Abstract
A hypervariable region (HVR) previously identified in the carboxy-terminal one-third of the Babesia bovis variable merozoite surface antigen family was more extensively analyzed in merozoite surface antigen 1 (MSA-1) from 16 strains and isolates. The MSA-1 HVR is proline rich and contains three semiconserved motifs nearly identical to those described for the related family member MSA-2. Two MSA-1-specific monoclonal antibodies previously shown to be reactive with the merozoite surface bound to a recombinant construct encoding the HVR, indicating that the HVR is surface exposed and accessible to antibody binding. Importantly, these surface-reactive, HVR-specific monoclonal antibodies were capable of inhibiting merozoite infectivity of the host erythrocyte in vivo. The results indicate that the MSA-1 HVR is involved in erythrocyte invasion and suggest that selection of MSA-1 variants may be driven by invasion-blocking antibodies.
Merozoite surface proteins of apicomplexan hemoparasites such as Plasmodium and Babesia spp. are involved in invasion of the host erythrocyte and provide potential targets for immune-mediated control (2-4, 6, 9, 18). Polyvalent antibodies against these surface proteins can block host cell invasion in vitro (12, 16). However, antigenic diversity of merozoite surface proteins and resulting immune evasion pose challenges to vaccine development (15, 19, 21).
Merozoite surface antigen 1 (MSA-1) of Babesia bovis, a causative agent of bovine babesiosis, is a member of an antigenically diverse group of proteins termed the variable merozoite surface antigen (VMSA) family. MSA-1 and the related MSA-2 proteins are exposed on the surfaces of both merozoites and sporozoites and induce invasion-blocking antibodies in immunized animals (8, 12, 16, 17). The region(s) of these proteins targeted by blocking antibodies is unknown. While MSA-1 and MSA-2 have strictly conserved amino- and carboxy-terminal regions encoding leader and glycosylphosphatidylinositol (GPI) anchor signals, respectively, the central, extramembranous region (8, 23), at least a portion of which is surface exposed, is variable (12, 14). There is enough MSA-1 sequence diversity between some strains of B. bovis to translate into a complete lack of immunologic cross-reactivity (11, 14, 15, 19, 21), and postinfection serum antibodies against vaccine breakthrough isolates do not cross-react with MSA-1 in the respective vaccine strain, suggesting immune selection for parasites that express allelic variants of MSA-1 (14).
The carboxy-terminal one-third of VMSA molecules contains the highest number of polymorphisms and in MSA-2 has been designated the hypervariable region (HVR) (1). The HVR is highly proline rich, a characteristic of other apicomplexan surface proteins (7, 22), and is surface exposed (1, 13). While proline-rich regions in other apicomplexan surface proteins are postulated to be involved in host cell binding, it is not known whether the HVR is involved in erythrocyte invasion or whether antibody directed toward the HVR will inhibit invasion, thereby potentially driving immune selection for VMSA variants. In this study, we extended previous analyses of VMSA sequence diversity (1, 14) to further characterize the MSA-1 HVR, and we tested the hypothesis that surface-reactive antibodies against the MSA-1 HVR will block merozoite invasion.
