Abstract
The Toll-like receptors (TLRs) and the adaptor myeloid differentiation factor 88 (MyD88) are important in the innate immune defenses of the host to microbial infections. Meningococcal ligands signaling via TLRs control inflammatory responses, and stimulation can result in fulminant meningococcal sepsis. In this study, we show that the responses to nonlipooligosaccharide (non-LOS) ligands of meningococci are MyD88 dependent. An isogenic LOS-deficient mutant of the serogroup C meningococcal strain FAM20 caused fatal disease in wild type C57BL/6 mice that was not observed in MyD88−/− mice. Fatality correlated with high proinflammatory cytokine and C5a levels in serum, high neutrophil numbers in blood, and increased bacteremia at 24 h postinfection in the wild-type mice. Infection with the parent strain FAM20 resulted in fatality in 100% of the wild-type mice and 50% of the MyD88−/− mice. We conclude that both LOS and another neisserial ligand cause meningococcal sepsis in an in vivo mouse model and confirm that meningococcal LOS can act via both the MyD88- dependent and -independent pathways, while the non-LOS meningococcal ligand(s) acts only via the MyD88-dependent pathway.
Neisseria meningitidis is a commensal inhabitant of the upper respiratory tract of between 5 and 15% of humans (8, 30). Nasopharyngeal carriage of meningococci is normally entirely asymptomatic, and meningococcemia and sepsis occur in only a small percentage of colonized people. The development of invasive disease is thought to depend on host factors, and despite improvement in treatment, N. meningitidis is still an important cause of morbidity and mortality around the world.
The Toll-like receptors (TLRs) are one of the most important families of signaling receptors in the innate immune defenses of the host to microbial infections (41). TLRs control a range of events, including the induction of proinflammatory cytokines, chemokines, costimulatory molecules, and adhesion molecules. The induction of these factors can be beneficial, by stimulating cellular responses to the infection, as well as potentially harmful, since uncontrolled proinflammatory responses can lead to sepsis and fatality. At least 13 members of the TLR family have been identified in mammals. These recognize pathogen-associated molecular patterns (PAMPs), which are conserved molecules shared among a broad range of pathogens. After recognition of ligands, the TLRs can activate a common signaling pathway that leads to activation of the NF-κB transcription factor and mitogen-activated protein kinase signaling molecules.
Myeloid differentiation factor 88 (MyD88) is an essential adaptor molecule for responses to a broad range of microorganisms or their components that are recognized by TLR2, TLR4, TLR5, TLR7, or TLR9. Some TLRs (including TLR3 and TLR4) signal via MyD88-independent pathways (42). Three other adaptor proteins associated with the TLRs have also been identified, and these include TRIF (Toll receptor-associated adaptor of interferon), MAL/TIRAP (MyD88-adaptor-like/ Toll/interleukin 1 [IL-1] receptor homologous region [TIR]-associated protein), and TRAM (Toll-receptor-associated molecule). All transduce signals from the TIR domains, activating protein kinases and transcription factors that cause inflammatory effects.
Neisseria meningitidis expresses a variety of PAMPs recognized by the TLRs. The highly abundant outer membrane (OM) protein PorB activates dendritic cells in a TLR2- and MyD88-dependent manner (24, 35). Similarly, the closely related species Neisseria gonorrhoeae expresses lipopeptides recognized by TLR2 (13). Meningococcal lipooligosaccharide (LOS) and OM blebs containing LOS have been reported to stimulate CD14/TLR4 (25, 46) via both MyD88-dependent and MyD88-independent pathways (47).
LOS has long been presumed to be the major inflammatory mediator of fulminant meningococcal sepsis and meningitis, with disease severity correlating with circulating concentrations of LOS and proinflammatory cytokines (4). Meningococcal LOS lacks the repeating O antigens of enteric LPS, but maintains a conserved inner core composed of heptose and 3-deoxy-d-manno-2-octulosonic acid (KDO) bound to lipid A, to which variable α- and β-chain saccharides are attached (18). Lipid A is the active moiety through its ability to upregulate the inflammatory response (34). Development of meningococcal LOS knockout mutants has allowed the study of the contribution of non-LOS molecules to the host response (39, 40, 44). The contributions of non-LOS ligands of meningococci to cellular responses have been shown in a number of studies to have effects on cytokine induction (10, 16, 32, 40, 43, 46), endothelial cell adhesion molecule expression (9), and expression of stress response genes (3). It has been shown that an LOS-deficient strain of N. meningitidis activates macrophages through TLR2 (17, 32); however, another study recently showed that the recognition of the LOS-deficient OM of N. meningitidis is not associated with the expression of TLR2 on human meningeal cells (16). The involvement of other pathogen response receptors in meningococcal infection, including the class A macrophage scavenger receptor SR-A (31) and other unidentified receptors, has also been suggested (16).
To investigate the role of MyD88 in the pathway of stimulation in response to non-LOS factors in vivo, we infected MyD88−/− mice with the serogroup C meningococcal strain FAM20 and an LOS-deficient isogenic lpxA strain (1). Our data show that MyD88 is crucial in the development of meningococcal sepsis associated with the unidentified non-LOS neisserial ligand(s).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The serogroup C meningococcal strain N. meningitidis FAM20 (12, 33) and the LOS-deficient FAM20 (lpxA) mutant (1) have previously been described. The lpxA mutant has a disrupted lpxA gene that encodes an enzyme responsible for the first step of the lipid A biosynthesis. Both strains were grown on GC agar containing Kellogg's supplement (20) at 37°C in a 5% CO2 atmosphere for 18 to 20 h, and the GC agar used for growth of the LOS mutant was supplemented with 50 μg of kanamycin/ml. Meningococcal strains were suspended in prewarmed GC liquid and immediately used for mouse inoculation.
