Abstract
Bacterial pathogens in the genera Anaplasma and Ehrlichia encode a protein superfamily, pfam01617, which includes the predominant outer membrane proteins (OMPs) of each species, major surface protein 2 (MSP2) and MSP3 of Anaplasma marginale and Anaplasma ovis, Anaplasma phagocytophilum MSP2 (p44), Ehrlichia chaffeensis p28-OMP, Ehrlichia canis p30, and Ehrlichia ruminantium MAP1, and has been shown to be involved in both antigenic variation within the mammalian host and differential expression between the mammalian and arthropod hosts. Recently, complete sequencing of the A. marginale genome has identified an expanded set of genes, designated omp1-14, encoding new members of this superfamily. Transcriptional analysis indicated that, with the exception of the three smallest open reading frames, omp2, omp3, and omp6, these superfamily genes are transcribed in A. marginale-infected erythrocytes, tick midgut and salivary glands, and the IDE8 tick cell line. OMPs 1, 4, 7 to 9, and 11 were confirmed to be expressed as proteins by A. marginale within infected erythrocytes, with expression being either markedly lower (OMPs 1, 4, and 7 to 9) or absent (OMP11) in infected tick cells, which reflected regulation at the transcript level. Although the pfam01617 superfamily includes the antigenically variable MSP2 and MSP3 surface proteins, analysis of the omp1-14 sequences throughout a cycle of acute and persistent infection in the mammalian host and tick transmission reveals a high degree of conservation, an observation supported by sequence comparisons between the St. Maries strain and Florida strain genomes.
The surface coat of intracellular bacteria mediates key events in the interaction with the host cell, including invasion and intracellular trafficking. For tick-borne, intracellular pathogens, these interactions occur in both the mammalian and arthropod hosts as the bacterium adapts to survival and replication in each (38, 40, 44). In addition, variation in the surface coat allows evasion of the host immune response and the establishment of persistent infection in the mammalian reservoir host and thus an increased opportunity for ticks to acquire and transmit the pathogen (16, 17, 32). Until recently, the identification of outer membrane proteins (OMPs) of the tick-borne pathogens in the genera Anaplasma and Ehrlichia has been based primarily on antibody recognition and surface-specific labeling and, as a consequence, has been biased towards detection of immunodominant and highly abundant proteins (10, 31, 35, 37, 40, 43). These approaches led to the establishment of a surface protein superfamily (pfam01617) that includes members encoded by the Anaplasma marginale msp2 and msp3 operons, the Anaplasma phagocytophilum msp2(p44) operon, the p30 genes of Ehrlichia canis, p28-omp genes of Ehrlichia chaffeensis, and the map1 genes of Ehrlichia ruminantium.
In A. marginale, there are single expression sites for both msp2 and msp3; however, there are multiple functional msp2 and msp3 pseudogenes distributed throughout the chromosome that serve as templates for gene conversion to generate major surface protein 2 (MSP2) and MSP3 surface coat variants (3, 6, 7, 32). These variants, which escape the preexisting antibody response, are believed to be critical for the long-term persistence of the organism within the immunocompetent mammalian host (16). A. phagocytophilum msp2(p44) appears to generate variants in a similar manner, although the number of functional pseudogenes and the role of segmental gene conversion differ between the two Anaplasma spp. (2, 24). In addition to a role in immune evasion, differential expression of pfam01617 outer membrane proteins between the mammalian and tick stages of infection has been identified. The msp2 operon-associated genes include opag1, opag2, and opag3. These genes are arranged in tandem, are located 5′ of the full-length msp2, and are encoded by a single transcript, which includes msp2 (26). However, OpAG3 can be detected only in the infected erythrocytes of the mammalian host and not within Dermacentor andersoni midguts or salivary glands, while OpAG2 can be detected in all three tissues (26). Furthermore, there is no evidence that OpAG1 is expressed in any host cell, despite the presence of transcript (26). In contrast to the msp2/msp3 gene structure in Anaplasma spp., the p28-omp genes of E. chaffeensis are arranged as tandemly repeated full-length genes in a single locus containing 22 paralogs (37). Posttranscriptionally modified gene products from two of the p28-omp genes, p28-omp19 and p28-omp20, are expressed in the infected macrophages of the mammalian host, while similarly modified products from one p28-omp gene, p28-omp14, are expressed in tick cells (45). This host cell-specific expression of different members of these multigene families suggests that these proteins may be necessary for colonization and survival within distinct host cell environments.
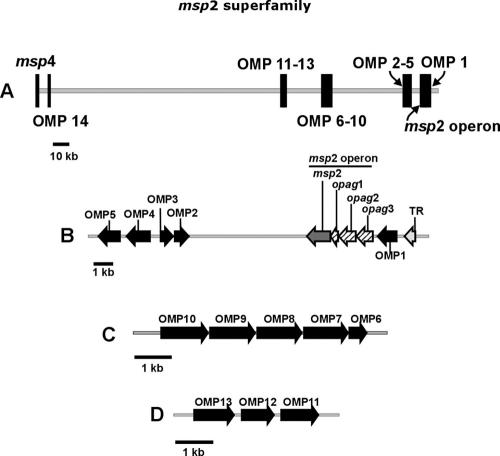
Complete sequencing of the A. marginale genome has identified additional genes encoding members of pfam01617, designated omp1 through omp14 (6). Both omp1 and omp14 are present in the chromosome as discrete single genes, while the remaining new members of the msp2 superfamily are arranged in three clusters, each at a distinct locus (Fig. 1): (i) omp2-5 are positioned near the msp2 operon with omp4 and omp5 and omp2 and omp3 being arranged in tandem, with the latter pair on the opposite strand from the msp2 operon; (ii) omp6-10 are arranged in tandem with a structure consistent with that of a five-gene operon; and (iii) omp11-13 are similarly arranged in an apparent three-gene operon (6). However, it is unknown if these genes are transcribed and if proteins are expressed during intracellular infection in either the mammalian host or tick vector. Thus, the first objective of this study is to determine whether these new members of the A. marginale msp2 superfamily, omp1-14, are transcribed and expressed in infected mammalian and tick cells.
FIG. 1.
(A) Spatial relationship among omp1-14 genes on the A. marginale chromosome, adapted from reference 6. (B) Segment of the genome with omp1, the msp2 operon, and omp2-5. (C) Locus with omp6-10 arranged in tandem. (D) Locus with omp11-13 arranged in tandem.
Although encoding outer membrane proteins within the same family, the genetic structure of A. marginale omp1-14 is distinctly different from that of msp2 and msp3 in that the former genes are single-copy genes, often arranged in tandem, and have no known pseudogenes. This structure of A. marginale omp1-14 is similar to that of the omp1/p28-omp/map1 gene families in Ehrlichia spp., for which there is no evidence of recombinatorial mechanisms capable of generating frequent structural or antigenic variation within a persistently infected animal (28, 30, 36). Based on this common genetic structure, we predict that, similar to the previously characterized outer membrane proteins in Ehrlichia spp. but unlike A. marginale MSP2, the sequences of OMPs 1 to 14 are stable during an infection cycle. Thus, in the second part of this study, we test the hypothesis that the genes encoding these predicted outer membrane proteins, omp1-14, are invariant during acute and persistent infection in the mammalian host, in the tick vector, and following tick-borne transmission.
