Abstract
Johne's disease (JD) infection, caused by Mycobacterium avium subsp. paratuberculosis, represents a major disease problem in farmed ruminants. Although JD has been well characterized in cattle and sheep, little is known of the infection dynamics or immunological response in deer. In this study, typing of M. avium subsp. paratuberculosis isolates from intestinal lymphatic tissues from 74 JD-infected animals showed that clinical isolates of M. avium subsp. paratuberculosis from New Zealand farmed red deer were exclusively of the bovine strain genotype. The susceptibility of deer to M. avium subsp. paratuberculosis was further investigated by experimental oral-route infection studies using defined isolates of virulent bovine and ovine M. avium subsp. paratuberculosis strains. Oral inoculation with high (109 CFU/animal) or medium (107 CFU/animal) doses of the bovine strain of M. avium subsp. paratuberculosis established 100% infection rates, compared to 69% infection following inoculation with a medium dose of the ovine strain. The high susceptibility of deer to the bovine strain of M. avium subsp. paratuberculosis was confirmed by a 50% infection rate following experimental inoculation with a low dose of bacteria (103 CFU/animal). This study is the first to report experimental M. avium subsp. paratuberculosis infection in red deer, and it outlines the strong infectivity of bovine-strain M. avium subsp. paratuberculosis isolates for cervines.
Paratuberculosis, or Johne's disease (JD), caused by Mycobacterium avium subsp. paratuberculosis, represents a mycobacterial disease of major importance to the livestock farming industry after the elimination of bovine tuberculosis. The economic impact of JD on sheep and cattle farming has been recognized for some time (17, 24), leading to major efforts towards the development of improved means of management and control of the disease in these domesticated ruminant species. Recently, JD has also been identified as an emerging problem in deer farming (20, 22).
In deer, as in cattle and sheep, JD presents as a chronic inflammatory disease of the lower intestinal tract, which can lead to loss of condition due to impaired gut digestive and absorptive functions. In severe cases, mortality can occur in clinically affected adult animals. However, in contrast to the case for cattle and sheep, JD may often be diagnosed in yearlings and can cause death in deer less than 1 year old (21). Primary diagnosis of JD in farmed deer is based on the detection of M. avium subsp. paratuberculosis shed in feces, milk, or semen or on postmortem examination of affected gastrointestinal tract tissues, such as epithelial and subepithelial tissues of the small intestine, especially the lower part of the jejunum, ileum, and ileocecal junction region and its associated draining lymph nodes (2). However, improved and more precise in vivo immunodiagnostics tests are currently being developed for the early identification of M. avium subsp. paratuberculosis infection in deer (16). Furthermore, preliminary studies on the feasibility of prophylactic vaccination against JD in deer have been undertaken (23).
The emerging problem of JD in farmed deer is underscored by the fact that little is known about M. avium subsp. paratuberculosis infection dynamics in this species. In particular, little is known about the pattern of immunological reactivity in M. avium subsp. paratuberculosis-infected deer that would be relevant to the development of improved immunodiagnostics or vaccine disease management tools. In other M. avium subsp. paratuberculosis-susceptible ruminant species, such as cattle, controlled experimental infection studies using defined isolates of M. avium subsp. paratuberculosis have identified both cell-mediated and humoral immune reactivity (32). M. avium subsp. paratuberculosis-specific peripheral blood CD4+ lymphocytes capable of secreting gamma interferon (IFN-γ) were activated, along with the production of immunoglobulin G (IgG) class antibodies specific for mycobacterial surface glycolipids. Such responses have been reported to become identifiable at approximately 20 weeks after experimental infection in cattle, emphasizing the chronic subclinical nature of the disease (32). In contrast, nothing is known of the kinetics of immune reactivity following controlled M. avium subsp. paratuberculosis infection in deer, nor are the patterns of cellular and humoral immunological reactivity well defined.
Recent advances in molecular typing have facilitated the identification of different M. avium subsp. paratuberculosis isolates. Through the use of IS900 restriction fragment length polymorphism (RFLP) and/or IS1311 PCR-restriction enzyme analysis (PCR-REA) methodologies, it is possible to differentiate bovine host-specific strains of M. avium subsp. paratuberculosis from ovine strains in clinical tissue samples (34). To a major extent, strains causing clinical cases of JD in farmed cattle and sheep can be typed as having either the bovine or ovine M. avium subsp. paratuberculosis genotype, respectively, although the genotypic status of M. avium subsp. paratuberculosis isolates from clinical cases of JD in deer (cervines) is not as well defined. Conflicting results have been reported, with some studies suggesting that ovine strains of M. avium subsp. paratuberculosis can be routinely isolated from deer (9, 10), while others report that cervine isolates are predominantly of the bovine genotype (20, 28, 34). Overall, the general perception is that deer are probably susceptible to infection with both bovine and ovine strains of M. avium subsp. paratuberculosis (6), although this assumption is unproven; nor have the relative susceptibilities of deer to these two strains been compared.
The present study was initiated to provide a more complete understanding of the infection dynamics of M. avium subsp. paratuberculosis in red deer, with particular emphasis on defining the patterns of immunological response in animals following controlled experimental infection and on monitoring longitudinal changes in these responses. We further addressed the issue of the relative susceptibility of deer to bovine or ovine strains of M. avium subsp. paratuberculosis and here report characteristics of the infection and ensuing immunological reactivity in red deer infected with either strain of the pathogen.
MATERIALS AND METHODS
Ethical approvals.
The animal experiments carried out in this study were approved by the Invermay AgResearch Animal Ethics Committee (INV607/03).
Farm setting and collection of field samples.