Media and buffers.
The cell culture medium was Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% inactivated fetal bovine serum (Sigma), 2 mM l-glutamine (Sigma), 1 mM sodium pyruvate (Gibco), and 1× nonessential amino acids (Gibco). Medium was supplemented with the antibiotics penicillin (100 U/ml) and streptomycin (100 U/ml) where indicated. GC liquid was composed of 1.5% (wt/vol) protease peptone no. 3 (Becton Dickinson), 3 mM soluble starch (Sigma), 23 mM K2HPO4 (Merck), 7 mM KH2PO4 (Merck), and 50 mM NaCl (Merck).
Mouse strains.
C57BL/6 mice (B6; wild type) were obtained from Taconic (M&B), Ry, Denmark. MyD88−/− mice (backcrossed 6 generations on the B6 background) were kept under pathogen-free conditions. All mice were bred and housed at the animal facility at the Rudbecks Laboratory, Uppsala, Sweden. Mice were 5 to 8 weeks old when challenged with bacteria. All animal procedures were performed in accordance with the institutional guidelines of Uppsala University under an approved protocol (C264/4).
Animal model.
Five- to 8-week-old mice were randomly distributed in groups and injected intraperitoneally (i.p.) with 100 μl of a bacterial suspension containing 1 × 108 or 5 × 108 FAM20 cells and the lpxA mutant strain. Equivalent amounts of bacteria were determined by optical density at 600 nm. The optical density measurement was used to control the dose since the lpxA mutant strain exhibits clumping and viable counts of this organism are lower at an equivalent optical density. The number of viable bacteria in the challenge dose was confirmed by viable count. Blood samples were obtained from the tail at different time points after bacterial challenge. The skin was disinfected with 70% ethanol, and 5 μl of blood was collected from the tail. Blood was diluted in GC liquid, serial dilutions were plated on GC agar plates and incubated overnight at 37°C in a 5% CO2 atmosphere, and CFU were enumerated.
Serum collection.
Mice that had been infected with 1 × 108 wild-type FAM20 cells or equivalent amounts of biological material of the lpxA mutant strain were anesthetized by light inhalation of isoflurane (Forene; Abbott), and blood was collected by retro-orbital bleed at various times postinfection. Blood was allowed to clot at 4°C, and serum was collected after centrifugation at 10,000 × g for 10 min. Serum was stored at −70°C until used.
Determination of cytokine and chemokine levels in serum by ELISA.
Concentrations of murine interleukin 6 (IL-6), tumor necrosis factor (TNF), IL-10, gamma interferon (Diaclone), KC, IFN-γ-inducible protein 10 (IP-10), and IFN-β (R&D Systems) were measured in serum of N. meningitidis-challenged mice (n ≥ 6) at different time points postinjection by using sandwich enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer's recommendations.
Analysis of C5a levels in serum by ELISA.
For detection of C5a in mouse serum, a rat anti-mouse C5a monoclonal antibody, I52-1486 (1 μg/ml), and biotinylated rat anti-mouse C5a monoclonal antibody I52-278 (0.5 μg/ml) from BD Biosciences were used, respectively, as ELISA capture and detection antibodies. Serum from infected mouse groups (n > 6) was diluted 1:10 in 1% bovine serum albumin in phosphate-buffered saline and incubated for 2 h at room temperature. Serum from the uninfected B6 mice was arbitrarily assigned a concentration of 40 U/ml C5a.
Blood smears.
Blood was obtained from the tail veins of mice at various time points postinfection of meningococci as described above, and smears were made. Slides were fixed with methanol and stained with Wright's stain according to manufacturer's recommendations (Sigma). A differential white blood cell count was performed by light microscopy.
Whole-blood bactericidal assay.
Whole blood was taken from the orbital vein of B6 and MyD88−/− mice (n = 3), pooled, and mixed 1:5 with sodium citrate (0.1 M). Bacterial suspensions containing 5 × 105 CFU of N. meningitidis FAM20 or the lpxA mutant were mixed 1:1 with blood in a final volume of 200 μl. The mixtures were incubated in 5% CO2 and 37°C for 1 h and 4 h, and surviving bacteria were plated on GC agar. The surviving viable bacteria in blood were determined after incubating plates overnight in 5% CO2 and 37°C.
Phagocytosis of meningococci by peritoneal macrophages.
Peritoneal cells were collected from uninfected B6 and MyD88−/− mice (n = 3). Briefly, animals were sacrificed by cervical dislocation, and 5 ml of cold sterile phosphate-buffered saline was injected intraperitoneally. Recovered cells were pelleted by centrifugation at 400 × g for 10 min at 4°C, washed, and cultured in cell culture medium containing antibiotics overnight in 5% CO2 and 37°C. For the infection of cells, the antibiotics were removed by washing cells with cell culture media without antibiotics and bacteria were added in fresh cell culture media at a multiplicity of infection of 100. After 60 min of incubation, extracellular bacteria were killed with 250 μg/ml gentamicin for 1 h at 37°C. The cells were lysed with 200 μl of 1% saponin (Quillaja bark; Sigma) for 10 min, and 800 μl of prewarmed GC liquid was added to the wells. Bacteria were enumerated by plating on GC agar.
Statistical analysis.
Data were evaluated using Microsoft Excel. A two-tailed Student's t test was used to assess significance in the blood survival assay, macrophage phagocytosis assay, and cytokine assays. Lethality experiments were assessed using a Fisher's exact test, and bacteremia and neutrophil numbers were monitored with a nonparametric Mann-Whitney test. Significance was accepted at P < 0.05. All results were obtained in at least two independent experiments.