MATERIALS AND METHODS
Transcription of omp1-14.
Detection of transcripts was done by reverse transcription and PCR using specific primers for each omp (Table 1). RNA was extracted from A. marginale (St. Maries strain)-infected bovine erythrocytes from two calves (C942 and C988), A. marginale-infected IDE8 tick cells, and A. marginale-infected midgut and salivary glands from adult Dermacentor andersoni ticks (pools of 10). IDE8 cells, derived from embryonic Ixodes scapularis ticks, were infected with the St. Maries strain of A. marginale and maintained at 34°C, as previously described (33, 34). Total RNA was extracted using TRIzol (BRL) treated with RNase-free DNase (Ambion) for 30 min at 37°C, followed by chemical inactivation with DNase inactivation solution (Ambion). RNA was reverse transcribed with an Omniscript (QIAGEN) or Retroscript (Ambion) reverse transcription kit using random hexamers according to the manufacturer's instructions. PCR amplification parameters were 35 cycles of melting at 94°C for 15 s, annealing at 65°C for 58 s, and extension at 72°C for 71 s, with a final extension step at 72°C for 7 min. PCR products were electrophoretically separated using a 1% agarose gel and stained with ethidium bromide for visualization.
TABLE 1.
Primers used for omp transcriptional analysis and sequencing
| Gene | Primera | Sequence (5′-3′) | Amplicon size (nt)b | Gene | Primera | Sequence (5′-3′) | Amplicon size (nt)b | |
|---|---|---|---|---|---|---|---|---|
| omp1 | RT/S F | AAGTACGAGTCTGATACCGCACTCAAAC | 778 | S R | AACAACAATAGAGCGCTACG | |||
| RT/S R | AAAACAGCAGGGTGGCACCG | Q F | TGCCCGAGCACCGAGATTTCTTAT | |||||
| Q F | CAGAAATTCCCCGTAGATACCAA | Q R | ATGACAGGGTACCTTTCTGCACCA | |||||
| Q R | TGGTCACTTCCTTATTCTCAGCG | Probe | ACTGGAAGATGCGCTGCTTACCGCGA | |||||
| Probe | TTGCTACAAGGCCGCAGGAGCC | omp9 | RT F | AGCTGGGGCTCTTGCGTTTG | ||||
| omp2 | RT F | GATTGAGTCTATGGGGGAACAAAAGAG | 567 | RT R | AACATATTCACTATAATCTGACGCTGC | 1,096 | ||
| RT R | GAGCTTCGAATGGTGCAGAGAG | S F | AGCGTCTACTGATTGTGTTC | 1,186 | ||||
| S F | AATCAGGTTCGGATTCTAGG | 1,102 | S R | TTCACTATAATCTGACGCTG | ||||
| S R | CAGTGAGTCTTCACATGGAG | Q F | GAAGTCACTACACGACCTGACTGT | |||||
| omp3 | RT F | GTGCCTCATTCGTACCTCCTACC | 314 | Q R | TAAAGCATCTTCGCGGGTCGT | |||
| RT R | GGGGTAAGTAGAGAGGCACTGATG | Probe | TATTCAGTGCGCTGAACACTGCGATCCA | |||||
| S F | TCTCCAAATTCCCGGTGCCT | 675 | omp10 | RT F | TCCTTCGGGTTGCTGCGTTG | 969 | ||
| S R | GGTGGGTGTCCTAGAATCCG | RT R | GCTTACCCCCATTCCAGCAC | |||||
| omp4 | RTF | TTGAGAAACTTGCTCCAATGTTAAAACC | 922 | S F | CACATTTTGGTTGCATTTATCG | 1,146 | ||
| RTR | AGCGGAATTGGCACTGTGC | S R | ATTCGCAGTTCTATGCAGCA | |||||
| S F | AATTTTTGTGGTTGGCGGTG | 1,294 | QF | TGGAAGAAGGTAAGAGGCTTGGCA | ||||
| S R | CACTCTGCCAACTAAAATAAAAGGA | QR | TCGCCGACCCAACATAGTTAAGCA | |||||
| QF | AGCTGGGCATAGGAAGAGAAGCAT | Probe | AAGGCAGAAGCAGAAGCGCGAAGAT | |||||
| QR | TGTGTCACCTCCTGTGTGTTGGAA | omp11 | RT F | ATGAGCTTTGTAAGGTTTCTTGCC | 624 | |||
| Probe | TGCACGCGCAAGATTTCGGATAAGCA | RT R | AAGCACCGAGGAGAGCTGTACC | |||||
| omp5 | RT F | AACTCGAGCTTCAGCCCCAG | 868 | S F | CTACGGGAGTAAATACTTGG | 1,145 | ||
| RT R | TGCCCTTGAGCCACACTCAC | S R | TACCACATACACGGCAAAAC | |||||
| S F | AAGCGTGCGTAGCTAAAACT | 1,177 | QF | GTGCTTGCTAGTTTGTGCAGGGAT | ||||
| S R | TGAAGCTGGTAGAATCCCGG | QR | CAATCTTCGCCTGGTAAGAAACCC | |||||
| omp6 | RT F | CTCCAATCGGAGGGGTTGTG | 492 | Probe | TGCCATATGTGTGTGCTGGGTTTGGT | |||
| RT R | GCATAAATCCAGTTTAGCCTCC | omp12 | RT F | ATGGGATCTATGATGAGGGC | 703 | |||
| S F | CATCGTGAATATACTGAGGA | 694 | RT R | CAATAAATAGCGTCACACCCC | ||||
| S R | AAACATGGATCACAGACGTT | S F | TGTGAGAGTTGCCGCTTCCG | 959 | ||||
| omp7 | RT F | GTGGTTAGATCTTTTCTGTTGGG | 399 | S R | TGCGGTGAGCACGTAGCAAA | |||
| RT R | CGCTCTACCACTGACCTTCATG | omp13 | RT F | ATGGTTAAAGCAGGGGCAGCATG | 474 | |||
| S F | TGGGGAGGGGATGTGTGTTG | 1,105 | RT R | GCTCCTCGCAACCTTAAATTCC | ||||
| S R | CGCGGCACTGCTCTTTATAC | S F | CGTTTTACAACCTTGACTTT | 1,156 | ||||
| Q F | TTCGTGCGCTGTCCTCTTCTACTC | S R | GAAAATACGCGCATACACAC | |||||
| Q R | TGTCCGTGCACCAATCTCACTAGA | omp14 | RT F | GAATCCGAACCTGATTCCTAGTTCT | 690 | |||
| Probe | AGTTGCATAATCTTGCGGATGCGCTA | RT R | ACAAGGAGTTGTCCAAGCCGG | |||||
| omp8 | RT F | AACTGGTAGGCTGGAGTTCG | 213 | S F | TAGTGCCGGGAACCGCAAGT | 1,155 | ||
| RT R | AGTGATAAGAAATCTCGGTGCTC | S R | GGGATGAACGGTCGTGAAGA | |||||
| S F | AGTGAATATGTTGAGAGGGA | 1,374 |
RT, forward (F) and reverse (R) primers used for detection of transcript after reverse transcription; S, forward (F) and reverse (R) primers used to amplify open reading frames for cloning and sequencing; Q, forward (F) and reverse (R) primers used for quantitative PCR; Probe, TaqMan probes used for quantitative PCR.
nt, nucleotides.
