-3, McColl et al. suggested that the MSP-3 antigen present on the merozoite surface (44 kDa) is the result of proteolytic processing of a larger MSP-3 precursor (62 kDa) (22), an observation recently confirmed and specified by Pearce et al. The latter study also confirmed the presence of a low-abundance band of 52 kDa (30). The 44-kDa band seen by McColl et al. corresponds most likely to the low-molecular-weight band (48 kDa) seen in our study with RAM1. The reactivity of RAM2 and RAM3 with a band of approximately 62 kDa, though not with the 48-kDa band, could indicate the existence of epitopes in the precursor protein that are accessible for only RAM2 and 3 but not for RAM1. In contrast, RAM1 may react with only MSP-3, having made the suggested intramolecular cross-linking (Fig. 4). This explanation fits well with the observation that only RAM 2 and RAM3 react with the probably linear, short MSP-6182-192 peptide (Fig. 5). The apparent binding of both RAM2 and RAM3 with bands of lower molecular masses cannot be explained from known data of the MSP-3 antigen. These molecules could be proteolytic degradation products derived from either MSP-3 or MSP-6, in accordance with the fact that these antibodies react with the MSP-6182-192 peptide (Fig. 5) or other MSP-3 homologs, i.e., H101 and H103 (31).
The competitive effect of RAM3 on both RAM1 and RAM2 (Fig. 1), in spite of the different peptide reactivity (Fig. 5), illustrated the overlapping nature of the epitopes, as expected with a 62-amino-acid fragment used for the panning procedure. The reaction patterns obtained in this study are unexpectedly diverse, given the monoclonal nature of the antibodies, and need further elucidation. Recent functional studies of affinity-purified anti-MSP-3 antibodies have confirmed the importance of fine specificity to MSP-3 (39). The complex conformation of this antigen could therefore pose a challenge in terms of raising an immune response leading to protective antibodies in terms of fine specificities and isotypes, as recently seen in MSP-3 vaccine studies (1, 13).
In order to assess the functional properties of the RAM1 antibody, the variable chain genes isolated were recombined with the allotypes of the most prevalent constant region genes found in African populations. The Cγ1 allotype, G1m(a,z), with a frequency close to 100% in Africa (11), and the most prevalent African Cγ3 allotype, G3m(b), were chosen to study the functional properties of the two cytophilic subclasses IgG1 and IgG3. The haplotype G1m(a,z);G3m(b) is found in 68% of Nigerians (11).
Results from the ADCI assay showed that the IgG3 version of RAM1 induced 1.6 times stronger inhibition than the IgG1 version over a broad range of concentrations (Fig. 6). This finding is in accordance with previously reported sero-epidemiological studies linking clinical immunity with specific IgG3 responses against several different target antigens: MSP-3, GLURP R0 and R2 (28, 42, 48), MSP-2 (46), and MSP-1 Block 2 (6). However, in other settings, IgG1 was found to be associated with protection: a high IgG1-to-IgG3 ratio in Kenyan children is associated with inhibitions in ADCI (38) and a sero-epidemiological study also from Kenya demonstrated association of protection from severe malaria with possession of elevated schizont-specific IgG1 levels relative to IgG2 and IgG4 levels (25). The diverging associations are explained by the fact that Ab is biologically active in cooperation with monocytes. The merozoite-Ab complex interacts with FcγRIIA on the monocyte and induces release of antiparasitic substances (4). FcγRIIA is polymorphic, and distinct functional phenotypes are associated with the various alleles. In one study (47), serum with dominance of IgG3 induced the highest phagocytosis with cells of the genotype FcγRIIA-Arg/Arg131, whereas serum with dominance of IgG1 induced the highest phagocytosis with cells of the genotype FcγRIIA-His/His131. In Kenya, possession of the genotype FcγRIIA-Arg/Arg131 per se is associated with protection from high-density infection (37).
Only the addition of monocytes in ADCI initiated the antiparasitic action, thus substantiating previous reports stating that the effect of anti-MSP-3 is elicited in cooperation with monocytes (4, 2).
In conclusion, the present study takes another step to “close the circle” from the transfer of antibody-based immunity in the clinic, defining the functional properties associated with the effect, isolating an involved antigen (MSP-3), and finally to constructing defined recombinant antibodies with functional properties comparable to those of the antibodies initially found in malaria-immune individuals. The definitive step would be the validation of the antiparasitic effect in a clinical setting.
Acknowledgments
In memory of Jan Engberg, who was supposed to be a coauthor of this paper.
We thank Betina Poulsen for excellent technical assistance.
