Abstract
We previously demonstrated that outer surface protein C (OspC) of Borrelia burgdorferi is essential for establishing mammalian infection. However, the role of OspC in mammalian infection is unknown. Here, we report experiments designed to distinguish between two models of OspC function in the mammalian host: (i) OspC fulfills an essential physiological role for growth and host adaptation or (ii) OspC provides a protective role for evasion of components of the innate immune response. We found that a B. burgdorferi ospC mutant, previously demonstrated to be noninfectious in both immunocompetent and SCID mice, could survive in the relatively immune-privileged environment of dialysis membrane chambers implanted within the peritoneum of a rat. The ospC mutant also adapts to the mammalian environment, as determined by the protein profiles of the chamber-cultivated spirochetes. Therefore, OspC does not appear to provide a physiological function for the survival of B. burgdorferi within the mammalian host. The second model, evasion of the innate immune system, was tested by assessing the infectivity of the ospC mutant in mice deficient for myeloid differentiation protein 88 (MyD88). Recent studies have shown that B. burgdorferi is prevented from reaching high cell numbers in the mammalian host by MyD88-dependent signaling pathways. The ospC mutant was incapable of infecting MyD88-deficient mice, suggesting that the role of OspC cannot be related solely to evasion of MyD88-mediated innate immunity. These results reiterate the importance of OspC in mammalian infection and eliminate simple models of function for this enigmatic protein.
Spirochetes of the genus Borrelia are obligate parasites transmitted by arthropods, and many are pathogenic to humans. Relapsing fever spirochetes produce a recurring bacteremia in the blood, whereas Lyme disease spirochetes persist at low levels in various tissues, including skin, nervous system, heart, and joints (42). To survive for extended periods within the mammalian host, the borreliae have apparently evolved multiple mechanisms to evade the host immune system. Of the Borrelia proteins associated with immune evasion, all are plasmid-encoded surface components, although the molecular mechanisms differ among species and some have not been fully elucidated.
Borrelia burgdorferi, the causative agent of Lyme disease in the United States, can contain over 21 plasmids, several of which encode proteins that may assist in host immune evasion. The VlsE lipoprotein is encoded on linear plasmid 28-1 from an expression site that is preceded by >8 kb of silent, reiterated partial copies of vlsE (48). Each silent cassette varies from the others and can recombine with the vlsE expression site through a gene conversion-like mechanism, resulting in variation at the outer membrane. During mammalian infection, switching at the vlsE locus produces new outer surface variants that, in theory, are not recognized by previously produced antibodies (28, 49, 50). Other plasmid-encoded surface proteins of B. burgdorferi have been implicated in evading components of the innate immune system. The CRASP/Erp proteins bind the host complement-regulatory protein factor H and factor H-like protein-1/reconectin (19, 22, 23, 43). Binding of these host proteins by B. burgdorferi may prevent activation of the alternative pathway of complement.
Recently, our lab demonstrated that the major outer surface protein C (OspC) of B. burgdorferi is an essential virulence factor required for the initial stages of mammalian infection (18). Infectivity in immunocompetent mice was completely abrogated in a mutant B. burgdorferi strain lacking OspC. The mutant strain did not elicit an antibody response in the mice, nor could this strain infect severe combined immunodeficient (SCID) mice. Since SCID mice lack functional B and T cells, and the OspC mutant could not infect the SCID mice, the function of OspC does not seem to be resistance to the acquired immune system of the host. This suggested that OspC may play a role in avoiding clearance by the innate immune system or, alternatively, may fulfill a required physiological function during the initial infection of the mammal. Another B. burgdorferi plasmid-encoded protein, PncA, was previously shown to be required for spirochete survival in vivo but not during in vitro growth in Barbour-Stoenner-Kelly (BSK) medium (35). Similarly, the ospC mutant grows normally during in vitro cultivation but is unable to infect mice (18).