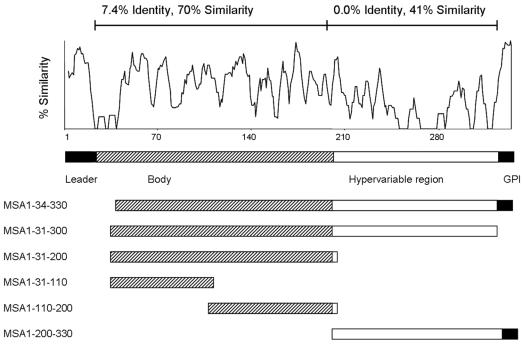
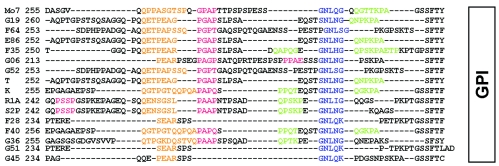
Comparative analysis of the MSA-1 sequence among strains from both geographically diverse regions and regions of endemicity demonstrated that the majority of strictly conserved amino acids are in the amino- and carboxy-terminal signal sequence regions (12, 14). Only single amino acids or small clusters of amino acids are conserved in all isolates throughout the rest of the molecule, and the last strictly conserved amino acid is at position 205 (14). To confirm that an HVR is a uniform feature of all merozoite surface antigens, previously reported sequences from 16 different B. bovis strains and isolates (14) (GenBank accession numbers AF275908 to AF275910 and DQ028735 to DQ028747) were assembled and analyzed with Vector NTI Suite 6 (InforMax) for regional sequence similarity and identity. Within the mature, processed proteins of 16 B. bovis strains and isolates, the level of sequence similarity for the amino-terminal two-thirds (amino acids [aa] 31 to 205) of the molecule was 70% (7.4% identity), while the level of sequence similarity for aa 205 to 300 was only 41%, with no strictly conserved residues (Fig. 1), consistent with an MSA-1 HVR in the carboxy-terminal one-third of the molecule. In addition, we analyzed all available MSA-1 sequences for the extent of their proline content to determine whether proline-rich motifs as identified in MSA-2 were consistently present in MSA-1. Within the MSA-1 HVR, 19.1% of amino acids are proline, compared to 2.9% of the amino acids in the rest of the molecule (data not shown). Further, the three degenerate proline-rich motifs in MSA-2 are present in the HVRs of all 16 strains and isolates examined (Fig. 2). The first motif has the consensus sequence QETPGPEAPQA and is present once. This consensus sequence is similar, especially at the nucleotide level, but not identical, to the consensus sequence of motif 1 of MSA-2 (QGTTGTQ-[PD]-SQD) (1). Motifs 2 and 3 are identical to those in MSA-2 and flank a semiconserved segment with a consensus sequence of GNLNG (Fig. 2). While the functional role of these motifs is unknown, proline-rich regions have been described in surface proteins of other apicomplexan hemoparasites that are involved in host cell binding and invasion, including the Duffy receptor proteins of Plasmodium vivax and Plasmodium knowlesi (7, 22) and the circumsporozoite proteins of several Plasmodium spp. (20). This led us to the hypothesis that the HVR is involved in erythrocyte binding and invasion.
FIG. 1.
Schematic diagrams of MSA-1 demonstrating overall sequence variation and showing constructs for expression of individual regions of the molecule. The top panel represents a similarity plot of all MSA-1 proteins examined (a total of 16 strains and isolates). A diagram of MSA-1 based on sequence variation and function and diagrams of individual MSA-1 constructs used in determining reactivities of antibodies to the hypervariable region are shown below.
FIG. 2.
The HVR of MSA-1 contains degenerate proline-rich motifs. The HVRs of 16 different B. bovis strains and isolates were manually aligned. Motif 1 is shown in orange letters, motif 2 in pink, and motif 3 in green. Motif 3 flanks a semiconserved GNLNG motif (blue). Residues in black are not assigned to any motif. Dashes indicate gaps.