A total of 74 infected red deer (Cervus elaphus) originating from 10 farms throughout the South Island of New Zealand were subjected to gross pathological examination at necropsy. These farms were noncontiguous, and some were separated by more than 400 km. None of the farms involved shared property boundaries. Samples of the jejunal lymph nodes and the ileocecal lymph node (ICLN), as well as tissue sections of jejunum, terminal ileum, and ileocecal valve (ICV), were taken for bacteriological culture and histological examinations.
Experimental animals.
A group of 81 newly weaned 4-month-old red deer (average weight at commencement of study, 50 kg; range, 41 to 60 kg) were obtained from the AgResearch Invermay Deer Farm, which had a ≥10-year-clear bovine tuberculosis-“free” status, has never had any clinical cases of JD, and has never had any lesions due to M. avium subsp. paratuberculosis found at slaughter. The animals received routine animal health treatments, which included pour-on moxidectin, a 4-g copper capsule, and vaccination with Yersiniavax. The study animals were subsequently maintained on pasture at the AgResearch Invermay research farm and fed ad libitum.
Isolation and preparation of M. avium subsp. paratuberculosis for experimental infection.
Two inocula were prepared directly from lymph nodes of a clinically affected merino sheep (no. JD3) (4) and a clinically affected red deer (no. 564). These clinically diseased animals were euthanatized, and in addition to the lymph nodes taken to harvest M. avium subsp. paratuberculosis organisms, fresh and fixed samples were taken for culture, histopathological examination, IS900 PCR, and IS1311 PCR-REA to confirm the diagnosis and identify the strains. The JD3 strain was confirmed as an “ovine” strain, and the 564 strain was confirmed as a “bovine” strain. An estimate of the number of organisms present in each tissue homogenate was made by microscopic counting under phase contrast prior to dosing the animals. CFU of bacteria were confirmed retrospectively by plate culture. There were consistently low levels of bacterial contamination when M. avium subsp. paratuberculosis was obtained directly from lymphatic tissues, recovered aseptically, from animals at necropsy. These two strains of M. avium subsp. paratuberculosis (JD3 and 564) were used to experimentally challenge deer by the oral route in this study.
Experimental infection, longitudinal blood monitoring, and necropsy.
Eighty-one deer were randomly assigned to one of five groups. Four of these groups were experimentally infected orally with defined numbers of M. avium subsp. paratuberculosis organisms obtained from homogenized gut lymphatic tissues (4) as follows: high-dose bovine-strain group (n = 16), 109 CFU strain 564; medium-dose bovine-strain group (n = 16), 107 CFU strain 564; medium-dose ovine-strain group (n = 16), 107 CFU strain JD3; and low-dose bovine-strain group (n = 16), 103 CFU strain 564. In each case, infection inocula were administered as four one-quarter doses of the total challenge dose, given on each of four sequential days.
Animals were subsequently maintained on open pasture in separate paddocks. In addition, a fifth group of animals (n = 17) was used as a sentinel control group; these animals were not inoculated with M. avium subsp. paratuberculosis but were maintained in the same paddock as the deer inoculated with the high-dose bovine strain for the duration of the study.
At intervals of 6, 12, 18, 24, 30, 36, and 44 weeks postinoculation, 20-ml heparinized blood samples were drawn from manually restrained animals by jugular venipuncture into evacuated tubes. At 44 weeks, all animals were euthanatized humanely using a captive bolt stun gun. Following exsanguinations, the intestines were removed from below the abomasum through to the rectum. Samples were taken from serial sections of the ICLN and ICV. Tissues were examined first macroscopically and then histologically to determine the grade of pathology at a microscopic level. Histological lesions were graded on a scale of 0 to 3 using the Perez classification (29) but without identifying the subtypes within each pathology grade. Based on the gross lesions and histopathological grading, animals were assigned a numerical disease score on a grading scale of 0 to 3 (0, no pathology; 3, severe pathology) to categorize the pathology found in individual animals. Additionally, samples of ICLN and ICV were homogenized and aliquots plated onto Middlebrook 7H11 agar for the bacteriological culture identification of M. avium subsp. paratuberculosis.
Molecular typing of M. avium subsp. paratuberculosis strains.
Seventy-four isolates of M. avium subsp. paratuberculosis, from 10 different properties, were cultured from cervine intestinal (mostly ileo-cecal) lymph nodes. Approximately 1g of lymph node tissue was homogenized in sterile, distilled water using a pestle and mortar and examined microscopically for acid-fast organisms by Ziehl-Neelsen staining. Of this homogenate, 250 μl was cultured onto Middlebrook 7H11 agar (Difco) with and without Mycobactin J (Allied Monitor) (1 μg/ml) and in a third vial containing Mycobactin J, vancomycin (50 μg/ml), nalidixic acid (50 μg/ml), and amphotericin B (50 μg/ml). Vials were incubated for up to 20 weeks at 37°C in a 5% CO2 atmosphere. Growth was checked microscopically by Ziehl-Neelsen staining. Prior to PCR analysis, isolates were heat killed at 90°C for 1 h.
DNA was extracted from mycobacterial pellets for PCR typing by standard methods (5, 7). Extracted samples were resuspended in 50 μl of sterile, distilled water. PCR to detect IS900 was performed with the primers P90 (5′-GAA GGG TGT TCG GGG CCG TCG CTT AGG) and P91 (5′-GGC GTT GAG GTC GAT CGC CCA CGT GAC) (25, 33), using 5 μl of genomic DNA extract as the template. The PCR mixture consisted of 1× HotMaster PCR buffer (Eppendorf, Hamburg, Germany), 0.5 μM each primer, 200 μM deoxynucleoside triphosphates, and 1.5 U HotMaster Taq DNA polymerase (Eppendorf, Hamburg, Germany), and PCRs were carried out in 50-μl reaction volumes in a Dyad DNA Engine thermal cycler (MJ Research). Reactions were cycled using a touchdown PCR protocol consisting of an initial six cycles of denaturation at 94°C for 30 s, lowering of the annealing temperature sequentially from 65°C down to 58°C for 30 s over six cycles, and a 1-min extension at 65°C, followed by 24 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 65°C for 1 min. The resulting 413-bp amplification products were visualized by electrophoresis on a 1% agarose gel stained with ethidium bromide. The M. avium subsp. paratuberculosis vaccine strain 316F served as a positive control for IS900 amplification.