Tilly and colleagues further demonstrated that OspC is required only at the initial stage of mammalian infection and is dispensable for persistence of B. burgdorferi in the mouse (44). The absolute and immediate requirement for OspC in the mammal explains why this protein is produced by B. burgdorferi within the feeding tick as the spirochete prepares for transmission to the mammalian host. Although our data do not support a requirement for OspC by B. burgdorferi in ticks (18, 44), other reports suggested that OspC may also be important for invasion of the tick salivary glands and binding of tick salivary proteins, indicating roles for OspC in both hosts (34, 37). However, the function of OspC within the mammalian host has not been determined.
Here, we report the results of experiments designed to distinguish between two simple models of OspC function in the mammalian host. The first model (physiological model) presumes that OspC provides a required physiological function during mammalian infection. The second model (innate immune model) proposes that OspC is necessary for B. burgdorferi to survive some aspect of the innate immune system. We tested these models by two methods. The first method assessed the ability of the ospC mutant to grow in dialysis membrane chambers (DMCs) implanted in the peritoneal cavities of rats. Growth of B. burgdorferi in DMCs was previously shown to require physiological functions, such as the nicotinamidase PncA, but not mechanisms of putative immune evasion, such as VlsE (35). The second method tested the ability of the ospC mutant to infect mice deficient in myeloid differentiation marker 88 (MyD88). MyD88 is a common adapter molecule required by most Toll-like receptors (TLRs) and other signaling pathways to trigger the innate immune response to microbial invasion. TLRs recognize common microbial components such as lipopolysaccharides, peptidoglycan, lipoproteins, and other pathogen-associated molecular compounds. Once activated by ligand binding, TLRs utilize signal transduction pathways (most of which include the common adapter MyD88) to produce inflammatory cytokines and other immune effectors. Several studies have demonstrated that TLRs and other MyD88-dependent pathways are significant mechanisms by which the host innate immune system limits B. burgdorferi proliferation (26, 31, 45, 46). In this study, we found that the OspC mutant was capable of growth and host adaptation within the largely immune-privileged DMCs, indicating that OspC does not fulfill a required physiological function in DMCs. However, further experiments indicated that OspC does not provide a survival mechanism for B. burgdorferi to evade the MyD88-mediated innate immunity. These results, combined with previous data, indicate a more complex function for OspC than predicted by our simple models: although required for the initial infection of mammals, OspC does not provide a singular role in survival of specific branches of the innate or acquired immune system so far investigated, nor does it provide a strictly physiological function for B. burgdorferi.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. burgdorferi strains were grown at 35°C in liquid BSKII medium supplemented with 6% rabbit serum (Pel Freez Biologicals, Rogers, AR) or in solid BSK medium incubated at 35°C under 2.5% CO2 (38). The B. burgdorferi type strain B31 (ATCC 35210) was originally isolated from a tick collected on Shelter Island, N.Y. (8). The genomic sequence of B. burgdorferi B31 culture MI has been determined (10, 15). B31 A3 is a low-passaged, transformable, infectious clonal derivative of B31 MI (14). The ospC mutant strain (ospC7) and the complemented strain (ospC7/ospC+4) were constructed in B31 A3 and previously described by Grimm et al. (18). Briefly, allelic exchange was used to disrupt the ospC locus by insertion of a kanamycin resistance marker. The complemented strain was constructed by integrating a wild-type copy of ospC adjacent to the mutated allele. The plasmid contents of the mutant and complemented strains were identical to that of the wild-type parent strain, as confirmed by PCR analysis (14, 18, 36).
Bacterial growth in DMCs.
Sterile Spectra/Por dialysis membrane tubing with a molecular mass cutoff of 8,000 Da (Spectrum Laboratories, Rancho Dominguez, CA) was filled with ∼4 ml BSKII medium containing ∼5 × 103 B. burgdorferi cells/ml and tied shut. The DMCs were then surgically implanted into the peritoneal cavities of anesthetized Sprague-Dawley rats (Harlan Sprague-Dawley Inc., Indianapolis, IN). Total genomic DNA was isolated from the unused portion of the B. burgdorferi inoculum, and plasmid content was determined by PCR analysis to confirm that all strains were isogenic (14). After 4 to 12 days, depending on the experimental time course, rats were euthanized and DMCs removed. Growth of DMC-cultured spirochetes was assessed by direct cell count using a Petroff-Hausser counting chamber, and host adaptation was determined by analysis of cell lysates by silver-stained gel and immunoblotting assays (18). Cell lysates used for two-dimensional gel analysis were obtained from DMCs inoculated at 1 × 107 cells/ml and removed after 72 h. Host adaptation was confirmed as described above.