This work was in part supported from the European Union STD Program (contract number TS3*-CT94-0317), the Novo Nordisk Research Foundation, and the Toyota Foundation, Denmark. M.H.D. has been supported by a fellowship from the Danish MRC; L.K.N. and R.L. have been supported by scholarships from the Research Foundation of H:S.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 73:8017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., G. Noun, P. Druilhe, C. Nakhle, N. Haddad, and S. Chamat. 2004. Plasmodium falciparum: production of human antibodies specific for the MSP-3 protein in the Hu-SPL-SCID Mouse. Exp. Parasitol. 108:47-52. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess, B. R., P. Schuck, and D. N. Garboczi. 2005. Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J. Biol. Chem. 280:37236-37245. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, D. R., C. Dobano, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirgwin, J. M., A. E. Przybyla, R. J. MacDonald, and W. J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294-5299. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland, S. M., T. D. Jones, and N. J. Dimmock. 2000. Properties of a neutralizing antibody that recognizes a conformational form of epitopeERDRD in the gp41 C-terminal tail of human immunodeficiency virus type 1. J. Gen. Virol. 81:1251-1260. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 10.Cranmer, S. L., C. Magowan, J. Liang, R. L. Coppel, and B. M. Cooke. 1997. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91:363-365. [DOI] [PubMed] [Google Scholar]
- 11.de Lange, G. G. 1991. Allotypes and other epitopes of immunoglobulins. Baillieres Clin. Haematol. 4:903-925. [DOI] [PubMed] [Google Scholar]
- 12.Druilhe, P., and S. Khusmith. 1987. Epidemiological correlation between levels of antibodies promoting merozoite phagocytosis of Plasmodium falciparum and malaria-immune status. Infect. Immun. 55:888-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Druilhe, P., F. Spertini, D. Soesoe, G. Corradin, P. Mejia, S. Singh, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLOS Med. 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziegiel, M., M. B. Borre, S. Jepsen, B. Hogh, E. Petersen, and J. Vuust. 1991. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 44:306-313. [DOI] [PubMed] [Google Scholar]
- 15.Dziegiel, M., L. K. Nielsen, P. S. Andersen, A. Blancher, E. Dickmeiss, and J. Engberg. 1995. Phage display used for gene cloning of human recombinant antibody against the erythrocyte surface antigen, rhesus D. J. Immunol. Methods 182:7-19. [DOI] [PubMed] [Google Scholar]
- 15a.Dziegiel, M. S. H., R. Lundquist, and L. K. Nielsen. September. 2005. Recombinant anti-Plasmodium falciparum antibodies. United Kingdom patent 2378949.
- 16.Edozien, J. C., H. M. Gilles, and I. O. K. Udeozo. 1962. Adult and cord-blood gamma-globulin and immunity to malaria in Nigerians. Lancet 2:951-955. [Google Scholar]
- 17.Engberg, J., P. S. Andersen, L. K. Nielsen, M. Dziegiel, L. K. Johansen, and B. Albrechtsen. 1996. Phage-display libraries of murine and human antibody Fab fragments. Mol. Biotechnol. 6:287-310. [DOI] [PubMed] [Google Scholar]
- 18.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 19.Hougs, L., L. Juul, A. Svejgaard, and T. Barington. 1999. Structural requirements of the major protective antibody to Haemophilus influenzae type B. Infect. Immun. 67:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khusmith, S., and P. Druilhe. 1983. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect. Immun. 41:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitan, B. 1998. Stochastic modeling and optimization of phage display. J. Mol. Biol. 277:893-916. [DOI] [PubMed] [Google Scholar]
- 22.McColl, D. J., A. Silva, M. Foley, J. F. Kun, J. M. Favaloro, J. K. Thompson, V. M. Marshall, R. L. Coppel, D. J. Kemp, and R. F. Anders. 1994. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 68:53-67. [DOI] [PubMed] [Google Scholar]
- 23.McGregor, I. A. 1964. The passive transfer of human malarial immunity. Am. J. Trop. Med. Hyg. 13:237-239. [DOI] [PubMed] [Google Scholar]
- 24.Mulhern, T. D., G. J. Howlett, G. E. Reid, R. J. Simpson, D. J. McColl, R. F. Anders, and R. S. Norton. 1995. Solution structure of a polypeptide containing four heptad repeat units from a merozoite surface antigen of Plasmodium falciparum. Biochemistry 34:3479-3491. [DOI] [PubMed] [Google Scholar]
- 25.Ndungu, F. M., P. C. Bull, A. Ross, B. S. Lowe, E. Kabiru, and K. Marsh. 2002. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 24:77-82. [DOI] [PubMed] [Google Scholar]
- 26.Norderhaug, L., T. Olafsen, T. E. Michaelsen, and I. Sandlie. 1997. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J. Immunol. Methods 204:77-87. [DOI] [PubMed] [Google Scholar]