Ovine/bovine strain typing of M. avium subsp. paratuberculosis isolates was performed as described by Marsh et al., based upon their observation that some copies of IS1311 from bovine strains carry a point mutation creating a recognition site for the restriction endonuclease Hinf1 (25). Primers M56 (5′-GCG TGA GGC TCT GTG GTG AA) and M94 (5′-CAG CGA TCG TCG ACA GTG TG) were used to amplify a 268-bp fragment of IS1311, which was subsequently digested with Hinf1. Following digestion, the presence of three restriction fragments (268 bp, 218 bp, and 50 bp) indicated that isolates were of the bovine strain genotype, whereas a single, undigested 268-bp fragment was indicative of the ovine strain genotype. Restriction fragments were visualized by electrophoresis on a 2% agarose gel stained with ethidium bromide. The M. avium subsp. paratuberculosis vaccine strain 316f served as a positive control for the bovine strain of M. avium subsp. paratuberculosis, and a clinical isolate of M. avium subsp. paratuberculosis, originating from a sheep infected with JD, served as a positive control for the ovine strain of M. avium subsp. paratuberculosis.
Serological tests for anti-M. avium subsp. paratuberculosis IgG1 antibodies.
A standard enzyme-linked immunosorbent assay (ELISA) protocol (11) with modifications for use in deer (13) was used in this study, with variations in antigens and antibodies used as stated. Ninety-six-well microtiter plates (Maxisorp; Nunc Products, Denmark) were coated with 50 μl of either of the following two target antigens at a final concentration of 5 μg/ml in carbonate buffer, pH 9.6: paratuberculosis protoplasmic antigen (PpAg) (obtained from Allied Monitor Inc, Fayette, MO) or purified protein derivative of M. paratuberculosis (PPDj) (obtained from CIDC Lelystad, The Netherlands). After incubation overnight at 4°C, unbound antigen was removed from the plates by washing six times in phosphate-buffered saline containing 0.05% Tween 20 (wash buffer). Test serum samples were diluted in wash buffer, added to separate wells for each antigen, incubated for 1 h at 37°C, and then washed a further six times. Unconjugated mouse monoclonal antibody specific for cervine IgG1 (9-f-98) (16) was then added and incubated for 1 h at 37°C, and unbound antibody was removed by washing six times. Antibody binding was visualized using a polyclonal goat anti-mouse IgG horseradish peroxidase-conjugated tertiary antibody (Biosource International, Camarillo, CA) and an O-phenylenediamine dihydrochloride (Sigma, St. Louis, Mo.) substrate system, as described previously (13). The reaction was stopped by addition of H2SO4 and the absorbance read at 490 nm using an automated microplate reader (Bio-Rad model 3550). Optical densities (OD) were converted to ELISA units by subtracting the OD of known negative serum samples from the OD of the test serum and multiplying by 100.
Cell mediated immunity (CMI) tests.
Lymphocyte transformation (LT) tests were conducted on Ficoll/Conroy-separated deer peripheral blood mononuclear cells, as described previously (13) but with the following modifications in the use of antigens. Leukocytes were plated at 2.5 × 105 viable cells per well into multiple wells of 96-well tissue culture plates (Nunc Products, Denmark). Cells were stimulated in the presence or absence of 2.5 μg of PPDj or purified protein derivative of Mycobacterium bovis (PPD-B) (CSL Ltd., Melbourne, Australia) for 5 days, prior to measurement of lymphocyte transformation via nuclear incorporation of [3H]thymidine as described previously (13). Beta emission was measured as mean counts per minute using a liquid scintillation counter.
IFN-γ production was induced by in vitro coculture of peripheral blood samples with the same antigens as outlined above. The assay used involved the proprietary BOVIGAM ELISA kit (Pfizer Animal Health Ltd). Briefly 1.5-ml aliquots of whole heparinized blood were placed into each of two wells of a 24-well tissue culture plate (BD Falcon). Each well was stimulated with 30 μg of PPDj or PPD-B. After 24 h, culture supernatants were removed and frozen at −20°C until tested. Analysis of IFN-γ was performed using the standard protocol recommended for the BOVIGAM kit; data were expressed as average OD units.
For both CMI assays, data were expressed as the mean differential signal produced in response to cell stimulation with PPDj minus that produced by stimulation with PPD-B.
Statistical analysis.
Differences in immune responses between treatment groups were compared by one-way analysis of variance, with Tukey's post hoc test to identify statistical significance with a probability value of <0.05.
RESULTS
Infection dynamics: experimental infection studies with deer.
At postmortem, pathological and histological investigation identified that 59 out of 81 deer had pathology consistent with M. avium subsp. paratuberculosis infection, which was subsequently confirmed as positive by culture. Twelve of 81 animals were culture positive in the absence of identifiable pathology, and 10 of 81 animals remained noninfected and nondiseased. Among the different infection subgroups, viable M. avium subsp. paratuberculosis organisms were isolated from the intestinal tissues of 16/16 of animals infected with either the high or medium dose of the bovine strain and from 8/16 animals infected with the low-dose of the bovine strain (Table 1). All 17 of the in-contact sentinels were also confirmed to be culture positive for M. avium subsp. paratuberculosis. Among the animals inoculated with the ovine strain of M. avium subsp. paratuberculosis, 11/16 were culture positive at necropsy.