Mouse infections.
Host-adapted B. burgdorferi cells harvested from DMCs were inoculated into three RML mice/strain. RML mice are an outbred strain of Swiss Webster mice maintained at Rocky Mountain Laboratories since 1937. Mice were inoculated and infectivity assessed by seroconversion to B. burgdorferi antigens and reisolation of spirochetes as previously described (17). All animal experiments were performed in accordance with the guidelines of the National Institutes of Health. Animal protocols were approved by the institution's Animal Care and Use Committee. Rocky Mountain Laboratories is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care.
MyD88-deficient mice were provided by Shizuo Akira (1) and were maintained as heterozygous breeding pairs at the sixth generation backcross on the C57BL/6 background. Homozygous MyD88-deficient offspring were identified by PCR-based genotyping (5). C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD). Mice were housed in ventilated cages in the Animal Resource Center at the University of Utah Medical Center (Salt Lake City, UT) according to the National Institutes of Health guidelines for care and use of laboratory animals.
PCR detection of B. burgdorferi DNA in mouse tissues.
DNA was prepared from rear ankle, heart, and ear tissues at the time of sacrifice as previously described (46). Briefly, tissues were incubated for 5 h at 37°C in an 0.1% collagenase A (Roche, Indianapolis, IN) solution. An equal volume of 0.2-mg/ml proteinase K (Invitrogen Life Technologies, Carlsbad, CA) was added, and samples were incubated overnight at 55°C. DNA was recovered by phenol-chloroform extraction and ethanol precipitation. DNA concentration was determined by optical density at 260 nm. PCR detection of B. burgdorferi DNA in mouse tissues was conducted by continuous fluorescent monitoring PCR with the LightCycler 3.5 (Roche Molecular Biochemicals) using software from the manufacturer, as described previously (5, 31). The oligonucleotide primers used for amplification of the B. burgdorferi recA gene were nTM17.F (5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′) and nTM17.R (5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′).
Two-dimensional gel electrophoresis and immunoblot analysis of DMC-cultivated cell lysates.
For separation in the first dimension by nonequilibrium pH gradient gel electrophoresis (NEPHGE), spirochetes from DMCs were suspended in C4TT NEPHGE buffer consisting of 7 M urea (Promega, Madison, WI), 2 M thiourea (Fisher Scientific, Pittsburgh, PA), 4.0% (wt/vol) CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (EMD Biosciences, La Jolla, CA), 1.0% (vol/vol) Triton X-100 (Bio-Rad Laboratories, Inc., Hercules, CA), 65 mM dithiothreitol, and 2.0% (vol/vol) preblended ampholytes pH 3 to 9.5 (Amersham Life Sciences, Piscataway, NJ) to a final cell density of 5 × 106 cells per μl. The samples were incubated overnight at 23°C with gentle agitation, clarified by ultracentrifugation (435,700 × g, 30 min, 23°C), and stored at −80°C until separation. Five microliters (2.5 × 107 cells) of each sample was loaded onto a 2.0-mm by 12-cm tube gel consisting of 8 M urea, 4.4% Duracryl acrylamide (Genomic Solutions, Ann Arbor, MI), 4.0% (wt/vol) CHAPS, 1.0% (vol/vol) Triton X-100, and 2.0% (vol/vol) preblended ampholytes pH 3 to 9.5. The first dimension was focused at 200 V for 1 h and then increased to 600 V for 4 h (a total of 2,600 V · h). The tube gels were extruded and stored at −80°C until separated in the second dimension