Quantitative real-time PCR.
Total RNA was isolated from infected IDE8 cells and erythrocytes as previously described (1). Midguts and salivary glands from D. andersoni-infected ticks were collected in RNAlater (Ambion), and RNA was extracted using an RNeasy kit and methods similar to those used for the IDE8 cells. Quantitative real-time PCR was performed on all omp genes, except omp2, omp3, and omp6, using a Bio-Rad iCycler as previously described (1). The primer sets and TaqMan probes used are listed in Table 1.
Expression of OMPs 1 to 14.
Specific antibodies for each predicted OMP were generated and used to detect expression in A. marginale-infected bovine erythrocytes and IDE8 tick cells using sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), followed by immunoblotting. The reactivity of each serum was confirmed by immunoblotting against the recombinant OMP expressed in Escherichia coli.
Generation of OMP-specific antisera and monoclonal antibodies.
One to three peptides for each predicted OMP were designed using TMpred to identify likely membrane-exposed segments of the proteins (Table 2) (19). Specific peptides were chosen based on predicted hydrophilicity and lack of sequence identity with other A. marginale proteins based on BLAST alignments. An amino-terminal cysteine was included for conjugation, and 2 mg of each peptide was cross-linked to maleimide-activated keyhole limpet hemocyanin using the Imject maleimide-activated immunogen conjugation kit (Pierce). Approximately 60 μg of conjugated peptide emulsified in Freund's complete adjuvant was used to immunize two mice with each peptide subcutaneously. The mice were boosted using four to six immunizations of 60 μg of antigen in Freund's incomplete adjuvant. By using an enzyme-linked immunosorbent assay (ELISA), anti-peptide antibodies were detected, and the lack of cross-reaction among peptides was confirmed. Briefly, 1 μg of nonconjugated peptide was applied to each well and incubated with blocker (phosphate-buffered saline with 5% milk and 0.2% Tween 20) for 1 h at room temperature. Serum diluted 1:300 in blocker was incubated with the peptide for 30 to 60 min at room temperature and then washed with 0.05% Tween 20 in phosphate-buffered saline. Antibody binding was detected with goat anti-mouse antibody conjugated to horseradish peroxidase and SureblueTMB substrate solution (Kirkegaard and Perry Laboratories). For monoclonal antibody production for OMP4, OMP7, OMP9, and OMP11, mice were boosted by intravenous injection of approximately 60 μg of conjugated peptide without adjuvant 72 h prior to euthanasia and collection of splenocytes. Cell fusion and cloning by limiting dilution were performed by standard procedures (50). Supernatants from the clones were screened for antibodies with an anti-peptide ELISA as described above, followed by SDS-PAGE and Western blotting with native and recombinant proteins.
TABLE 2.
Peptides used to generate antibodies that reacted with the peptide in an ELISA
| Peptide | Location of peptide (aa)d | Sequence |
|---|---|---|
| OMP1-P1a | 151-160 | CTKKYKDNPERAYR |
| OMP1-P2a | 70-79 | CKEKQQGGTAK |
| OMP1-P3b | 279-287 | CTESPKGSQG |
| OMP2-P1a | 7-19 | CEQKRGRCESAR |
| OMP3-P1a | 35-50 | CITRHPSPTYHHSSPHR |
| OMP3-P2c | 141-155 | CATIYNHPMLSSQPHK |
| OMP3-P3c | 17-26 | CQCHHLCTTNT |
| OMP4-P1b | 176-190 | CSDTIESELFQHTGGD |
| OMP5-P1a | 188-100 | CQGGLSIDSSTSTA |
| OMP5-P3 | 45-53 | CGHKGAGTRR |
| OMP5-P2a | 224-245 | CSSEDRLAAAK |
| OMP6-P1 | 21-30 | CGTGSSAAEAF |
| OMP6-P2a | 131-139 | CGRHWKQGNS |
| OMP7-P1b | 198-209 | CDLKHVGASSVD |
| OMP8-P1b | 172-185 | CALPEHRDFLSLEDA |
| OMP9-P1b | 207-220 | CGTTREDALAATQIV |
| OMP10-P1 | 192-200 | CEEGKRLGNL |
| OMP10-P2 | 268-276 | CSDKDEARRA |
| OMP10-P3 | 182-190 | CSTNAGDGKS |
| OMP11-P1b | 149-168 | CHDEGVVGDLYASE |
| OMP12-P1 | 149-158 | CNIALVRAQT |
| OMP12-P2 | 6-16 | CRATKKGSISVR |
| OMP12-P3 | 39-48 | CRKFRSQGRAY |
| OMP13-P1 | 199-210 | CNAAGAGSSAGQQ |
| OMP13-P3a | 317-325 | CGASSRTRDD |
| OMP14-P1a | 239-249 | CQNTQESKRDEA |
| OMP14-P2a | 378-388 | CLGKTKEKVSAS |
| OMP14-P3a | 36-46 | CASSHGMNGRED |
Peptides that elicited antibodies that reacted with the recombinant protein.
Peptides that elicited antibodies that reacted with the recombinant protein and A. marginale proteins in erythrocytes and/or IDE8 cells.
Peptides that elicited antisera that did not react with A. marginale-infected erythrocytes and/or IDE8 cells but were not tested against the recombinant protein. The remaining peptides elicited antisera that reacted with the peptide in an ELISA but did not react with recombinant proteins.
aa, amino acids.
Expression of recombinant OMPs in E. coli.