TABLE 1.
Influence of inoculation strain and dose on subsequent recovery of live M. avium subsp. paratuberculosis organisms from gastrointestinal tract tissues of deer
| Tissue | No. of infected animals after inoculation with M. avium subsp. paratuberculosis
|
||||
|---|---|---|---|---|---|
| Bovine strain
|
Ovine strain, medium dose (n = 16) | ||||
| In-contact sentinels (n = 17) | High dose (n = 16) | Medium dose (n = 16) | Low dose (n = 16) | ||
| ICLN | 16 | 16 | 16 | 3 | 8 |
| ICV | 12 | 16 | 14 | 6 | 3 |
| Totala (%) | 17 (100) | 16 (100) | 16 (100) | 8b (50) | 11b (69) |
Total number of animals with infection in ICLN and/or ICV.
Significantly lower incidence of culture-confirmed infection in deer inoculated with the low dose of the bovine strain of M. avium subsp. paratuberculosis (P = 0.02) or with the medium dose of the ovine strain of M. avium subsp. paratuberculosis (P = 0.043) compared to animals inoculated with either the high or medium dose of the bovine strain (Fisher's exact test).
Longitudinal patterns of immune reactivity.
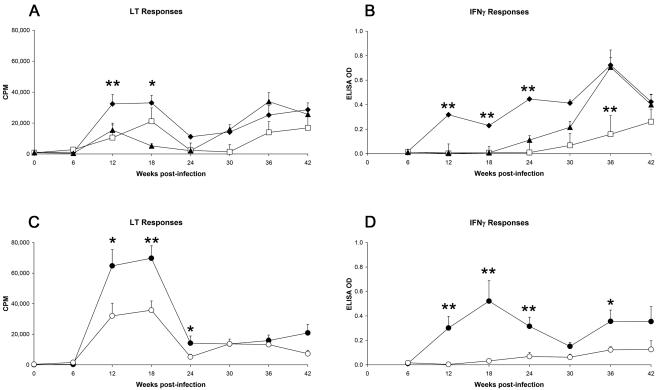
In deer that were experimentally infected with M. avium subsp. paratuberculosis, antigen-specific LT and IFN-γ responses were first apparent at 12 weeks postinfection (Fig. 1). Overall CMI responses were highest among deer that received the high- or medium-dose inocula of the bovine strain of M. avium subsp. paratuberculosis. LT responses in both the groups receiving both the high and medium doses of the bovine strain peaked at 18 weeks and then declined; IFN-γ responses in the medium-dose bovine-strain group followed a similar pattern, while IFN-γ responses in the high-dose bovine-strain group rose progressively until week 36 postinfection. Deer infected with the low dose of the bovine strain of M. avium subsp. paratuberculosis showed a lesser degree of CMI reactivity, with LT responses first apparent at 12 weeks but IFN-γ responses not apparent until week 30 postinfection. The deer which were maintained in contact with the group infected with the high dose of the bovine strain pf M. avium subsp. paratuberculosis showed a pattern of immune reactivity similar to that of the animals infected with the low dose of the bovine strain of M. avium subsp. paratuberculosis, with LT responses first apparent at 12 weeks but IFN-γ responses not apparent until week 30 postinfection. Deer infected with the ovine strain of M. avium subsp. paratuberculosis showed a low level of IFN-γ reactivity (with responses first detectable at week 24 and sustained until week 44); LT responses were first detectable at week 12 and peaked at week 18 in this group. When deer that had been experimentally infected with medium doses of the bovine or ovine strain of M. avium subsp. paratuberculosis were directly compared, overall CMI responses were more vigorous in response to the medium dose of the bovine strain of M. avium subsp. paratuberculosis than to the ovine strain.
FIG. 1.
CMI responses following experimental infection of deer with different doses of the bovine or ovine strain of M. avium subsp. paratuberculosis. All data represent mean (+ standard error of the mean) responses for n = 16 or 17 deer/group. Asterisks indicate statistically significant differences observed in animals infected with the high dose (⧫) or low dose (□) of the bovine strain of M. avium subsp. paratuberculosis versus sentinel control animals (▴) (panels A and B) or to statistically significant differences observed between animals infected with the medium dose of the bovine strain (•) and the medium dose of the ovine strain (○) (panels C and D). *, P < 0.05; **, P < 0.01.
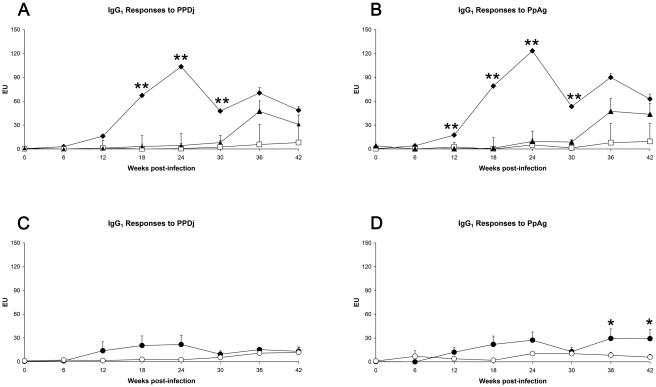
The IgG1 serological response profiles in M. avium subsp. paratuberculosis-infected deer were similar when either PpAg or PPDj was used as the target antigen (Fig. 2). The IgG1 response in bovine-strain-infected deer was dose dependent, with the strongest responses seen in the high-dose inoculation group, followed by the medium dose group; responses were first apparent in these groups at 12 weeks postinfection. IgG1 responses in the low-dose bovine-strain group remained at a low level throughout, while responses among the in-contact sentinel animals became apparent with a delayed effect at week 36 and then rose to a magnitude similar to that observed in the animals infected with the medium dose of the bovine strain. Deer infected with the ovine strain of M. avium subsp. paratuberculosis remained serologically nonreactive throughout the study.