RESULTS
MyD88−/− mice show resistance to meningococcal sepsis.
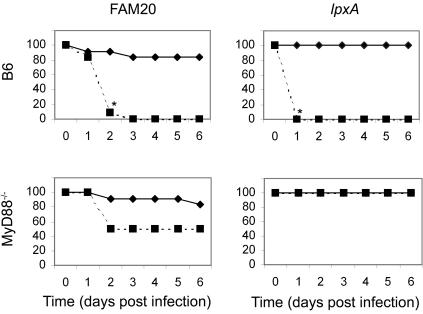
To assess the role of MyD88 in the development of meningococcal sepsis, the survival of wild-type C57BL/6 (B6) mice and MyD88−/− mice infected intraperitoneally with N. meningitidis FAM20 and its LOS-deficient lpxA mutant was monitored (Fig. 1). Wild-type B6 mice and the MyD88−/− mice infected with 1 × 108 CFU of FAM20/mouse had fatality rates of between 10 and 20%; however, the B6 mice showed signs of distress following infection, and this was not noted in the MyD88−/− mice. Both mouse strains survived following infection with the LOS mutant at an equivalent amount of biological material as that given in the dose of 1 × 108 CFU of FAM20/mouse. At a higher dose of 5 × 108 CFU of FAM20/mouse, or the equivalent amount of the lpxA mutant, significant fatality was noted in all B6 mice infected with the wild-type strain FAM20 and the lpxA mutant. In the MyD88−/− mice, fatal infection occurred at 1 to 2 days postinfection in 50% of mice infected with FAM20. The MyD88−/− mice were resistant to infection with the lpxA mutant. All subsequent assays were conducted in mice given a sublethal dose of 1 × 108 CFU/mouse of FAM20 or equivalent amounts of the lpxA mutant.
FIG. 1.
MyD88−/− mice are resistant to intraperitoneal challenge with the LOS-deficient meningococcal lpxA mutant. Shown is the survival of B6 and MyD88−/− mice following infection with 1 × 108 CFU/mouse (solid lines: n = 12) and 5 × 108 CFU/mouse (dashed lines; n = 12) of the wild-type bacterial strain N. meningitidis FAM20 or equivalent amounts of the LOS-deficient lpxA mutant. Survival was analyzed by Fisher's exact test. Significant differences between the low- and high-dose survival are indicated by an asterisk at the time point at which the differences became significant (P < 0.05).
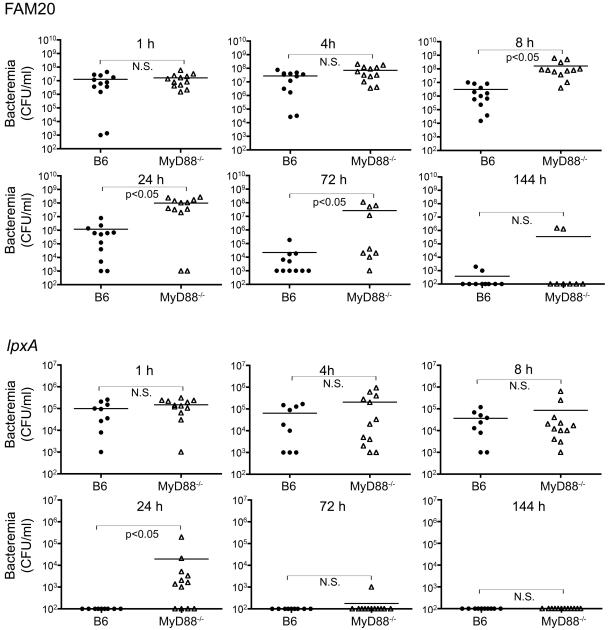
MyD88−/− mice have higher bacteremia and delayed clearance of N. meningitidis.
Bacteremia levels were analyzed in mice administered the wild-type strain FAM20 or the LOS-deficient lpxA mutant (Fig. 2). An equivalent rate of entrance to the blood was noted in B6 and MyD88−/− mice following infection with the wild-type bacterial strain FAM20, with no significant difference in bacteremia levels noted at 1 and 4 h postdose; however, at 8, 24, and 72 h postdose, the MyD88−/− mice had a higher level of bacteremia, most likely related to the decreased capacity of these mice to clear the infection. At the early time points following administration of the lpxA mutant, no significant difference was noted in bacterial levels in blood of either mouse strain; however, at 24 h postinfection, a higher level of bacteremia was seen in the MyD88−/− mice. In both mouse strains, the level of bacteremia following infection with FAM20 was between two- and threefold increased compared to the levels observed following infection with the lpxA mutant. The decreased clearance of the lpxA mutant in the MyD88−/− mice correlated with increased bacterial survival in whole blood in vitro (Table 1). Significantly higher levels of the lpxA mutant were noted in MyD88−/− mouse blood compared to B6 mouse blood at 4 h postinfection.
FIG. 2.
Bacteremia in mice infected with wild-type FAM20 or the lpxA mutant. Mice were infected with the wild-type bacterial strain FAM20 at 1 × 108 CFU or equivalent amounts of biological material of the lpxA mutant, and bacterial blood titers were monitored. All mice had similar bacteremia levels following infection with the lpxA mutant, except at 24 h postinfection (P < 0.05); however, bacteremia was significantly enhanced in MyD88−/− mice following infection with the wild-type bacterial strain FAM20 (P < 0.05) at 8, 24, and 72 h postinfection. The counts were analyzed by the nonparametric Mann-Whitney test. N.S., nonsignificant difference.
TABLE 1.