by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Prior to the separation of proteins in the second dimension, focused NEPHGE tube gels were equilibrated twice (10 ml, 10 min, 23°C) with SDS equilibration buffer composed of 3 M urea, 2.0% (wt/vol) SDS (Fisher), 1.0% dithiothreitol, and 10% (vol/vol) glycerol in 125 mM Tris (pH 8.8). Standard SDS-PAGE was performed by laying the tube gel onto a 13.5-cm × 1.5-mm × 14-cm (length by width by height) 12.5% acrylamide gel on a Hoefer SE600 gel apparatus and running the apparatus at 35 mA per gel. Broad-range protein standards were used to estimate relative molecular masses (Bio-Rad, Hercules, CA). For two-dimensional immunoblotting, the proteins were electrophoretically transferred to nitrocellulose, stained, and blocked as described previously (9). Pooled mouse immune serum, as the primary antibody, was diluted 1:2,000 in Tris-buffered saline (150 mM NaCl in 10 mM Tris-HCl, pH 8.0) with the addition of 0.1% Tween 20 (TBS-T20) and applied to the blot (1 h, 24°C). The blot was washed twice in 100 to 200 ml TBS-T20 for 10 min to remove residual primary antibody. Horseradish peroxidase-conjugated goat anti-mouse antibody (Sigma Chemical Co., St. Louis, Mo.) was diluted 1:5,000 in TBS-T20 and applied to the blot (45 min, 24°C), followed by three washes with 100 to 200 ml of TBS-T20. Reactive bands were visualized with the Enhanced Chemiluminescence kit (Amersham) in accordance with the manufacturer's specifications.
RESULTS
Host adaptation of the ospC mutant strain.
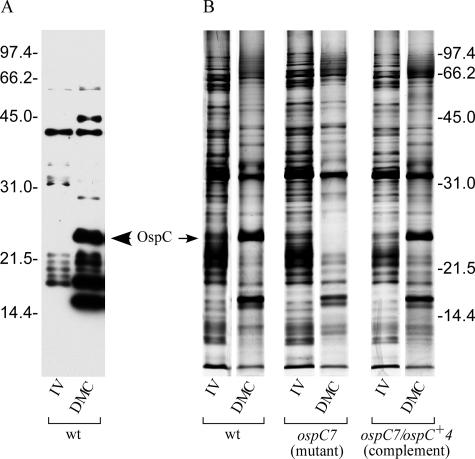
B. burgdorferi cells have been cultured and reisolated from chambers implanted within the peritoneal cavities of mice and rats (2, 21). These host-adapted B. burgdorferi cells display many characteristics indicative of adaptation to the mammalian host (2, 7). Among the changes that we observed from DMC-cultivated B. burgdorferi are the up-regulation of OspC and several unidentified, smaller proteins, readily visible on an immunoblot probed with mouse antisera against B. burgdorferi (Fig. 1A). Purser and coworkers demonstrated the utility of B. burgdorferi growth in DMCs to differentiate between spirochetal proteins that provide a required physiological function (such as PncA) and proteins that function in immune evasion (such as VlsE) (35). Previously, we showed that the OspC mutant could not infect SCID mice, suggesting an unanticipated physiological function for OspC (18). Therefore, we tested the ospC mutant, complement, and wild-type strains in DMCs to determine if OspC provided an essential physiological role (Table 1). Ten rats were used per strain, and each strain survived and grew to similar cell densities, although some variability among individuals within groups was observed. These results indicated that OspC did not appear to provide a typical physiological function required for growth in the DMC.
FIG. 1.
Survival and host adaptation of B. burgdorferi strains in dialysis membrane chambers. (A) Cell lysates of in vitro (IV)- and DMC-grown B31 A3 (wild type) were immunoblotted and incubated with pooled sera from mice infected with B. burgdorferi. (B) Silver-stained SDS-polyacrylamide gel comparing B. burgdorferi strains grown in vitro (IV) to those grown in DMCs. Protein bands correlating to OspC and other lower-molecular-weight proteins are induced in DMC-cultured B. burgdorferi cells (except in the mutant strain, which lacks OspC). Molecular mass standards (in kilodaltons) are indicated on the outside of both panels.
TABLE 1.