- 27.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 28.Oeuvray, C., M. Theisen, C. Rogier, J. F. Trape, S. Jepsen, and P. Druilhe. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 68:2617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orum, H., P. S. Andersen, A. Oster, L. K. Johansen, E. Riise, M. Bjornvad, I. Svendsen, and J. Engberg. 1993. Efficient method for constructing comprehensive murine Fab antibody libraries displayed on phage. Nucleic Acids Res. 21:4491-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce, J. A., A. N. Hodder, and R. F. Anders. 2004. The alanine-rich heptad repeats are intact in the processed form of Plasmodium falciparum MSP3. Exp. Parasitol. 108:186-189. [DOI] [PubMed] [Google Scholar]
- 31.Pearce, J. A., K. Mills, T. Triglia, A. F. Cowman, and R. F. Anders. 2005. Characterisation of two novel proteins from the asexual stage of Plasmodium falciparum, H101 and H103. Mol. Biochem. Parasitol. 139:141-151. [DOI] [PubMed] [Google Scholar]
- 32.Roeffen, W. F., J. M. Raats, K. Teelen, R. M. Hoet, W. M. Eling, W. J. van Venrooij, and R. W. Sauerwein. 2001. Recombinant human antibodies specific for the Pfs48/45 protein of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 276:19807-19811. [DOI] [PubMed] [Google Scholar]
- 33.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanger, F., G. M. Air, B. G. Barrell, N. L. Brown, A. R. Coulson, C. A. Fiddes, C. A. Hutchison, P. M. Slocombe, and M. Smith. 1977. Nucliotide sequence of bacteriophage phi X174 DNA. Nature 265:687-695. [DOI] [PubMed] [Google Scholar]
- 36.Seehaus, T., F. Breitling, S. Dubel, I. Klewinghaus, and M. Little. 1992. A vector for the removal of deletion mutants from antibody libraries. Gene 114:235-237. [DOI] [PubMed] [Google Scholar]
- 37.Shi, Y. P., B. L. Nahlen, S. Kariuki, K. B. Urdahl, P. D. McElroy, J. M. Roberts, and A. A. Lal. 2001. Fc gamma receptor IIa (CD32) polymorphism is associated with protection of infants against high-density Plasmodium falciparum infection. VII. Asembo Bay Cohort Project. J. Infect. Dis. 184:107-111. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Y. P., V. Udhayakumar, A. J. Oloo, B. L. Nahlen, and A. A. Lal. 1999. Differential effect and interaction of monocytes, hyperimmune sera, and immunoglobulin G on the growth of asexual stage Plasmodium falciparum parasites. Am. J. Trop. Med. Hyg. 60:135-141. [DOI] [PubMed] [Google Scholar]
- 39.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 40.Singh, S., S. Soe, C. Roussilhon, G. Corradin, and P. Druilhe. 2005. Plasmodium falciparum merozoite surface protein 6 displays multiple targets for naturally occurring antibodies that mediate monocyte-dependent parasite killing. Infect. Immun. 73:1235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, T., G. Killeen, C. Lengeler, and M. Tanner. 2004. Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am. J. Trop. Med. Hyg. 71:80-86. [PubMed] [Google Scholar]
- 42.Soe, S., M. Theisen, C. Roussilhon, K. S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soe, S., S. A. Khin, A. Htay, W. Nay, A. Tin, S. Than, C. Roussilhon, J. L. Perignon, and P. Druilhe. 2001. Premunition against Plasmodium falciparum in a malaria hyperendemic village in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 95:81-84. [DOI] [PubMed] [Google Scholar]
- 44.Sowa, K. M., D. R. Cavanagh, A. M. Creasey, J. Raats, J. McBride, R. Sauerwein, W. F. Roeffen, and D. E. Arnot. 2001. Isolation of a monoclonal antibody from a malaria patient-derived phage display library recognising the Block 2 region of Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 112:143-147. [DOI] [PubMed] [Google Scholar]
- 45.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 47.Tebo, A. E., P. G. Kremsner, and A. J. F. Luty. 2002. Fc gamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin. Exp. Immunol. 130:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theisen, M., D. Dodoo, A. Toure-Balde, S. Soe, G. Corradin, K. K. Koram, J. A. L. Kurtzhals, L. Hviid, T. Theander, B. Akanmori, M. Ndiaye, and P. Druilhe. 2001. Selection of glutamate-rich protein long synthetic peptides for vaccine development: antigenicity and relationship with clinical protection and immunogenicity. Infect. Immun. 69:5223-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trucco, C., D. Fernandez-Reyes, S. Howell, W. H. Stafford, T. J. Scott-Finnigan, M. Grainger, S. A. Ogun, W. R. Taylor, and A. A. Holder. 2001. The merozoite surface protein 6 gene codes for a 36-kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91-101. [DOI] [PubMed] [Google Scholar]
- 50.van der Heyde, H. C., M. M. Elloso, W. J. vande, K. Schell, and W. P. Weidanz. 1995. Use of hydroethidine and flow cytometry to assess the effects of leukocytes on the malarial parasite Plasmodium falciparum. Clin. Diagn. Lab. Immunol. 2:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wohlhueter, R. M., K. Parekh, V. Udhayakumar, S. N. Fang, and A. A. Lal. 1994. Analysis of binding of monoclonal antibody to a malarial peptide by surface-plasmon resonance biosensor and integrated rate equations. J. Immunol. 153:181-189. [PubMed] [Google Scholar]