Growth of meningococcal strains in whole mouse blood from B6 and MyD88−/− mice
| Time postinfection (h) | Bacterial strain | Growth in mouse blood (CFU/ml)a
|
|
|---|---|---|---|
| B6 | MyD88−/− | ||
| 1 | FAM20b | 3.6 × 105 (4.5 × 104) | 3.0 × 105 (1.2 × 104) |
| lpxA mutantc | 1.1 × 105 (2.2 × 104) | 1.6 × 105 (5.1 × 104) | |
| 4 | FAM20b | 4.6 × 106 (1.4 × 105) | 3.6 × 106 (4.3 × 105) |
| lpxA mutantc | 3.6 × 105 (1.2 × 105) | 8.0 × 105 (1.4 × 105)* | |
Data are presented as mean (standard deviation). *, statistically significant difference (P < 0.05) in bacterial numbers compared to numbers in B6 mice observed using a Student's t test.
Original inoculum, 1.8 × 105 CFU/ml.
Original inoculum, 1.1 × 105 CFU/ml.
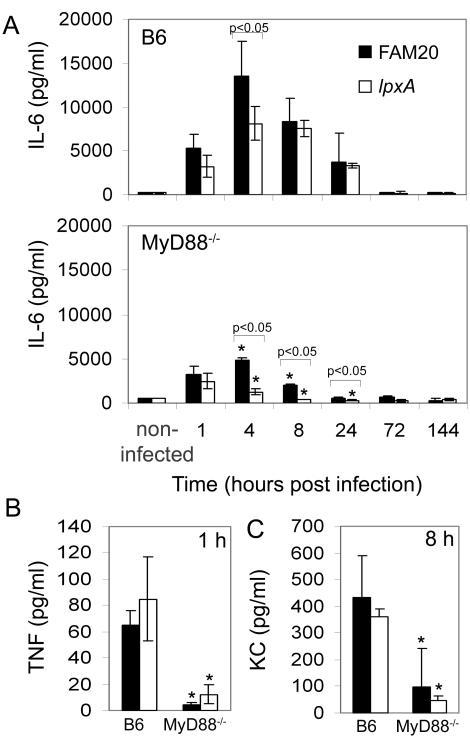
MyD88−/− mice have lower proinflammatory cytokine levels following meningococcal infection.
As expected, the levels of the proinflammatory cytokines IL-6 and TNF and the chemokine KC were lower in the MyD88−/− mice than those seen in B6 mice (Fig. 3). In B6 mice, there was a significant increase in the level of IL-6 in mouse serum following infection with FAM20 only at 4 h postdose (Fig. 3A), which was not noted at other time points postdose, indicating that IL-6 induction is independent of LOS expression. In the MyD88−/− mice, LOS deficiency resulted in a decreased IL-6 response at 4, 8, and 24 h postdose. The levels of the proinflammatory cytokine TNF (Fig. 3B) and the chemokine KC (Fig. 3C) were also lower in infected MyD88−/− mice than in B6 mice, although no difference was noted between the wild-type strain FAM20 and the LOS-deficient lpxA mutant strain in either mouse strain, further confirming that another non-LOS factor is responsible for the proinflammatory effect in vivo. No statistical differences in basal levels of TNF or KC were noted in uninfected mice. The level of TNF in uninfected B6 mice was 4.1 ± 1.8 pg/ml, while the level in uninfected MyD88−/− mice was 3.3 ± 0.8 pg/ml. The level of KC in uninfected B6 mice was 44.0 ± 30.3 pg/ml, while the level in uninfected MyD88−/− mice was 93.9 ± 10.3 pg/ml.
FIG. 3.
The wild-type strain FAM20 (black bars) and the LOS-deficient lpxA mutant (white bars) induce similar levels of proinflammatory cytokines in serum of B6 and MyD88−/− mice. (A) IL-6 induction in B6 and MyD88−/− mice at various times postadministration (intraperitoneal) of 1 × 108 FAM20 cells/mouse or equivalent amounts of biological material of the lpxA mutant. (B) TNF induction was measured at 1 h postadministration, and (C) KC levels were monitored at 8 h postadministration in serum. Both TNF and KC were significantly decreased in MyD88−/− mice; however, no difference was observed in the response to the wild-type strain FAM20 and the lpxA mutant. Statistically significant reductions of IL-6 compared to FAM20 are indicated by bars, and significance compared to the control B6 mice at each time point is indicated by a single asterisk when P is <0.05. Significance between treatments was determined using an unpaired Student's t test. Data are represented as means ± standard deviation.
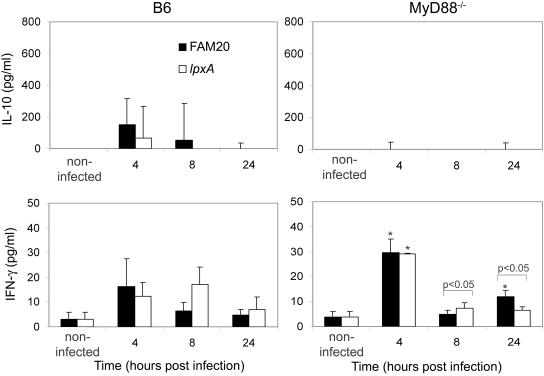
MyD88−/− mice have a decreased IL-10 response but increased levels of IFN-γ.
IL-10 is an anti-inflammatory cytokine (23, 36) with suppressive effects on the synthesis of proinflammatory cytokines and chemokines, such as TNF, IL-1α, IL-1β, IL-2, IL-6, and IL-8. IL-10 was not detected in uninfected mice. Following infection with FAM20, the IL-10 serum concentration in B6 mice was highest at 4 h postchallenge (Fig. 4). LOS-deficient bacteria triggered detectable IL-10 production only at 4 h postinfection, but IL-10 was undetectable at later time points. Sera from MyD88−/− mice did not contain detectable levels of IL-10 after challenge with either bacterial strain, and these data indicate that MyD88−/− mice have an impaired capacity to induce IL-10.