B. burgdorferi ospC mutant grows and adapts in DMCs but remains noninfectious for mice
| B. burgdorferi strain | DMC result:
|
Mouse infectivity by DMC-grown cellsc | |
|---|---|---|---|
| Growtha | Host adaptationb | ||
| WTd | + | + | 3/3 |
| ospC7 (mutant strain) | + | + | 0/3 |
| ospC7/ospC+4 (complemented strain) | + | + | 3/3 |
Growth within DMCs represents at least four doublings of B. burgdorferi from the initial inoculum, and all strains reached densities of >106 cells/ml.
Host adaptation was assessed by SDS-PAGE and subsequent immunoblot analysis (Fig. 1).
Number of mice infected/number of mice injected with DMC-grown B. burgdorferi. Mouse infectivity was assessed by serological response 4 weeks postinfection against the early antigen P39 (40) and total B. burgdorferi cell lysate.
WT, wild type.
Although all three strains grew in the DMCs, it was not apparent whether the ospC mutant could alter its protein composition in adaptation to the mammalian environment. Therefore, total protein lysates of in vitro- and DMC-grown B. burgdorferi cells were visualized by silver staining an SDS-polyacrylamide gel separated in a single dimension (Fig. 1B). All strains cultivated in the DMCs, including the ospC mutant, displayed significantly different protein profiles from those of in vitro-grown cultures. Among the many protein changes observed was the induction of OspC in the wild-type and complemented strains (although OspC was absent from the mutant strain, as expected). Therefore, under DMC growth conditions, OspC does not appear to be required for sensing the host environment, nor does OspC seem to be involved in a signaling cascade to adapt to the mammalian environment.
Antigenic composition of DMC-cultivated spirochetes.
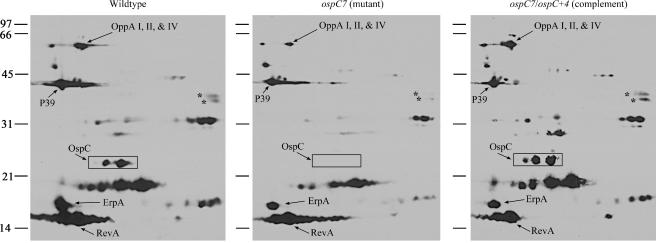
Potentially, the loss of a major membrane protein such as OspC could result in compensatory changes in expression of other membrane proteins, either to physically compensate for the absent protein or if OspC interaction is required by other proteins to function. We therefore examined the proteome of the host-adapted ospC mutant in greater detail by two-dimensional gel and immunoblot analysis of total cell lysates (Fig. 2). Although some proteins showed minor variation (e.g., spots marked with asterisks, Fig. 2), the overall antigenic profiles of the three strains were similar, suggesting that OspC does not significantly affect expression of other antigenic proteins.
FIG. 2.
Two-dimensional NEPHGE immunoblot of host-adapted B. burgdorferi. The immunoblot was incubated with pooled sera from mice infected with B. burgdorferi. Acid ends are to the left. Presumptive identities of OspC and other proteins are indicated based on the molecular weight and position in NEPHGE, relative to previously identified proteins (32). Asterisks denote proteins that may be at lower concentrations in the OspC mutant than in wild-type and complemented strains. Positions of molecular mass standards (in kilodaltons) are indicated. P39 has also been referred to as BmpA (41).
Host-adapted ospC mutant spirochetes cannot infect mice.
Since the protein profiles of DMC-cultivated ospC mutant spirochetes resembled those of the wild type and complemented clones, we wondered whether mammalian infectivity might be restored in host-adapted ospC mutant cells. Infectivity was assessed by inoculating DMC-grown bacteria directly into mice and examining the serological response after 4 weeks. Sera from all mice inoculated with either wild-type A3 or the complemented strain contained antibodies that recognized B. burgdorferi proteins (Table 1). However, sera from mice inoculated with the DMC-grown ospC mutant strain did not exhibit a detectable antibody response toward B. burgdorferi (data not shown). Apparently, the induction of specific proteins following growth in DMCs does not restore infectivity to spirochetes lacking OspC.