FIG. 2.
Serological responses following experimental infection of deer with different doses of the bovine or ovine strain of M. avium subsp. paratuberculosis. EU, ELISA units. All data represent mean (+ standard error of the mean) responses for n = 16 or 17 deer/group. Asterisks refer to statistically significant differences observed in animals infected with the high dose (⧫) or low dose (□) of the bovine strain of M. avium subsp. paratuberculosis animals versus sentinel control animals (▴) (panels A and B) or to statistically significant differences observed between animals infected with the medium dose of the bovine strain (•) and the medium dose of the ovine strain (○) (panels C and D). *, P < 0.05; **, P < 0.01.
Immunological responses in relation to disease severity.
In order to fully understand how immunological responses relate to disease status, a further analysis was undertaken by first categorizing animals according to disease severity and then comparing several immune parameters across these groupings. Using these criteria, deer presenting with severe pathology constituted 16% of the animals (13/81); medium-grade pathology, 14% (11/81); low-grade pathology, 43% (35/81); infected and no pathology/culture positive, 15% (12/81); and uninfected, no pathology/culture negative, 12% (10/81).
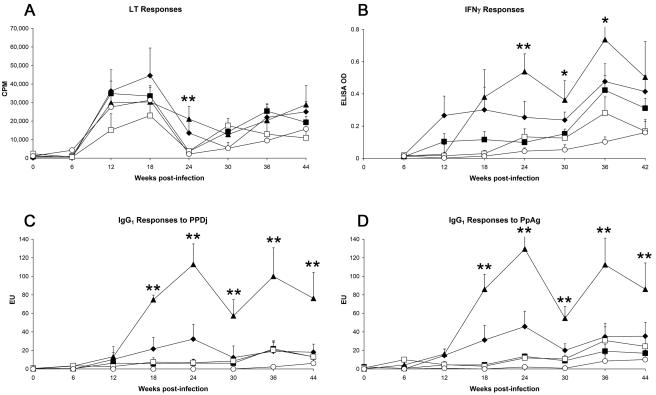
LT responses were largely independent of disease severity (Fig. 3) whereas IFN-γ responses showed a clear relationship to disease severity; animals exhibiting severe pathology had the strongest IFN-γ secretion, and IFN-γ responses generally lowered with declining disease severity. Serological reactivity followed a similar (but more exaggerated) pattern as cytokine secretion, with IgG1 antibody responses to both PpAg and PPDj strongly evident in deer exhibiting severe pathology and decreasing incrementally in association with decreasing severity of disease. Chronologically, increased LT responses were first evident among M. avium subsp. paratuberculosis-infected deer (all disease severity groups) at 12 weeks postinfection, while IFN-γ secretion and IgG1 antibody responses were delayed, becoming first apparent only at 18 weeks (and only in the high- and medium-pathology groupings at this time point) (Fig. 3). Uninfected animals had low levels of CMI, with LT reactivity at 12 to 18 and 42 weeks postchallenge and low levels of IFN-γ at 38 to 42 weeks.
FIG. 3.
Overview of the chronological changes in immune responses observed in M. avium subsp. paratuberculosis-exposed deer (both strains, all doses), based on postmortem grading of disease severity and infection status. EU, ELISA units. All data represent mean (+ standard error of the mean) responses. Asterisks refer to statistically significant differences among deer with high-grade pathology (⧫), medium-grade pathology (▴), low-grade pathology (▪), or M. avium subsp. paratuberculosis culture-positive status (□), each compared to animals that were M. avium subsp. paratuberculosis culture negative (○). *, P < 0.05; **, P < 0.01.
DISCUSSION
The present study has identified that red deer are particularly susceptible to infection with strains of M. avium subsp. paratuberculosis that can be identified by IS1311 PCR-REA as having the bovine genotype. Further, the ensuing patterns of immunological reactivity were most vigorous and diverse (CMI and humoral reactivity) following experimental infection with a defined virulent bovine strain of M. avium subsp. paratuberculosis. A total of 74 tissue samples, derived from New Zealand farmed deer with JD lesions and culture-confirmed M. avium subsp. paratuberculosis infection, were typed by IS1311 PCR-REA analysis. All 74 isolates proved to be bovine strains of M. avium subsp. paratuberculosis based on the identification pattern of three bovine-strain-specific fragments in Hinf1-digested products (data not shown). These results suggest that on-farm disease in deer, at least in New Zealand, is predominantly due to cross-infection with M. avium subsp. paratuberculosis isolates of bovine strain specificity.