FIG. 4.
The wild-type strain FAM20 (black bars) and the LOS-deficient mutant lpxA (white bars) induce similar levels of the regulatory cytokines IL-10 and IFN-γ in serum of B6 and MyD88−/− mice. IL-10 levels were significantly increased in B6 mice at all time points postinfection compared to levels observed in MyD88−/− mice following infection. IFN-γ was increased in MyD88−/− mice at 4 h postinfection compared to levels in wild-type B6 mice. Statistically significant changes in mice infected with the lpxA mutant compared to FAM20 are indicated by bars. Significant increases are indicated with an asterisk when P is <0.05. Significance between treatments was determined using an unpaired Student's t test. Data are represented as means ± standard deviation.
IL-10 is a strong inhibitor of IFN-γ production (26), and the antagonistic effect of IL-10 on IFN-γ is reciprocal (14). IFN-γ enhances the function of macrophages and polymorphonuclear leukocytes by stimulating nonspecific defense mechanisms, such as phagocytosis and secretion of reactive oxygen intermediates (27). Uninfected B6 and MyD88−/− mice had levels of IFN-γ of 3.0 ± 3.0 pg/ml and 3.8 ± 2.3 pg/ml, respectively. Both mouse strains showed similar levels of IFN-γ; however, at 4 h postinfection, the levels induced in MyD88−/− mice were significantly higher following administration of both bacterial strains (Fig. 4). We measured the phagocytosis of cells taken from the peritoneal cavity of mice and despite the induction of IFN-γ in MyD88−/− mice, the peritoneal cells isolated from these mice showed inhibited phagocytosis of the lpxA mutant (data not shown).
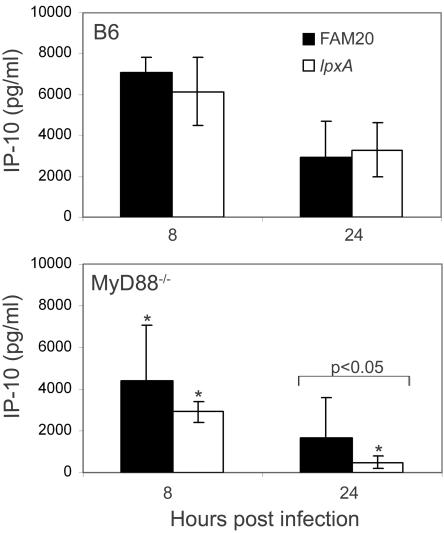
Induction of the MyD88-independent cytokines IP-10 and IFN-β.
We examined the production of cytokines IP-10 and IFN-β, which are induced via the MyD88-independent pathway. It has previously been shown that IP-10 can be induced by polymorphonuclear neutrophils following stimulation with meningococcal OM vesicles, but only if the neutrophils are stimulated in combination with IFN-γ (22). Uninfected B6 and MyD88−/− mice had levels of IP-10 of 200.0 ± 19.2 pg/ml and 220.0 ± 108.0 pg/ml, respectively. IP-10 was induced in significantly larger amounts in B6 mice compared to in MyD88−/− mice following infection with both strains (Fig. 5), despite the presence of higher levels of IFN-γ in the MyD88−/− mice at 4 h postinfection (Fig. 4). No difference was observed in the level of IP-10 following infection with FAM20 or the LOS-deficient lpxA mutant, except for at 24 h postinfection in MyD88−/− mice, where lower levels were observed following infection with the lpxA mutant. This further shows that in these mice the LOS of the wild-type bacterial strain has the potential to stimulate IP-10 production in a MyD88-independent manner. Interestingly, IFN-β was not significantly induced in either mouse strain following infection with either bacterial strain (data not shown).
FIG. 5.
Levels of IP-10 are decreased in MyD88−/− mice. Statistically significant changes in mice infected with the lpxA mutant (white bars) compared to FAM20 (black bars) are indicated. Significantly decreased levels of these cytokines compared to the control B6 mice at each time point are indicated with a single asterisk when P is <0.05. Significance between treatments was determined using an unpaired Student's t test. Data are represented as means ± standard deviation.
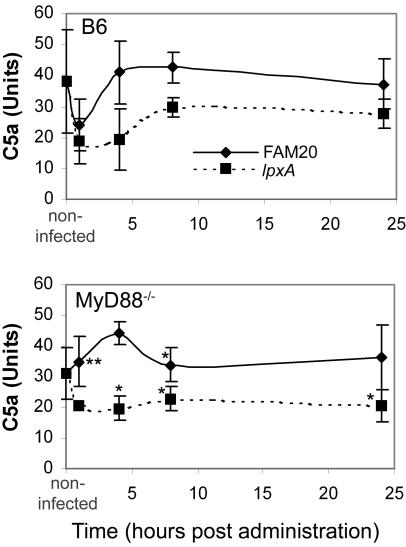
MyD88−/− mice show inhibited C5a induction.