To obtain antigens for use as OMP-specific positive controls for antiserum and monoclonal antibody reactivity, each OMP was expressed as a His-tagged fusion protein in E. coli using the pBAD (Invitrogen) or pET (Novagen) expression system. Inserts containing the full-length or truncated open reading frames were generated by subcloning from clones generated for DNA sequencing, as described below. Briefly, clones of each omp were amplified by PCR with Pfu DNA polymerase for pET and Taq polymerase for pBAD vectors. Specific primers were designed to amplify the entire length of each omp, except for omp7, omp9, omp10, which were truncated by 123, 86, and 149 bp at the 3′ end, respectively, and omp14, which was truncated on the 5′ end by 147 bp to exclude promoters that could allow for uncontrolled expression of the membrane proteins, which are potentially toxic to E. coli (Table 1). The PCR fragments were cloned into the pET28b+ or pBAD-TOPO plasmids and sequenced to confirm the reading frame and the absence of nucleotide changes. PET28 plasmids were used to transform competent E. coli BLR(DE3)pLysS or HMS174(DE3)pLysS cells, and pBAD plasmids were transformed into TOP10 cells (Invitrogen). The transformed BLR(DE3)pLysS and HMS174(DE3)pLysS cells were grown in 2× YT broth and induced with a 1 mM solution of IPTG (isopropyl-β-d-thiogalactopyranoside). The transformed TOP10 cells were grown in LB broth and induced with a final concentration of 0.2% arabinose. The cells were harvested after 3 h of incubation at 37°C, frozen overnight, and lysed with sonication. Fractions containing the recombinant protein were used in Western blotting. Recombinant OMP1-3 and 14 were each purified by using a Ni2+-charged column under denaturing conditions using imidazole in wash buffer (200 mM NaCl, 20 mM Tris [pH equal to two points ± the isoelectric point]) and step elution using 10 mM to 250 mM imidazole.
SDS-PAGE and immunoblotting.
Sonicated pellets of A. marginale-infected erythrocytes and infected IDE8 cells stored in proteinase inhibitor buffer at −80°C were thawed and mixed with SDS-PAGE buffer (26). The number of A. marginale organisms in each sample was normalized by using quantitative real-time PCR to determine the copy number of msp5 in DNA samples, as previously described (27). Uninfected erythrocytes and IDE8 cells were treated identically as negative controls. As positive controls for antibody reactivity, lysates of recombinant E. coli expressing OMPs 4 to 9, 11, and 13 and isolated recombinant OMP fusion proteins 1 to 3 and 14 were used. Precast SDS-containing 4 to 20% or 7% polyacrylamide gels (Bio-Rad) were used for electrophoresis of protein samples at 60 V for 2.5 h. After transfer onto nitrocellulose, proteins were detected with murine antisera diluted to 1:50 or undiluted supernatant from hybridomas using the Western-Star chemiluminescence immunoblot detection system (Applied Biosystems) according to the manufacturer's instructions. Sera used to detect OMP expression in A. marginale-infected IDE8 cells were first adsorbed against uninfected IDE8 cells at room temperature for 60 min. Sera from nonimmunized mice and a monoclonal antibody to an unrelated organism (Trypanosoma brucei) served as negative antibody controls.
Analysis of omp1-14 sequence variation during in vivo infection and tick transmission.
The sequences of omp1-14 were determined during the complete cycle of A. marginale St. Maries strain transmission, which included blood from acute and persistent infection in the bovine mammalian host, salivary glands of infected ticks, and blood from the subsequent successfully transmitted infection. Calf C956 was infected by feeding adult male Dermacentor andersoni (Reynolds Creek strain) ticks that had acquired the St. Maries strain of A. marginale by feeding on an infected calf. C956 developed a peak rickettsemia of 109 A. marginale cells per ml of blood (13.1% of the erythrocytes were infected) during the acute stage of infection. When this animal entered the persistent phase of infection (≤107 A. marginale cells/ml of blood), adult male D. andersoni ticks were allowed to attach and acquisition feed for 7 days. The ticks were gently removed and held for 5 days at 26°C to allow for clearance of the blood meal from the mouthparts. A cohort of the ticks was then placed on a second naïve calf, calf C988, and allowed to transmission feed for 4 days. Calf C988 subsequently developed a peak rickettsemia of >108 A. marginale cells per ml (4.3% of the erythrocytes were infected). Calf C956 was surgically splenectomized 332 days after initial infection to allow for recrudescence to >109 A. marginale cells per ml (30% of the erythrocytes were infected).
DNA was extracted from whole blood collected in EDTA and from tick salivary glands isolated by dissection after acquisition feeding on calf C956 using a Puregene DNA isolation kit (Gentra Systems). omp1-14 were amplified from these tissues using specific primers listed in Table 1. The cycling conditions were 35 cycles of melting at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 90 s. The amplicons were cloned into pCR4 (Invitrogen), and inserts were sequenced in both directions with the BigDye kit and an ABI PRISM automated sequencer (PE Applied Biosystems) using T3 and T7 primers. The sequences were compiled and analyzed using the Vector NTI software package (Invitrogen).
Alignments were done for each gene in a pairwise fashion with the sequences derived from the completely sequenced genome of the St. Maries strain of A. marginale (6). Sequences from the Florida strain were draft sequences from a genome sequencing project currently in progress (http://www.vetmed.wsu.edu/research_vmp/anagenome). The msp2 expression sites from all samples, except the tick salivary gland, were amplified as previously described, and a single clone from each time point during infection was sequenced (7).
Nucleotide sequence accession numbers.
Eighty-four gene sequences for omp1-14 have been deposited in GenBank with consecutive accession numbers from DQ282512 to DQ282595. These sequences are from the St. Maries strain of A. marginale and were taken from tick salivary glands, IDE8 cells, and erythrocytes from a calf with acute, persistent, or recrudescent infection.
RESULTS
Transcription of omp1-14.
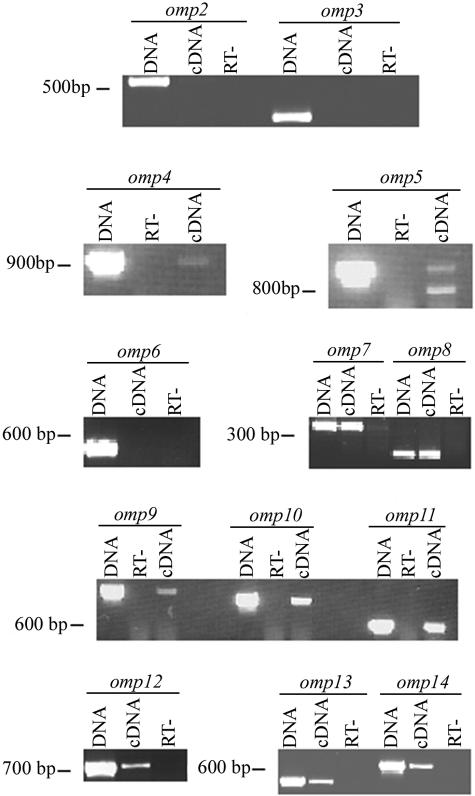
Total RNA from bovine erythrocytes, IDE8 cells, and D. andersoni salivary glands and midguts infected with the St. Maries strain of A. marginale was isolated and reverse transcribed. The sequence encoding each omp was amplified by PCR from cDNA, genomic DNA, and RNA using specific primers. Transcripts from omp2, omp3, and omp6 were not detected in any tissue. Transcripts for all other omp genes were detected in the infected erythrocytes (Fig. 2). Similarly, transcripts for omp1, omp4, omp5, and omp7 to omp14 were identified in D. andersoni midguts, salivary glands, and IDE8 cells.