While it has been suggested previously that deer might be equally susceptible to both bovine and ovine strains of M. avium subsp. paratuberculosis (6, 8, 9), there have been few independent verifications of this, and reviews commonly cite an instance from 1993 in which three cervine-derived isolates exhibited IS900 RFLP banding patterns similar to those generated by ovine-strain isolates in New Zealand (9). Whittington et al. summarized some documented occurrences in ruminants of strains of M. avium subsp. paratuberculosis as determined by IS900 RFLP analysis (34). Of 33 cervine-derived M. avium subsp. paratuberculosis isolates described (20 from New Zealand [9], 11 from South America [26], and 2 from Denmark [31]), only 3 had been reported to exhibit RFLP banding patterns similar to those of ovine strains of M. avium subsp. paratuberculosis. In the same report Whittington et al. confirmed that in a comparison of 297 M. avium subsp. paratuberculosis isolates which were strain typed by both IS900 RFLP and IS1311 PCR-REA there was complete concordance between these two typing approaches with respect to ovine/bovine genotype (34). Additionally, Collins et al. have reported complete agreement between RFLP typing and an alternative, also PCR-based, typing method (6). Pavlik et al. (28) and Machackova et al. (20) have strain typed more than 110 isolates of M. avium subsp. paratuberculosis from wild cervids, including red (Cervus elaphus), fallow (Dama dama), and roe (Capreolus capreolus) deer, by IS900 RFLP analysis. All cervine isolates from red, fallow, and roe deer resembled bovine-type M. avium subsp. paratuberculosis RFLP patterns except for a single isolate from a fallow deer which returned an “intermediate” strain type (20). In separate studies, 40 M. avium subsp. paratuberculosis isolates from a herd of farmed red deer in the Czech Republic also demonstrated bovine-strain banding patterns (21), as did a single fallow deer isolate of M. avium subsp. paratuberculosis from Spain (1) and three M. avium subsp. paratuberculosis isolates recovered from red deer in Italy (27). Thus, while deer may become infected with ovine strains of M. avium subsp. paratuberculosis (6, 9), this appears to be the exception rather than the rule. This is especially striking for New Zealand deer farms, where many properties have mixed animal grazing systems involving deer and sheep and many sheep properties have endemic ovine-strain M. avium subsp. paratuberculosis in their flocks.
Based on the findings obtained following typing of deer isolates of M. avium subsp. paratuberculosis, a controlled infection experiment was set up to specifically address the issue of the comparative susceptibilities and immune reactivities of red deer to bovine or ovine strains of M. avium subsp. paratuberculosis. In common with previous reports of experimental M. avium subsp. paratuberculosis infection in cattle (32), the data obtained showed that deer infected with bovine strains of M. avium subsp. paratuberculosis developed vigorous M. avium subsp. paratuberculosis-specific CMI responses which were first evident at 12 weeks postinfection. The onset of peripheral blood immune reactivity in deer is more rapid than that reported for cattle (32), and the course of clinical JD in deer has been reported to be more rapid than that observed in cattle (22), together suggesting that M. avium subsp. paratuberculosis infection in deer progresses more acutely than the equivalent disease in cattle.
In the present study, not only were deer susceptible to infection with high (109 CFU/animal) or medium (107 CFU/animal) doses of the bovine strain of M. avium subsp. paratuberculosis, but 50% became infected when the challenge inoculum was reduced to 103 CFU/animal (low dose). In contrast, deer inoculated with 107 CFU/animal of the ovine strain of M. avium subsp. paratuberculosis had a 69% infection rate. These results together suggest greater susceptibility of deer to establishment of infection with a bovine strain of the pathogen. CMI (LT and IFN-γ) and antibody responses were vigorous in deer following infection with high or medium doses of the bovine strain of M. avium subsp. paratuberculosis, while the equivalent (medium-dose) ovine strain of M. avium subsp. paratuberculosis only induced noticeable LT reactivity, with little observable IFN-γ induction or antibody production. Mixed CMI and antibody responses characterize disease caused by M. avium subsp. paratuberculosis in cattle (30). A limited LT response in the absence of IFN-γ and antibody (as observed in deer inoculated with sheep strains of M. avium subsp. paratuberculosis) might instead represent the development of protective immunity, possibly involving Th1-type pathways of immune reactivity.
The immunological reactivity of deer to the bovine strain of M. avium subsp. paratuberculosis was dose dependent, with both CMI and antibody responses strongest in deer infected with the highest dose (109 CFU/animal). Both CMI and antibody responses appeared to peak and then decline throughout the course of infection, but with different kinetics; CMI responses generally peaked at 12 to 18 weeks and then declined, while antibody responses peaked at 24 weeks. The early onset of CMI reactivity followed by seroconversion is a noted feature of chronic mycobacterial infection in ruminants, especially deer (12, 14), and has been noted specifically for M. avium subsp. paratuberculosis infection in cattle (3) and sheep (30). Khalifeh and Stabel have previously described a decline in the M. avium subsp. paratuberculosis-specific CMI response among chronically infected cattle, coincident with progression from subclinical to clinical disease (18). In sheep, Kurade et al. reported a decline in CMI reactivity concomitant with a rise in serological reactivity at approximately 20 weeks postinfection in animals experimentally infected with M. avium subsp. paratuberculosis, suggesting a change in the predominant immune phenotype around this time (19). Similarly, we have described that peripheral blood LT responses in M. avium subsp. paratuberculosis-inoculated sheep remain low for the first 6 months and then increase beyond 8 months in line with increased IgG1 antibody production and sharply decreased M. avium subsp. paratuberculosis-specific IFN-γ production (4).
Control of JD in farmed ruminants is dependent on detection and removal of subclinical disease in infected animals. We have recently developed a serological screening tool which detects subclinical JD infection in deer (16). In the present study the pattern of immunological reactivity in deer experimentally infected with M. avium subsp. paratuberculosis was analyzed in the context of the clinical outcome following experimental infection. LT responses were shown to be common to all M. avium subsp. paratuberculosis-exposed deer, regardless of disease outcome at necropsy. While LT appears to have limited value as a stand-alone test for the diagnosis of active M. avium subsp. paratuberculosis infection in deer, it is possible that LT combined with other components of the immunological response could form the basis of a composite immunodiagnostic test, similar to the one used for the diagnosis of M. bovis infection in farmed deer in New Zealand (13, 15). In contrast to LT, both IFN-γ production and IgG1 anti-M. avium subsp. paratuberculosis antibody reactivity were shown to correlate with disease severity, with responses strongest in the high- and medium-grade pathology groups. Serological reactivity to M. avium subsp. paratuberculosis surface glycolipids has been shown previously to be a feature of active M. avium subsp. paratuberculosis infection in cattle (32), and the IgG1 response to PPDj and protoplasmic antigens (PpAg) shown here correlates with the onset of disease and clinical pathology in deer. IgG1 serological reactivity to PpAg appears to be capable of differentiating between lesion negative/infected animals and lesion negative/uninfected animals at time points between 24 and 44 weeks postinfection, identifying subclinically affected animals as shown previously in naturally infected deer.