C5a is a potent chemotactic factor for neutrophils, monocytes, and macrophages, and production of C5a correlates with increased cytokine release and severe sepsis (15). The production of C5a in the serum of mice infected with FAM20 or the LOS-deficient mutant lpxA was monitored by ELISA analysis (Fig. 6). In the wild-type B6 mice, a sharp decrease in the level of C5a was noted at 1 h postinfection with both FAM20 and the LOS lpxA mutant. In mice infected with FAM20, the levels of C5a rose rapidly and C5a was seen at normal levels at all subsequent times. The level of C5a in the mice infected with the lpxA mutant did not rise to normal levels, and C5a was inhibited at all subsequent time points. In MyD88−/− mice infected with FAM20, the sharp decrease observed in the wild-type mice at 1 h postdose was not noted and an increase in C5a levels was observed at 1 and 4 h postdose, indicating that LOS can stimulate C5a induction in the absence of MyD88. The basal levels of C5a in these mice were slightly lower than those in the wild-type mice, and the basal level was reached at 8 h postdose. The levels of C5a in MyD88−/− mice infected with the lpxA mutant were significantly lower than those in the mice infected with FAM20 and in B6 mice at all time points.
FIG. 6.
Serum samples were assessed for the presence of the complement factor C5a from B6 and MyD88−/− mice infected with FAM20 (solid lines) and the LOS-deficient lpxA mutant (dashed lines). Significance between treatments was determined using an unpaired Student's t test. Statistical decrease compared to treatment with FAM20 at each time point is indicated with a single asterisk when P is <0.05, while an increase is indicated with a double asterisk. Data are represented as means ± standard deviation.
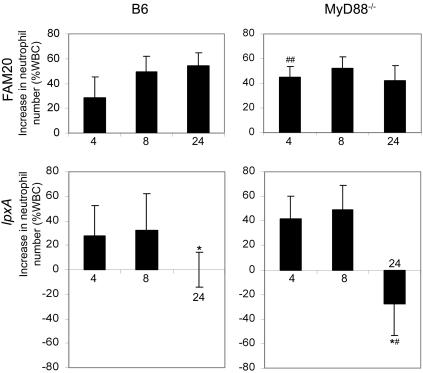
MyD88 influences neutrophil infiltration in mice infected with the lpxA mutant.
Polymorphonuclear neutrophils are natural effector cells mediating antimicrobial defenses via the release of toxic oxygen intermediates and lytic enzymes. The number of neutrophils as a percentage of total immune cells was determined by microscopic examination of blood smears. Both mouse strains showed similar neutrophil numbers prior to infection. Neutrophils constituted 23.8% ± 9.8% and 20.3% ± 5.5% of total blood cells in B6 mice and MyD88−/− mice, respectively. In mice infected with FAM20, the infiltration of neutrophils into the blood was not affected by MyD88 (Fig. 7). Neutrophil infiltration in B6 mice following infection with the LOS mutant was inhibited, probably reflecting the lower bacteremia in these mice compared to that in mice infected with FAM20 at 24 h postdose. The neutrophil infiltration in MyD88−/− mice infected with the lpxA mutant was significantly decreased at 24 h postinfection compared to infection in the B6 mice, as well as significantly decreased compared to infection with FAM20. This indicates that MyD88-dependent signaling is required for recognition of the LOS-deficient mutant and the recruitment of neutrophils to the blood.
FIG. 7.
Infiltration of neutrophils into blood of B6 and MyD88−/− mice infected with FAM20 and the LOS-deficient lpxA mutant. Significant decreases in neutrophil levels in MyD88−/− mice compared to B6 mice were determined using an unpaired Student's t test, and statistically reduced (P < 0.05) neutrophil levels are indicated with #, while significant inductions are indicated with ##. Statistically significant decreases compared to FAM20 infection are indicated with a single asterisk when P is <0.05. Data are represented as means ± standard deviation. WBC, white blood cells.
DISCUSSION
Sepsis caused by Neisseria meningitidis is a rapid and severe disease associated with high fatality rates. The fatality associated with sepsis has long been presumed to be dependent on the expression of LOS, and a correlation between disease severity and the levels of cytokines and circulating LOS has been observed (4). However, in this study we have observed that another neisserial factor is also involved in the development of septic shock, since an LOS-deficient mutant of the serogroup C strain FAM20 caused fatal sepsis and induced equivalent proinflammatory responses in the mouse model of meningococcal sepsis. We have also confirmed involvement of the adaptor MyD88 in the signaling pathway stimulated by this currently unidentified neisserial factor.
In contrast to other microbial diseases where the expression of various TLRs or adaptor or signaling molecules is considered to be beneficial and enhances clearance of the causative agent (21, 29, 45), expression of these factors in a mouse model of meningococcal sepsis is associated with fatality. It is unlikely that development of sepsis is a direct result of responses to the bacteria itself, since both strains showed similar levels of fatality in the wild-type B6 mice despite vastly different levels of bacteremia. It is more likely that the excessive production of proinflammatory cytokines, chemokines, and costimulatory and adhesion molecules or the generation of heat shock proteins contributes to disease severity.
In this study, we observed that MyD88−/− mice showed resistance to meningococcal killing, indicating that signaling via MyD88 is associated with enhanced morbidity and mortality in this mouse model and is involved in the development of sepsis. MyD88−/− mice had a decreased capacity to produce the proinflammatory cytokines IL-6 and TNF and the chemokine KC and had lower C5a levels and decreased neutrophils in the blood when infected with the LOS-deficient lpxA mutant. The cytokines IL-6 and TNF and the chemokine KC are under control of the NF-κB promoter. These cytokines are all induced early in a MyD88-dependent manner; however, since NF-κB is induced later in response to the IRF-3 adaptor, a late induction of these cytokines could also be seen. It has previously been shown that a meningococcal serogroup B LOS mutant shows poor activation of NF-κB (9), which is inconsistent with our data showing that both meningococcal strains induced equivalent amounts of these cytokines. Also, other in vitro studies using cell models of monocytes (40, 43), differentiated macrophages (32, 46), dendritic cells (10), and meningeal cells (16) have shown that meningococci deficient in LOS are able to induce significant cellular activation, although levels of cytokines induced in most of these studies were reduced compared to those observed following infection with the parent strain.