FIG. 2.
Detection of transcript in A. marginale-infected bovine erythrocytes from total RNA using reverse transcription and PCR. Genomic DNA (DNA) was used as a positive control, while total RNA in the absence of reverse transcriptase (RT-) controlled for DNA contamination. Reverse-transcribed RNA (cDNA) was used to detect omp-specific transcript. A. marginale omp1 transcripts in infected erythrocytes have been previously reported (25).
Expression of OMPs 1 to 14.
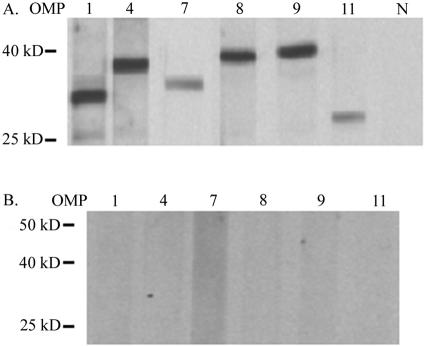
Specific antisera were generated for all predicted OMPs with each antiserum reacting in ELISA only with the peptide used as the immunogen (data not shown). Reactivity was detected in infected bovine erythrocytes with specific antisera to OMPs 1, 4, 7 to 9, and 11 (Fig. 3). Because the bands representing OMPs 4, 8, and 9 cannot be differentiated based on size, the specificity of each antiserum was confirmed by the absence of cross-reactivity among the sera using each recombinant protein as a positive control (data not shown). In addition, there was no reactivity of the anti-OMP sera with uninfected erythrocytes (Fig. 3) and no reactivity of sera from unimmunized mice with infected erythrocytes.
FIG. 3.
Expression of A. marginale OMPs in infected erythrocytes. (A) Western blots using A. marginale St. Maries strain-infected erythrocytes as an antigen. Polyclonal, monospecific mouse sera were used for detection of OMP1, OMP4, OMP8, OMP9, and OMP11, and monoclonal antibody 121/161 was used to detect OMP7. N, normal mouse serum. (B) Identical blots using uninfected erythrocytes with twice the protein load of panel A as a control. Predicted molecular sizes are as follows: OMP1, 32 kDa; OMP4, 37 kDa; OMP7, 39 kDa; OMP8, 40 kDa; OMP9, 40 kDa; OMP11, 32 kDa.
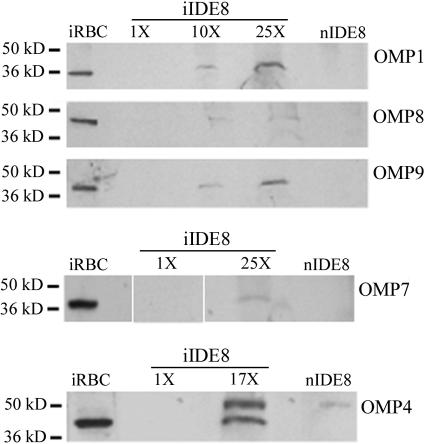
To compare the level of expression in infected IDE8 cells with that of bovine erythrocytes, the number of organisms per sample was determined using quantitative real-time msp5 PCR (27). When 7.3 × 106 organisms per lane were loaded and examined using immunoblotting, OMPs 1, 4, 7 to 9, and 11 were detected in the erythrocytes but not in the IDE8 cells. OMPs 1, 4, and 7 to 9 were detected in infected IDE8 cells when 10 to 25 times the amount of antigen needed for detection in infected erythrocytes was loaded (Fig. 4). However, even with this greater amount of antigen, the signal was less intense in IDE8 cells than in infected erythrocytes loaded at 7.3 × 106 organisms per lane. Expression of OMP11 was not detected in IDE8 cells when antigen was loaded at 25 times the amount needed for detection in infected erythrocytes.
FIG. 4.
Decreased expression of OMPs in tick cells infected with the St. Maries strain of A. marginale (iIDE8) compared to infected erythrocytes. A total of 7.3 × 106 organisms were loaded in the infected erythrocyte (iRBC) and the 1× iIDE8 lanes. A total of 7.3 × 107 (10×) to 1.6 × 108 (25×) organisms were loaded in the remaining IDE8 lanes. The noninfected IDE8 cells (nIDE8) were used as a negative control, corresponding to the maximum load of iIDE8. Polyclonal, monospecific mouse sera were used to detect OMP1 and OMP8; monoclonal antibody 121/1055 was used to detect OMP9; and monoclonal antibodies 121/161 and 122/255 were used to detect OMP7 and OMP4, respectively.
No expression of OMPs 2, 3, 5, 6, 10, and 12 to 14 was detected in infected erythrocytes or IDE8 cells using immunoblotting. The presence of antibodies that react with these proteins in SDS-PAGE and immunoblots was confirmed by their reactivity to each recombinant OMP (data not shown). The exceptions are OMPs 10 and 12, to which none of six sera raised against three different peptides each for OMPs 10 and 12 reacted. OMPs 10 and 14 have been previously reported to be expressed in St. Maries strain-infected erythrocytes using matrix-assisted laser desorption ionization-time of flight mass spectrometry, as have OMP4 and OMP7 (29).
Quantitative analysis of omp transcripts.
Real-time PCR quantification of omp-specific transcripts was performed to determine whether differences in protein expression of OMPs 1, 4, 7 to 9, and 11 between infected erythrocytes and IDE8 cells reflected differences in transcript levels. Because omp10 is upstream of omp7, omp8, and omp9, and the structure of the four genes is consistent with an operon, transcript levels from omp10 were also measured to determine if they are similar to those from omp7, omp8, and omp9. Transcript from each omp was detected in infected bovine erythrocytes, D. andersoni midguts and salivary glands, and IDE8 cells. Transcript levels for omp1, omp4, omp7, omp8, omp9, omp10, and omp11 in IDE8 cells were 8 to 22% of those in erythrocytes (Table 3). In general, transcript levels in the infected tick midgut were low, similar to those in infected IDE8 cells, while the salivary gland levels were higher than those in any other tissue and most similar to those in infected erythrocytes. The exception was omp1, in which transcript levels in the midguts and salivary glands were similar to, and lower than, those in the infected erythrocytes (Table 3).
TABLE 3.