B. burgdorferi ospC mutant does not infect MyD88−/− mice.
The ability of the ospC mutant to grow and host-adapt in the relatively immune-privileged DMC indicated that OspC does not fulfill a required physiological function but perhaps provides a means to evade the immune response of the mammal. Previous results for immunocompetent and SCID mice indicated that the adaptive immune response was not required to eliminate the ospC mutant, suggesting that OspC may allow B. burgdorferi to avoid clearance by some component of the innate immune system (18). Several studies have identified TLR-2 as the major receptor for B. burgdorferi lipoproteins and demonstrated that TLR-2 and MyD88-dependent signaling pathways prevent B. burgdorferi from attaining high cell numbers during infection (4-6, 20, 26, 46).
To determine if OspC is required to survive TLR-mediated killing or other MyD88-dependent pathways, we infected immunocompetent and MyD88-deficient mice with the ospC mutant, complement, and wild-type strains (Table 2). Although the wild-type and ospC-complemented strains infected both sets of mice, the ospC mutant strain did not infect either, indicating that elimination of TLR signaling does not obviate the requirement for OspC by B. burgdorferi in the mammal. Since the ospC mutant was unable to infect mice lacking components of either the acquired (18) or innate branches of the immune response, the essential role of OspC in mammalian infection is other than immune evasion or not solely restricted to resisting the single components of host immunity examined.
TABLE 2.
B. burgdorferi ospC mutant does not infect MyD88−/− mice
| Mousea and B. burgdorferi strain | Detection of B. burgdorferi
|
|
|---|---|---|
| PCRb | Culturec | |
| MyD88+/+ | ||
| BSKd (negative control) | 0/1 | 0/1 |
| WTe | 4/5 | 5/5 |
| ospC7 (mutant) | 0/5 | 0/5 |
| ospC7/ospC+4 (complement) | 3/5 | 2/5 |
| MyD88−/− | ||
| BSKd (negative control) | 0/2 | 0/2 |
| WT | 5/5 | 5/5 |
| ospC7 (mutant) | 0/5 | 0/5 |
| ospC7/ospC+4 (complement) | 4/5 | 5/5 |
All mice derived from a C57BL/6 background as described in Materials and Methods.
Number of PCR-positive mice/number of mice injected, assessed by PCR detection of the B. burgdorferi recA gene from mouse tissues (ankle, heart, and ear). Positive results were based on detection in at least one tissue.
Number of mice culture positive for B. burgdorferi/number of mice injected, based on the culture of mouse urinary bladders in BSK medium. After 2 weeks, cultures were examined by dark-field microscopy for the presence of spirochetes.
Negative-control mice were injected with sterile BSK medium.
WT, wild type.
DISCUSSION
B. burgdorferi is transmitted to the mammalian host as Ixodes ticks feed. The blood meal of the tick appears to act as a trigger for B. burgdorferi to restructure its outer membrane in preparation for infection of the mammalian host. Among the changes that occur prior to transmission to the host is the expression of OspC on the outer surface of the bacterium (39). OspC remains on the outer surface of B. burgdorferi during migration to the tick salivary glands and during the initial infection of the mammal (25, 30, 39), although exceptions to this model have been observed. Ohnishi and colleagues noted OspC-negative cells deposited in the host dermis tissue by feeding ticks, suggesting that OspC may not be required for migration from the tick midgut to the salivary glands or transmission (33). However, the timing of successful mouse infections correlated with the expression of OspC on the surface of B. burgdorferi. Grimm et al. constructed an ospC-minus mutant and demonstrated that whereas this strain could be detected in the salivary glands of feeding ticks, it was unable to infect mice by either tick bite or needle inoculation (18). Complementation with a wild-type copy of ospC restored infectivity, confirming the requirement for this gene product for mammalian infection. Additionally, the ospC mutant did not provoke an antibody response in immunocompetent mice, nor could it infect SCID mice. Therefore, to distinguish between the physiological model and the innate immune model of OspC function, we assessed the viability of the ospC mutant in DMCs implanted within rat peritoneal cavities. The molecular mass cutoff of 8,000 Da of the DMC excludes most components of the immune system from the chambers and therefore provides a relatively immune-privileged environment. These conditions allow some B. burgdorferi strains to grow when they normally would not persist in immunocompetent hosts (35). However, inflammation in murine DMC models has been shown to influence transcript levels and protein expression in DMC-cultivated B. burgdorferi, indicating that some components of the inflammatory response are capable of traversing the DMC (11). The OspC-deficient B. burgdorferi survived, grew, and adapted in the DMC similarly to the wild-type and complemented strains. This indicates that OspC is not providing an essential physiological function for the spirochete in the DMC environment. In contrast, PncA provides an essential function for B. burgdorferi and is required for survival of the spirochete in the DMC (35). However, it is possible that OspC is not essential for B. burgdorferi growth in DMC culture conditions but does provide a physiological function required to survive in the host outside the chambers.