A recent study by Zughaier et al. (47) identified IFN-α/β as key molecules in the differential induction of the MyD88-independent signaling pathway to N. meningitidis LOS. We did not observe an increase in the level of IFN-β in serum from mice infected with either the wild-type strain FAM20 or the LOS-deficient lpxA mutant. It is possible that IFN-α is responsible for the effects or that low concentrations of IFN-β that were not detectable in the ELISA analysis affect signaling. IP-10 is an important chemokine involved in T-cell recruitment. It is produced in response to IFN-γ in combination with either TNF or bacterial lipopolysaccharides, and the response is blocked by IL-10 and IL-4 (11). LOS is not the only ligand responsible for the induction of IP-10 in vivo, since the wild-type strain and the lpxA mutant both induced equivalent IP-10 levels at 8 h postinfection (Fig. 5). However, at 24 h postinfection, the level of IP-10 was significantly reduced following infection with the LOS mutant in the MyD88−/− mice, confirming previous findings that LOS stimulates IP-10 production in the absence of MyD88-dependent signaling (19). However, our findings also confirm that MyD88 also plays a role in the induction of IP-10 (28) since levels were decreased in MyD88−/− mice compared to B6 mice.
In this study, we have also observed an involvement of C5a in the innate response to LOS-deficient meningococcal infection (Fig. 6). We showed a decrease in the levels of C5a in the serum of mice infected with the LOS-deficient meningococcal strain. This was surprising since it had previously been determined that complement activation and the subsequent inflammation are induced by factors independent of meningococcal lipooligosaccharide (38, 39). The decrease of C5a was significantly pronounced in MyD88−/− mice, indicating that the C5a response to the LOS-deficient meningococcal strain is mediated via MyD88. Decreased C5a levels in MyD88−/− mice could also account for the reduced neutrophil numbers in these mice. Antibodies to C5a have been shown to be important in the prevention of meningococcal sepsis (37) and provide an explanation for the difference in disease severity between B6 mice and MyD88−/− mice.
High levels of neutrophils were shown to infiltrate into the blood circulation in both B6 and MyD88−/− mice following infection, although the lpxA mutant had a decreased ability to cause neutrophil infiltration compared to the wild-type strain (Fig. 7). At 24 h, neutrophil levels were not related to bacteremia levels, which were significantly higher in MyD88−/− mice following administration of both bacterial strains at this time (Fig. 2). It is likely that the decrease in neutrophil levels is a direct result of the absence of unmeasured chemotactic factors in the blood. The decreased responses in MyD88−/− mice also indicate that although neutrophils are present in the blood, the function of these cells may be compromised.
Lipooligosaccharide of meningococci has previously been shown to have a central role in the development of sepsis in humans (5-7), and the response to LOS is primarily mediated via the TLR4-dependent signaling pathway (2, 46). The findings of this study indicate that meningococci express a non-LOS ligand that makes a significant contribution to the development of meningococcal septic shock. Although the neisserial porin has been shown to be a TLR2 ligand which signals via the MyD88 pathway (24, 35), the fatality associated with the lpxA mutant described here is not due to the action of porin. This is evident from unpublished data showing that infection of TLR2−/− mice with the lpxA mutant results in sepsis equivalent to that observed in wild-type mice.
In this study, we have used an in vivo animal model to study the effects of meningococcal LOS. We have noted that MyD88−/− mice are not susceptible to LOS-deficient meningococcal infection despite increased bacteremia levels and decreased innate immunity, showing that the response to an unidentified neisserial PAMP occurs in a MyD88-dependent manner. Identification of the unknown ligand is of critical importance in understanding the development of meningococcal sepsis.
Acknowledgments
We thank S. Akira, K. Takeda, and O. Takeuchi, Department of Host Defense, Research Institute for Microbial Diseases, Osaka, Japan, for the MyD88−/− mice.
This work was supported by grants from the Swedish Medical Research Council (Dnr 2004-4831 and 2002-6240), Swedish Cancer Society, Swedish Society for Medicine, Wenner-Gren Foundations, and Uppsala University.