Comparison of transcript levels for A. marginale omp1, omp4, and omp7-11 in infected bovine erythrocytes, tick salivary glands and midguts, and IDE8 cells
| Site of infectionb | No. (%) of transcriptsa (level relative to infected erythrocytes)
|
||||||
|---|---|---|---|---|---|---|---|
| omp1 | omp4 | omp7 | omp8 | omp9 | omp10 | omp11 | |
| RBC | 2.94 × 103 (100) | 8.24 × 102 (100) | 1.18 × 104 (100) | 1.39 × 104 (100) | 2.79 × 104 (100) | 2.54 × 104 (100) | 5.31 × 103 (100) |
| SG | 1.62 × 103 (55) | 1.18 × 103 (143) | 1.95 × 104 (165) | 2.22 × 104 (160) | 5.9 × 104 (211) | 3.12 × 104 (122) | 1.33 × 104 (250) |
| MG | 1.44 × 103 (49) | 1.62 × 101 (2) | 1.45 × 103 (12) | 2.40 × 103 (17) | 4.11 × 103 (15) | 2.85 × 103 (11) | 6.10 × 102 (11) |
| IDE8 | 2.67 × 102 (9) | 7.05 × 101 (9) | 2.23 × 103 (19) | 2.82 × 103 (20) | 6.11 × 103 (22) | 3.87 × 103 (15) | 4.42 × 102 (8) |
Normalized to the copy number of the 16S rRNA gene.
RBC, infected bovine erythrocytes; SG, infected Dermacentor andersoni salivary glands; MG, infected D. andersoni midguts; IDE8, infected tick cell line.
Analysis of omp1-14 sequence variation during in vivo infection and tick transmission.
Each omp was sequenced during acute and persistent infection in calf C956, in the salivary glands of infected D. andersoni ticks, and during acute infection after successful tick transmission in calf C988. Calf C956 was splenectomized to allow for recrudescence to high-level rickettsemia, and each omp was also sequenced at this time. The sequence identity between the St. Maries strain and sequences from each time point of infection was >99% with either no sequence variation or one to three substitutions, with no cumulative increase in substitutions throughout the infection cycle. In contrast, as a positive control for the detection of variation resulting from recombination, the MSP2 hypervariable region, including amino acids (aa) 162 to 280, had marked variation (68.6 to 73.7%) between the acutely infected calf C956 and all other time points of infection. In addition, the amino acid identity between OMP1-14 proteins in the St. Maries strain and Florida strain is high, between 85.3% and 100%. The highest amount of variation occurs between OMP7 proteins (85.3%), due to nucleotide substitutions in the middle of the gene that maintain the reading frame but introduce amino acid variation.
The genes encoding OMPs 7 to 9 share large stretches of sequence identity on the 5′ and 3′ ends that could mediate recombination, as occurs between the msp2/msp3 expression sites and their respective pseudogenes (Fig. 5). While the corresponding N- and C-terminal amino acid sequences are relatively conserved among OMPs 7 to 9 (83% identity in aa 1 to 150 and 76% identity in aa 275 to 403), the central region encodes marked amino acid polymorphism (18% identity in aa 151 to 274). Thus, recombination among these three genes could potentially generate variation. However, examination of omp7, omp8, and omp9 at each time point revealed a high degree of identity (99.7 to 100%), and thus, recombination among these related genes either appears to not occur or is uncommon.
FIG. 5.
Conservation among OMP7, OMP8, and OMP9. SM represents the previously published St. Maries strain genome sequence (6). FL represents a draft sequence from the Florida strain genome.
DISCUSSION
The identification of omp1-14 in the A. marginale genome (Fig. 1) represents a substantial expansion in the number of pfam01617 members, augmenting the original members that had been identified through surface labeling, immunoprecipitation, and immunoblotting. However, unlike the original family members, there was no a priori assurance that the in silico-identified omp1-14 would be expressed, and thus, answering this question was the first objective of the study. Overall, members of pfam01617 are widely expressed in A. marginale. As shown in the present study, A. marginale omp1, omp4, omp7-9, and omp11 are all transcribed and expressed in bovine erythrocytes. In addition, omp10 and omp14 transcripts were detected, and OMP10 and OMP14 protein expression has been recently reported for A. marginale strains isolated from infected erythrocytes using mass spectrometry (29). OMP10 expression (originally designated as Ana43) was initially reported in a strain of A. marginale isolated from Australia (42). In combination with the previously demonstrated expression of A. marginale msp2, msp3, msp4, opag2, and opag3, which are also members of pfam01617, a total of 14 of 19 (74%) of the A. marginale members of this protein superfamily are known to be expressed.
Differential expression of genes between infection in the vertebrate and in invertebrate hosts is a common theme in arthropod-borne pathogens, reflecting the specialized requirements for invasion, survival, and replication in each host (15, 26, 38, 47, 48). The differential expression of A. marginale omp1, omp4, omp7-9, and omp11 between cell types appears to be, at least in part, transcriptionally regulated. Protein levels were a minimum of 10 to 25 times higher in infected erythrocytes than in the infected tick cells, with corresponding differences in transcript levels. Overall, normalized transcript levels were highest in the infected salivary glands and erythrocytes, while low transcript levels were detected in the infected tick midgut and IDE8 cells. This differential expression does not simply reflect the differences in the number of replications and resulting colony size among each cell type: A. marginale undergoes limited replication in the erythrocyte (two to four replications resulting in 4 to 16 A. marginale organisms per cell), while replication in IDE8 tick cells, tick midgut, and tick salivary gland continues to form well-developed colonies composed of numerous bacteria (4, 20, 23, 33). The similarity in levels between the tick salivary gland and the erythrocyte, apart from omp1, is consistent with a remodeling of the A. marginale surface in the salivary gland to develop an erythrocyte-infective stage at the time of tick transmission feeding. The low A. marginale transcript levels in the tick midgut, supported by the similarly low transcript levels and low protein expression in the infected IDE8 cells, suggest that other molecules may be more critical in initial tick infection and colonization, in agreement with the association of specific MSP1a (not a member of pfam01617) sequences with binding to both IDE8 cells and tick midguts (11, 12). Furthermore, the low level of expression of pfam01617 OMPs in infected IDE8 cells may explain the lack of efficacy of vaccines based on A. marginale grown in these cells, as they do not represent the OMP expression that occurs in either the mammalian infective stage, the tick salivary gland, or erythrocytes (13, 22).