Although OspC is not required for spirochete growth in DMCs, it was possible that OspC was involved in sensing and adapting to the mammalian host. The crystal structure of OspC displays a resemblance to the ligand-binding domain of the aspartate receptor of Salmonella enterica serovar Typhimurium, involved in chemotaxis (13, 24, 47). This observation suggested a potential role for OspC in detecting and/or signaling cues associated with the change from the tick to the mammal. However, the ospC mutant grew and host adapted similarly to the wild-type and complemented strains (Table 1 and Fig. 1), indicating that OspC is not necessary for sensing and adapting to aspects of the mammalian host environment mimicked in the DMC. These findings were supported by two-dimensional immunoblotting of B. burgdorferi cell lysates from DMC-grown bacteria in which the antigenic protein profiles of the wild-type, OspC mutant, and complemented strains did not differ substantially from one another (Fig. 2). These data also indicate that other immunogenic proteins (i.e., RevA and ErpA) do not appear to be altered in abundance to compensate for the lack of OspC, even in the mammalian-adapted spirochetes grown in the DMCs.
Further, the host-adapted ospC mutant strain could not infect immunocompetent mice, indicating that other proteins induced during growth in DMCs could not fulfill the function of OspC. Although B. burgdorferi lacks any sequence paralogs of ospC, it is possible that functional homologs existed in the genome. The sequential expression of potential OspC substitutes might explain why OspC is required only during the initial stage of infection but is not necessary for persistence of the spirochete (44). However, if functional homologs of OspC are present, they apparently are not expressed in the DMC.
Since the ospC mutant strain cannot infect immunodeficient SCID mice or elicit an antibody response in immunocompetent mice but can survive in DMCs, we hypothesized that OspC may be required for evading some component of the host innate immune response. TLRs play a major role in the initial host response to microbial infections, and TLR-2 was specifically shown to respond to the lipoproteins of B. burgdorferi and aid in controlling spirochete numbers (6, 26, 46). Many of the Toll-like receptor family members signal through the adapter protein MyD88 (see reference 3 for a recent review). However, other MyD88-dependent pathways, in addition to the TLR-2 pathway, play important roles in limiting B. burgdorferi proliferation in host tissues (5, 26). The MyD88 signaling pathway is known to be important in several aspects of innate host defense including activation of the phagocyte-dependent killing pathways that include induction of reactive oxygen intermediates and nitric oxide production and up-regulation of the production of defensins. Although wild-type and complemented strains could establish infections in MyD88−/− mice, the ospC mutant strain could not (Table 2). Therefore, the sole function of OspC is not evasion of these and other MyD88-dependent pathways of host defense.