In summary, we have reported here that isolates of M. avium subsp. paratuberculosis from clinical cases of JD in New Zealand farmed deer were exclusively of the bovine strain genotype; moreover, deer experimentally challenged with the bovine strain of M. avium subsp. paratuberculosis more readily established infection and developed a more pronounced immunological response than animals inoculated with the ovine strain of M. avium subsp. paratuberculosis. While the ovine strain of M. avium subsp. paratuberculosis is still infectious to red deer, the susceptibility of deer to this strain appears to be lower, and the repertoire of immunological reactivity as a consequence of infection is more limited. The bovine strain of M. avium subsp. paratuberculosis used in the current study was isolated from infected deer lymphatic tissue, and the ovine strain was isolated directly from sheep. Consequently, it is debatable whether the increased virulence was due to host modification of the bacterial phenotype or to intrinsic genotypic virulence of the strain. In practical terms, it is likely that bovine strains of M. avium subsp. paratuberculosis will be of more economic relevance to the commercial farming of red deer, and the range and magnitude of immune reactivity suggest that it may be possible to develop improved diagnostic screening tools for bovine strains of M. avium subsp. paratuberculosis infection and JD in deer. In contrast, infection with ovine strains of M. avium subsp. paratuberculosis in deer is more insidious and appears to be less amenable to immunodiagnosis. Results from the current study suggest that farmed deer may represent an important natural reservoir of M. avium subsp. paratuberculosis infection which poses a threat to other domesticated livestock and wildlife.
Acknowledgments
We acknowledge the technical support from staff in the Disease Research Laboratory. The contribution made by farmers and veterinarians is also gratefully acknowledged. Microbial culturing was carried out by Geoff de Lisle and Gary Yates at AgResearch, Wallaceville, New Zealand. Histopathological examination of tissues was carried out by Gary Clark (AgResearch). We acknowledge the contribution made by Frank Cross in the preparation of the manuscript.
Funding support from the New Zealand Foundation of Research Science and Technology (FoRST) is gratefully acknowledged.
Editor: V. J. DiRita
REFERENCES
- 1.Alvarez, J., L. De Juan, V. Briones, B. Romero, A. Aranaz, J. F. Fernandez-Garayzabal, and A. Mateos. 2005. Mycobacterium avium subspecies paratuberculosis in fallow deer and wild boar in Spain. Vet. Rec. 156:212-213. [DOI] [PubMed] [Google Scholar]
- 2.Amemori, T., L. Matlova, O. A. Fischer, W. Y. Ayele, M. Machackova, E. Gopfert, and I. Pavlik. 2004. Distribution of Mycobacterium avium subsp. paratuberculosis in the gastrointestinal tract of shedding cows and its application to laparoscopic biopsy. Vet. Med. Czech. 49:225-236. [Google Scholar]
- 3.Begg, D. J., and J. F. Griffin. 2005. Vaccination of sheep against M. paratuberculosis: immune parameters and protective efficacy. Vaccine 23:4999-5008. [DOI] [PubMed] [Google Scholar]
- 4.Begg, D. J., R. O'Brien, C. G. Mackintosh, and J. F. Griffin. 2005. Experimental infection model for Johne's disease in sheep. Infect. Immun. 73:5603-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy, E., R. J. Whittington, I. Marsh, J. Marshall, and M. T. Campbell. 1998. A method for purification and characterisation of Mycobacterium avium subsp. paratuberculosis from the intestinal mucosa of sheep with Johne's disease. Vet. Microbiol. 64:51-60. [DOI] [PubMed] [Google Scholar]
- 6.Collins, D. M., M. De Zoete, and S. M. Cavaignac. 2002. Mycobacterium avium subsp. paratuberculosis strains from cattle and sheep can be distinguished by a PCR test based on a novel DNA sequence difference. J. Clin. Microbiol. 40:4760-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousins, D. V., R. A. Skuce, R. R. Kazwala, J. D. van Embden, et al. 1998. Towards a standardized approach to DNA fingerprinting of Mycobacterium bovis. Int. J. Tuberc. Lung Dis. 2:471-478. [PubMed] [Google Scholar]
- 8.de Lisle, G. W. 2002. Johne's disease in New Zealand: the past, present and a glimpse into the future. N.Z. Vet. J. 50:53-56. [DOI] [PubMed] [Google Scholar]
- 9.de Lisle, G. W., G. F. Yates, and D. M. Collins. 1993. Paratuberculosis in farmed deer: case reports and DNA characterization of isolates of Mycobacterium paratuberculosis. J. Vet. Diagn. Investig. 5:567-571. [DOI] [PubMed] [Google Scholar]
- 10.de Lisle, G. W., G. F. Yates, and R. H. Montgomery. 2003. The emergence of Mycobacterium paratuberculosis in farmed deer in New Zealand—a review of 619 cases. N.Z. Vet. J. 51:58-62. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, J. F., and G. S. Buchan. 1989. The ELISA technique for diagnosis of severe tuberculosis in deer and exotic ruminants. Proc. N.Z. Vet. Assoc. Deer Branch 5:78-86. [Google Scholar]