In the current study, transcript but not protein expression was detected for A. marginale omp5, omp10, omp13, and omp14. For omp10 and omp14, this appears to reflect the limited sensitivity of the specific antibodies used in the immunoblotting assay, as we have recently reported the detection of protein expression of OMP10 and OMP14 using mass spectrometry in the same A. marginale St. Maries strain isolated from infected erythrocytes (29). Whether this also applies to the detection of transcripts but not proteins encoded by omp5 and omp13 or reflects posttranscriptional regulation, as previously reported for A. marginale opag1-3 and E. chaffeensis p28-omp genes, is unknown (26, 45). Transcripts from omp12 were also detected. However, it is unknown whether omp12 is expressed as a protein because the antibodies developed (three different peptides were used as immunogens) did not react with recombinant OMP12 in immunoblots. The lack of both transcript and protein expression for omp2, omp3, and omp6 suggests that these three genes represent pseudogenes. This is consistent with these three omp genes encoding the smallest open reading frames (ORFs) of the 14 newly identified members, having 729 (omp2), 684 (omp3), and 459 (omp6) nucleotides. Among α-proteobacteria, there is a correlation between ORF size and correct identification of a gene as opposed to a pseudogene, with the mean size of the protein-coding gene being nearly 900 bp (5). Additionally, omp6, the shortest ORF, appears to have arisen from a duplication of omp10. The ORF of omp6 has >99% identity to a sequence contained within that of omp10 but is truncated on the 3′ end relative to omp10. Whether a functional omp6 accumulated mutations and deletions, resulting in pseudogene formation, or was never functional is unknown. There are also two alternative explanations for the lack of detectable transcription and expression for omp2, omp3, and omp6: (i) these omp genes are expressed only at very low levels, below the sensitivity of detection for both assays, or (ii) expression is tightly restricted and occurs only in tissues other than those tested, as infection has been detected in tissues other than tick midguts and salivary glands (18, 21). Regardless, as the tick midgut and salivary glands and bovine erythrocytes represent the key sites for invasion, replication, and maturation in the transmission cycle, and given the low level of expression, if any, the data suggest that OMP2, OMP3, and OMP6 are unlikely to be involved in the key steps of transmission.
A. marginale msp2 and msp3, original members of pfam01617, are highly variable during infection, resulting from sequential gene conversion events that generate structural and antigenic variants expressed from single expression sites for each (3, 7, 16, 32). In contrast, the newly identified omp1-14 genes are highly conserved throughout an entire cycle of infection, including within acutely and persistently infected calves, within the tick salivary gland, and in the subsequently infected calf following tick transmission feeding. This high degree of conservation is more similar to that of the other members of pfam01617 in Ehrlichia than to that of A. marginale msp2 and msp3. Although limited data are available, there is 100% identity between the p28-omp genes of the Arkansas strain of E. chaffeensis compared at two different time points, as analyzed using GenBank accession numbers (U72291 and AF068234). The available sequences used for comparing portions (from 345 to 885 of 5,875 nucleotides) of the St. Vincent strain at three time points have 99 to 100% identity (GenBank accession numbers AF77735, AF479837, and AF151715). The map1 locus of the Gardel (accession numbers AY652746 and U50832) and Walgevonden (accession numbers AF125274 and U49843) strains of E. ruminantium are similar, with 100% identity between each strain, each sequenced at two time points. Identical sequences at the 5′ and 3′ ends of omp7, omp8, and omp9 provide a structural basis for homologous recombination (Fig. 5). However, the changes in these genes through an infection cycle are minimal, suggesting that recombination events involving these genes are infrequent. Although the Florida and St. Maries strains differ in sequence with regard to pfam01617 members such as msp2, msp3, and msp4, there is a high degree of sequence identity for all the omp genes, including omp7-9, providing further evidence that recombination of these three genes is a rare occurrence (14).
The expanded knowledge of the expressed pfam01617 members and their differential regulation in cell types infected in the tick and mammalian hosts provides new opportunities to examine critical steps in transmission and for vaccine development. The signaling events associated with the up-regulation of omp1, omp4, omp7-9, and omp11 as infection progresses from the tick midgut to the salivary gland are unknown but are hypothesized to be linked to the tick feeding associated with the development of infectivity and subsequent transmission. The data that this expression is at least partially transcriptionally regulated indicate that analysis of transcription factor binding to promoter sequences during infection of the different cell types may be a first approach to a better understanding of how infectivity develops at the time of transmission. In addition, the newly identified OMPs may have a role in the induction of protective immunity that follows immunization with purified A. marginale outer membranes (9, 46). None of the previously identified major surface proteins, including the original members of pfam01617, have consistently induced protective immunity (39, 41). OMPs 4, 7, 10, and 14 have recently been identified as targets of antibodies induced by the immunization of cattle with purified outer membranes and, specifically, by high titers of immunoglobulin G2, which is associated with protective immunity (8, 29). Furthermore, the expressed OMPs may have an important role in maintaining the native conformation of the membrane, as the A. marginale outer membrane is composed of proteins with extensive intra- and intermolecular covalent and noncovalent bonds (49). The knowledge that these outer membrane proteins are relatively invariant and are expressed in the infected erythrocyte supports investigations into their importance in immunity and relevance to vaccine development.
Acknowledgments
The technical assistance of Beverly Hunter, Carter Hoffman, and Ralph Horn is gratefully acknowledged.
This work was supported by the NIH (AI45580, AI053692, and AI44005) and the USDA-ARS (CRIS 5384-32000-016-00D and Cooperative Agreement 58-5348-3-212). Susan Noh was supported by KO8AI052412, and Joe Agnes was supported by T32-GM008336.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Barbet, A. F., J. T. Agnes, A. L. Moreland, A. M. Lundgren, A. R. Alleman, S. M. Noh, K. A. Brayton, U. G. Munderloh, and G. H. Palmer. 2005. Identification of functional promoters in the msp2 expression loci of Anaplasma marginale and Anaplasma phagocytophilum. Gene 353:89-97. [DOI] [PubMed] [Google Scholar]
- 2.Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbet, A. F., J. Yi, A. Lundgren, B. R. McEwen, E. F. Blouin, and K. M. Kocan. 2001. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect. Immun. 69:3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blouin, E. F., and K. M. Kocan. 1998. Morphology and development of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in cultured Ixodes scapularis (Acari: Ixodidae) cells. J. Med. Entomol. 35:788-797. [DOI] [PubMed] [Google Scholar]
- 5.Borodovsky, M., W. S. Hayes, and A. V. Lukashin. 1999. Statistical predictions of coding regions in prokaryotic genomes by using inhomogeneous Markov models, p. 11-33. In R. L. Charlebois (ed.), Organization of the prokaryotic genome, vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 6.Brayton, K. A., L. S. Kappmeyer, D. R. Herndon, M. J. Dark, D. L. Tibbals, G. H. Palmer, T. C. McGuire, and D. P. Knowles, Jr. 2005. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 102:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, W. C., D. Zhu, V. Shkap, T. C. McGuire, E. F. Blouin, K. M. Kocan, and G. H. Palmer. 1998. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect. Immun. 66:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, S. M., J. S. Dumler, H. M. Feng, and D. H. Walker. 1994. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am. J. Trop. Med. Hyg. 50:52-58. [PubMed] [Google Scholar]