The crystal structure of OspC also displays an overall three-dimensional similarity to that of the variant surface glycoprotein (VSG) of Trypanosoma brucei, the causative agent of African sleeping sickness (13). VSG undergoes antigenic variation and likely protects key membrane proteins from attack by the host immune system by masking the cell in a thick, shield-like barrier (reviewed in reference 12). Although the function of OspC may not be identical to that of the VSGs of T. brucei, we have shown that the ospC mutant can grow in the largely immune-privileged DMC but cannot infect mice deficient in components of acquired or innate immunity. The structural similarity to VSG of T. brucei supports the role of OspC in survival of some aspect of the host immune system, perhaps shielding the B. burgdorferi outer membrane from attack by the innate immune system or in binding a component of that system. B. burgdorferi is susceptible to killing by other branches of the innate immune system that are not altered in MyD88−/− mice. Polymorphonuclear leukocytes, among the first immune cells to respond to microbial invasion in dermis tissues, have been shown to possess microbicidal activity against B. burgdorferi and suggest an area for further investigation (16, 27, 29). Alternatively, OspC may provide protection from other components of host immunity, such as complement. Finally, it is possible that OspC may provide multiple functions essential for survival in the mammalian host, including evasion of both the acquired and innate branches of the immune system.
Acknowledgments
We gratefully acknowledge the veterinary expertise of Don Gardner and the graphical talents of Gary Hettrick and Anita Mora in figure preparation. We thank Shizuo Akira for providing MyD88-deficient mice and Melissa Caimano and Justin Radolf for providing DMC protocols and advice. Also, we are grateful to Sonja Best, Frank DeLeo, and Kim Hasenkrug for critical reading of the manuscript and helpful discussions.
This research was supported in part by the Intramural Research Program of the NIH, NIAID.
Editor: A. D. O'Brien
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akins, D. K., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 11.Crowley, H., and B. T. Huber. 2003. Host-adapted Borrelia burgdorferi in mice express OspA during inflammation. Infect. Immun. 71:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donelson, J. E. 2003. Antigenic variation and the African trypanosome genome. Acta Trop. 85:391-404. [DOI] [PubMed] [Google Scholar]
- 13.Eicken, C., V. Sharma, T. Klabunde, R. T. Owens, D. S. Pikas, M. Höök, and J. C. Sacchettini. 2001. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J. Biol. Chem. 276:10010-10015. [DOI] [PubMed] [Google Scholar]
- 14.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, R., L. Gusmani, R. Murgia, C. Guarnaccia, M. Cinco, and G. Rottini. 1998. Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect. Immun. 66:1408-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm, D., A. F. Elias, K. Tilly, and P. A. Rosa. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, J. T. Seppälä, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 21.Jonsson, M., T. Elmros, and S. Bergström. 1995. Subcutaneous implanted chambers in different mouse strains as an animal model to study genetic stability during infection with Lyme disease Borrelia. Microb. Pathog. 18:109-114. [DOI] [PubMed] [Google Scholar]
- 22.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 23.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 24.Kumaran, D., S. Eswaramoorthy, B. J. Luft, S. Koide, J. J. Dunn, C. L. Lawson, and S. Swaminathan. 2001. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 28.McDowell, J. V., S.-Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery, R. R., S. E. Malawista, K. J. M. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6:2121-2134. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 36.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramamoorthi, N., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, D. Fish, J. Anguita, M. V. Norgard, F. S. Kantor, J. F. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosa, P., D. S. Samuels, D. Hogan, B. Stevenson, S. Casjens, and K. Tilly. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J. Bacteriol. 178:5946-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson, W. J., W. Burgdorfer, M. E. Schrumpf, R. H. Karstens, and T. G. Schwan. 1991. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J. Clin. Microbiol. 29:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson, W. J., W. Cieplak, M. E. Schrumpf, A. G. Barbour, and T. G. Schwan. 1994. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol. Lett. 119:381-388. [DOI] [PubMed] [Google Scholar]
- 42.Steere, A. C. 1995. Borrelia burgdorferi (Lyme disease, Lyme borreliosis), p. 2143-2155. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed., vol. 2. Churchill Livingstone, Inc., New York, N.Y. [Google Scholar]
- 43.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. VanRaden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed]
- 45.Wang, X., Y. Ma, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2005. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect. Immun. 73:657-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 47.Yeh, J. I., H. P. Biemann, G. G. Prive, J. Pandit, D. E. Koshland, Jr., and S. H. Kim. 1996. High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor. J. Mol. Biol. 262:186-201. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J. R., and S. J. Norris. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 66:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 66:3689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]