- 12.Griffin, J. F., D. N. Chinn, C. R. Rodgers, and C. G. Mackintosh. 2001. Optimal models to evaluate the protective efficacy of tuberculosis vaccines. Tuberculosis (Edinburgh) 81:133-139. [DOI] [PubMed] [Google Scholar]
- 13.Griffin, J. F., J. P. Cross, D. N. Chinn, C. R. Rodgers, and G. S. Buchan. 1994. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N.Z. Vet. J. 42:173-179. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, J. F., C. G. Mackintosh, and G. S. Buchan. 1995. Animal models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 3:418-424. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, J. F., C. G. Mackintosh, and C. R. Rodgers. 2006. Factors influencing the protective efficacy of a BCG homologous prime-boost vaccination regime against tuberculosis. Vaccine 24:835-845. [DOI] [PubMed]
- 16.Griffin, J. F. T., E. Spittle, C. R. Rodgers, S. Liggett, M. Cooper, D. Bakker, and J. P. Bannantine. 2005. An IgG1 ELISA for diagnosis of Johne's disease in red deer (Cervus elaphus). Clin. Diagn. Lab. Immunol. 12:1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, D. J., and G. Benedictus. 2001. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Tech. 20:151-179. [DOI] [PubMed] [Google Scholar]
- 18.Khalifeh, M. S., and J. R. Stabel. 2004. Upregulation of transforming growth factor-beta and interleukin-10 in cows with clinical Johne's disease. Vet. Immunol. Immunopathol. 99:39-46. [DOI] [PubMed] [Google Scholar]
- 19.Kurade, N. P., B. N. Tripathi, K. Rajukumar, and N. S. Parihar. 2004. Sequential development of histologic lesions and their relationship with bacterial isolation, fecal shedding, and immune responses during progressive stages of experimental infection of lambs with Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 41:378-387. [DOI] [PubMed] [Google Scholar]
- 20.Machackova, M., P. Svastova, J. Lamka, I. Parmova, V. Liska, J. Smolik, O. A. Fischer, and I. Pavlik. 2004. Paratuberculosis in farmed and free-living wild ruminants in the Czech Republic (1999-2001). Vet. Microbiol. 101:225-234. [DOI] [PubMed] [Google Scholar]
- 21.Machackova-Kopecna, M., M. Bartos, M. Straka, V. Ludvik, P. Svastova, J. Alvarez, J. Lamka, I. Trcka, F. Treml, I. Parmova, and I. Pavlik. 2005. Paratuberculosis and avian tuberculosis infections in one red deer farm studied by IS900 and IS901 RFLP analysis. Vet. Microbiol. 105:261-268. [DOI] [PubMed] [Google Scholar]
- 22.Mackintosh, C. G., G. W. de Lisle, D. M. Collins, and J. F. Griffin. 2004. Mycobacterial diseases of deer. N.Z. Vet. J. 52:163-174. [DOI] [PubMed] [Google Scholar]
- 23.Mackintosh, C. G., R. E. Labes, and J. F. Griffin. 2005. The effect of Johne's vaccination on tuberculin testing in farmed red deer (Cervus elaphus). N.Z. Vet. J. 53:216-222. [DOI] [PubMed] [Google Scholar]
- 24.Manning, E. J., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. 20:133-150. [DOI] [PubMed] [Google Scholar]
- 25.Marsh, I., R. Whittington, and D. Cousins. 1999. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311. Mol. Cell Probes. 13:115-126. [DOI] [PubMed] [Google Scholar]
- 26.Moreira, A. R., F. Paolicchi, C. Morsella, M. Zumarraga, A. Cataldi, B. Fabiana, A. Alicia, O. Piet, D. van Soolingen, and R. M. Isabel. 1999. Distribution of IS900 restriction fragment length polymorphism types among animal Mycobacterium avium subsp. paratuberculosis isolates from Argentina and Europe. Vet. Microbiol. 70:251-259. [DOI] [PubMed] [Google Scholar]
- 27.Nebbia, P., P. Robino, E. Ferroglio, L. Rossi, G. Meneguz, and S. Rosati. 2000. Paratuberculosis in red deer (Cervus elaphus hippelaphus) in the western Alps. Vet. Res. Commun. 24:435-443. [DOI] [PubMed] [Google Scholar]
- 28.Pavlik, I., J. Bartl, L. Dvorska, P. Svastova, R. du Maine, M. Machackova, W. Yayo Ayele, and A. Horvathova. 2000. Epidemiology of paratuberculosis in wild ruminants studied by restriction fragment length polymorphism in the Czech Republic during the period 1995-1998. Vet. Microbiol. 77:231-251. [DOI] [PubMed] [Google Scholar]
- 29.Perez, V., J. F. Garcia Marin, and J. J. Badiola. 1996. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 114:107-122. [DOI] [PubMed] [Google Scholar]
- 30.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 31.Thoresen, O. F., and I. Olsaker. 1994. Distribution and hybridization patterns of the insertion element IS900 in clinical isolates of Mycobacterium paratuberculosis. Vet. Microbiol. 40:293-303. [DOI] [PubMed] [Google Scholar]
- 32.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittington, R., I. Marsh, E. Choy, and D. Cousins. 1998. Polymorphisms in IS1311, an insertion sequence common to Mycobacterium avium and M. avium subsp. paratuberculosis, can be used to distinguish between and within these species. Mol. Cell. Probes 12:349-358. [DOI] [PubMed] [Google Scholar]
- 34.Whittington, R. J., A. F. Hope, D. J. Marshall, C. A. Taragel, and I. Marsh. 2000. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J. Clin. Microbiol. 38:3240-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]