- 11.de la Fuente, J., J. C. Garcia-Garcia, A. F. Barbet, E. F. Blouin, and K. M. Kocan. 2004. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet. Microbiol. 98:313-322. [DOI] [PubMed] [Google Scholar]
- 12.de la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2001. Differential adhesion of major surface proteins 1a and 1b of the ehrlichial cattle pathogen Anaplasma marginale to bovine erythrocytes and tick cells. Int. J. Parasitol. 31:145-153. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente, J., K. M. Kocan, J. C. Garcia-Garcia, E. F. Blouin, P. L. Claypool, and J. T. Saliki. 2002. Vaccination of cattle with Anaplasma marginale derived from tick cell culture and bovine erythrocytes followed by challenge-exposure with infected ticks. Vet. Microbiol. 89:239-251. [DOI] [PubMed] [Google Scholar]
- 14.de la Fuente, J., R. A. Van Den Bussche, J. C. Garcia-Garcia, S. D. Rodriguez, M. A. Garcia, A. A. Guglielmone, A. J. Mangold, L. M. Friche Passos, M. F. Barbosa Ribeiro, E. F. Blouin, and K. M. Kocan. 2002. Phylogeography of New World isolates of Anaplasma marginale based on major surface protein sequences. Vet. Microbiol. 88:275-285. [DOI] [PubMed] [Google Scholar]
- 15.Felek, S., R. Greene, and Y. Rikihisa. 2003. Transcriptional analysis of p30 major outer membrane protein genes of Ehrlichia canis in naturally infected ticks and sequence analysis of p30-10 of E. canis from diverse geographic regions. J. Clin. Microbiol. 41:886-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge, N. L., K. M. Kocan, E. F. Blouin, and G. L. Murphy. 1996. Developmental studies of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) infected as adults by using nonradioactive in situ hybridization and microscopy. J. Med. Entomol. 33:911-920. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman, K., and W. Stoffel. 1993. TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 347:166. [Google Scholar]
- 20.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58:1117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocan, K. M., N. L. Ge, E. F. Blouin, and G. L. Murphy. 1998. Development of a non-radioactive DNA probe and in situ hybridization for detection of Anaplasma marginale in ticks and cattle. Ann. N. Y. Acad. Sci. 849:137-145. [DOI] [PubMed] [Google Scholar]
- 22.Kocan, K. M., T. Halbur, E. F. Blouin, V. Onet, J. de la Fuente, J. C. Garcia-Garcia, and J. T. Saliki. 2001. Immunization of cattle with Anaplasma marginale derived from tick cell culture. Vet. Parasitol. 102:151-161. [DOI] [PubMed] [Google Scholar]
- 23.Kocan, K. M., D. Stiller, W. L. Goff, P. L. Claypool, W. Edwards, S. A. Ewing, T. C. McGuire, J. A. Hair, and S. J. Barron. 1992. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am. J. Vet. Res. 53:499-507. [PubMed] [Google Scholar]
- 24.Lin, Q., and Y. Rikihisa. 2005. Establishment of cloned Anaplasma phagocytophilum and analysis of p44 gene conversion within an infected horse and infected SCID mice. Infect. Immun. 73:5106-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löhr, C. V., K. A. Brayton, A. F. Barbet, and G. H. Palmer. 2004. Characterization of the Anaplasma marginale msp2 locus and its synteny with the omp1/p30 loci of Ehrlichia chaffeensis and E. canis. Gene 325:115-121. [DOI] [PubMed] [Google Scholar]
- 26.Löhr, C. V., K. A. Brayton, V. Shkap, T. Molad, A. F. Barbet, W. C. Brown, and G. H. Palmer. 2002. Expression of Anaplasma marginale major surface protein 2 operon-associated proteins during mammalian and arthropod infection. Infect. Immun. 70:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löhr, C. V., F. R. Rurangirwa, T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Specific expression of Anaplasma marginale major surface protein 2 salivary gland variants occurs in the midgut and is an early event during tick transmission. Infect. Immun. 70:114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long, S. W., X. F. Zhang, H. Qi, S. Standaert, D. H. Walker, and X. J. Yu. 2002. Antigenic variation of Ehrlichia chaffeensis resulting from differential expression of the 28-kilodalton protein gene family. Infect. Immun. 70:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez, J. E., W. F. Siems, G. H. Palmer, K. A. Brayton, T. C. McGuire, J. Norimine, and W. C. Brown. 2005. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infect. Immun. 73:8109-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride, J. W., X. Yu, and D. H. Walker. 1999. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: a potential serodiagnostic antigen. Clin. Diagn. Lab. Immunol. 6:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire, T. C., G. H. Palmer, W. L. Goff, M. I. Johnson, and W. C. Davis. 1984. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect. Immun. 45:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meeus, P. F., K. A. Brayton, G. H. Palmer, and A. F. Barbet. 2003. Conservation of a gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol. Microbiol. 47:633-643. [DOI] [PubMed] [Google Scholar]
- 33.Munderloh, U. G., E. F. Blouin, K. M. Kocan, N. L. Ge, W. L. Edwards, and T. J. Kurtti. 1996. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J. Med. Entomol. 33:656-664. [DOI] [PubMed] [Google Scholar]
- 34.Munderloh, U. G., Y. Liu, M. Wang, C. Chen, and T. J. Kurtti. 1994. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80:533-543. [PubMed] [Google Scholar]
- 35.Murphy, C. I., J. R. Storey, J. Recchia, L. A. Doros-Richert, C. Gingrich-Baker, K. Munroe, J. S. Bakken, R. T. Coughlin, and G. A. Beltz. 1998. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect. Immun. 66:3711-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal, U., and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 5:659-666. [DOI] [PubMed] [Google Scholar]
- 39.Palmer, G. H., A. F. Barbet, G. H. Cantor, and T. C. McGuire. 1989. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect. Immun. 57:3666-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer, G. H., and T. C. McGuire. 1984. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J. Immunol. 133:1010-1015. [PubMed] [Google Scholar]
- 41.Palmer, G. H., S. M. Oberle, A. F. Barbet, W. L. Goff, W. C. Davis, and T. C. McGuire. 1988. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect. Immun. 56:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riding, G., M. Hope, D. Waltisbuhl, and P. Willadsen. 2003. Identification of novel protective antigens from Anaplasma marginale. Vaccine 21:1874-1883. [DOI] [PubMed] [Google Scholar]
- 43.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singu, V., H. Liu, C. Cheng, and R. R. Ganta. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tebele, N., T. C. McGuire, and G. H. Palmer. 1991. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect. Immun. 59:3199-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unver, A., N. Ohashi, T. Tajima, R. W. Stich, D. Grover, and Y. Rikihisa. 2001. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect. Immun. 69:6172-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unver, A., Y. Rikihisa, R. W. Stich, N. Ohashi, and S. Felek. 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 70:4701-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidotto, M. C., T. C. McGuire, T. F. McElwain, G. H. Palmer, and D. P. Knowles, Jr. 1994. Intermolecular relationships of major surface proteins of Anaplasma marginale. Infect. Immun. 62:2940-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoyama, W. M. 1994. Production of monoclonal antibodies, p. 2.5.1-2.5. 17. In J. E. Coligan (ed.), Current protocols in immunology, vol. 1. Wiley Intersciences Inc., New York, N.Y. [Google Scholar]