Editor: J. N. Weiser
REFERENCES
- 1.Albiger, B., L. Johansson, and A. B. Jonsson. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerre, A., B. Brusletto, R. Ovstebo, G. B. Joo, P. Kierulf, and P. Brandtzaeg. 2003. Identification of meningococcal LPS as a major monocyte activator in IL-10 depleted shock plasmas and CSF by blocking the CD14-TLR4 receptor complex. J. Endotoxin Res. 9:155-163. [DOI] [PubMed] [Google Scholar]
- 3.Bonnah, R. A., J. Hoelter, L. Steeghs, C. A. Enns, M. So, and M. U. Muckenthaler. 2005. Lipooligosaccharide-independent alteration of cellular homeostasis in Neisseria meningitidis-infected epithelial cells. Cell Microbiol. 7:869-885. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., A. Bjerre, R. Ovstebo, B. Brusletto, G. B. Joo, and P. Kierulf. 2001. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin Res. 7:401-420. [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Investig. 89:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., T. E. Mollnes, and P. Kierulf. 1989. Complement activation and endotoxin levels in systemic meningococcal disease. J. Infect. Dis. 160:58-65. [DOI] [PubMed] [Google Scholar]
- 8.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263-1271. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, G. L., R. S. Heyderman, P. van der Ley, and N. J. Klein. 2004. High-level endothelial E-selectin (CD62E) cell adhesion molecule expression by a lipopolysaccharide-deficient strain of Neisseria meningitidis despite poor activation of NF-kappaB transcription factor. Clin. Exp. Immunol. 135:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon, G. L., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 12.Dyer, D. W., W. McKenna, J. P. Woods, and P. F. Sparling. 1987. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb. Pathog. 3:351-363. [DOI] [PubMed] [Google Scholar]
- 13.Fisette, P. L., S. Ram, J. M. Andersen, W. Guo, and R. R. Ingalls. 2003. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 278:46252-46260. [DOI] [PubMed] [Google Scholar]
- 14.Florquin, S., Z. Amraoui, D. Abramowicz, and M. Goldman. 1994. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J. Immunol. 153:2618-2623. [PubMed] [Google Scholar]
- 15.Guo, R. F., and P. A. Ward. 2005. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23:821-852. [DOI] [PubMed] [Google Scholar]
- 16.Humphries, H. E., M. Triantafilou, B. L. Makepeace, J. E. Heckels, K. Triantafilou, and M. Christodoulides. 2005. Activation of human meningeal cells is modulated by lipopolysaccharide (LPS) and non-LPS components of Neisseria meningitidis and is independent of Toll-like receptor (TLR)4 and TLR2 signalling. Cell Microbiol. 7:415-430. [DOI] [PubMed] [Google Scholar]
- 17.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2000. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J. Endotoxin Res. 6:411-415. [PubMed] [Google Scholar]
- 18.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 19.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 22.Lapinet, J. A., P. Scapini, F. Calzetti, O. Peréz, and M. A. Cassatella. 2000. Gene expression and production of tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-8, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect. Immun. 68:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchant, A., M. L. Alegre, A. Hakim, G. Pierard, G. Marecaux, G. Friedman, D. De Groote, R. J. Kahn, J. L. Vincent, and M. Goldman. 1995. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. J. Clin. Immunol. 15:266-273. [DOI] [PubMed] [Google Scholar]
- 24.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 25.Mirlashari, M. R., and T. Lyberg. 2003. Expression and involvement of Toll-like receptors (TLR)2, TLR4, and CD14 in monocyte TNF-alpha production induced by lipopolysaccharides from Neisseria meningitidis. Med. Sci. Monit. 9:BR316-BR324. [PubMed] [Google Scholar]
- 26.Moore, K., A. O'Garra, R. De Waal Malefyt, P. Viera, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 27.Murray, H. W. 1981. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J. Exp. Med. 153:1302-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagarajan, U. M., D. M. Ojcius, L. Stahl, R. G. Rank, and T. Darville. 2005. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J. Immunol. 175:450-460. [DOI] [PubMed] [Google Scholar]
- 29.Naiki, Y., K. S. Michelsen, N. W. Schroder, R. Alsabeh, A. Slepenkin, W. Zhang, S. Chen, B. Wei, Y. Bulut, M. H. Wong, E. M. Peterson, and M. Arditi. 2005. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J. Biol. Chem. 280:29242-29249. [DOI] [PubMed] [Google Scholar]
- 30.Neal, K. R., J. S. Nguyen-Van-Tam, N. Jeffrey, R. C. Slack, R. J. Madeley, K. Ait-Tahar, K. Job, M. C. Wale, and D. A. Ala'Aldeen. 2000. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. Br. Med. J. 320:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peiser, L., M. P. J. De Winther, K. Makepeace, M. Hollinshead, P. Coull, J. Plested, T. Kodama, E. R. Moxon, and S. Gordon. 2002. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 70:5346-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 33.Rahman, M., H. Kallstrom, S. Normark, and A. B. Jonsson. 1997. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol. Microbiol. 25:11-25. [DOI] [PubMed] [Google Scholar]
- 34.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zahringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 35.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174:3545-3550. [DOI] [PubMed] [Google Scholar]
- 36.Spits, H., and R. de Waal. 1992. Functional characterization of human IL-10. Int. Arch. Allergy Immunol. 99:8-15. [DOI] [PubMed] [Google Scholar]
- 37.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, M. van Deuren, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102:3702-3710. [DOI] [PubMed] [Google Scholar]
- 38.Sprong, T., D. L. Jack, N. J. Klein, M. W. Turner, P. van der Ley, L. Steeghs, L. Jacobs, J. W. van der Meer, and M. van Deuren. 2004. Mannose binding lectin enhances IL-1b and IL-10 induction by non-lipopolysaccharide (LPS) components of Neisseria meningitidis. Cytokine 28:59-66. [DOI] [PubMed] [Google Scholar]
- 39.Sprong, T., A.-S. Moller, A. Bjerre, E. Wedege, P. Kierulf, J. W. van der Meer, P. Brandtzaeg, M. van Deuren, and T. E. Mollnes. 2004. Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide. Infect. Immun. 72:3344-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70:283-288. [PubMed] [Google Scholar]
- 41.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 42.Theofilopoulos, A. N., R. Baccala, B. Beutler, and D. H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307-336. [DOI] [PubMed] [Google Scholar]
- 43.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. Van Der Ley, M. Van Deuren, R. E. Callard, and N. Klein. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Ley, P., and L. Steeghs. 2003. Lessons from an LPS-deficient Neisseria meningitidis mutant. J. Endotoxin Res. 9:124-128. [DOI] [PubMed] [Google Scholar]
- 45.Wieland, C. W., S. Florquin, N. A. Maris, K. Hoebe, B. Beutler, K. Takeda, S. Akira, and T. van der Poll. 2005. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J. Immunol. 175:6042-6049. [DOI] [PubMed] [Google Scholar]
- 46.Zughaier, S. M., Y.-L. Tzeng, S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zughaier, S. M., S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2005. Differential induction of the Toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect. Immun. 73:2940-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]