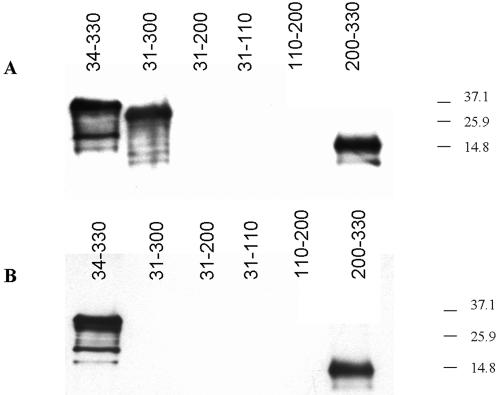
Two surface-reactive monoclonal antibodies (MAbs), Babb35A4, and 23/10.41, generated by immunization of mice with whole merozoites, have been described for MSA-1 (10). Neither the region of the molecule bound by these monoclonal antibodies nor whether they can inhibit invasion was known. To determine if these surface-reactive antibodies bind epitopes in the HVR of MSA-1, their reactivities with recombinant proteins corresponding to individual regions of MSA-1 were determined by Western blot analysis (Fig. 3). Based on the degree of variability and predicted structure of the mature processed protein (14), MSA-1 was subdivided into three regions representing the amino-terminal region (aa 31 to 110), the central hydrophilic region (aa 110 to 200), and the HVR (aa 200 to 330) (Fig. 1). Each of the three regions was amplified from genomic DNA by PCR using the primers described in Table 1. Amplicons were cloned in frame into the pTrcHis expression vector by using the pTrcHis TOPO TA cloning kit (Invitrogen). Following expression of recombinant protein according to the manufacturer's recommendations, bacteria were pelleted and frozen at −20°C overnight, and recombinant protein was purified on a Sepharose column. Fractionated proteins were further purified on a 4-to-20% gradient gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroeluted overnight, and quantified using a micro-bicinchoninic acid protein reagent kit (Pierce). The antigens were then tested for reactivity with both monoclonal antibodies by immunoblotting. As conformational controls, the amino-terminal two-thirds of MSA-1 from aa 31 to 200 and the entire predicted mature processed protein from aa 31 to 300 were also cloned, expressed, and tested for reactivity, along with a slightly truncated full-length construct containing aa 34 to 330. As shown in Fig. 3, monoclonal antibody 23/10.41 reacted with full-length recombinant MSA-1 (rMSA-1) (aa 34 to 330), the slightly truncated processed protein (aa 31 to 300), and the HVR (aa 200 to 330), while monoclonal antibody Babb35A4 reacted with full-length recombinant protein (aa 34 to 330) and the HVR (aa 200 to 330) but not with the slightly truncated processed protein (aa 31 to 300). Neither MAb demonstrated reactivity with the central or amino-terminal two-thirds of MSA-1, indicating that these two surface-reactive MAbs bind exclusively to the HVR and that the HVR of MSA-1 is surface exposed.
FIG. 3.
Surface-reactive monoclonal antibodies bind to the HVR. Recombinant proteins corresponding to the indicated regions of MSA-1 were probed with monoclonal antibody 23/10.41 (A) or Babb35A4 (B) in immunoblots.
TABLE 1.
Oligonucleotide primers used to amplify Mo7 msa-1 regions for expression
| Primer | Sequence | Region (aa) |
|---|---|---|
| Mo7-34-5′ | 5′-TCAATCGTCCTTCCCGAAG-3′ | 34-330 |
| Rev2 | 5′-AGCCACAGTCAATCCGCC-3′ | 34-330, 200-330 |
| Mo7-5′ | 5′-GCCGATACTTCAATCGTCCTTCC-3′ | 31-110, 31-300, 31-200 |
| Mo7-3′ | 5′-ATGTACCCTGTTGTCCTTGGAGG-3′ | 31-300 |
| Mo7-200-5′ | 5′-TACAAACTTGTTGAGTCTTTTGATG-3′ | 200-330 |
| Mo7-200-3′ | 5′-ATAGAAATCAGAGGCCGGGC-3′ | 110-200, 31-200 |
| Mo7-110-5′ | 5′-CGCCCTGATCTATTTAATGC-3′ | 110-200 |
| Mo7-110-3′ | 5′-AGGGCGAATCATAGGATTGTT-3′ | 31-110 |
Since Babb35A4 reacted with full-length rMSA-1 (aa 34 to 330) but not with the construct lacking the final 30 aa, while MAb 23/10.41 reacted with both full-length protein and the slightly truncated processed protein, the epitopes bound by these monoclonal antibodies are not identical. The Babb35A4 epitope must either be located between aa 300 to 330 or be affected by the loss of this region. It is interesting that the terminal 15 aa in the aa-300-to-330 stretch comprises the signal sequence for addition of the GPI moiety. Presumably, this signal sequence is cleaved and replaced by the GPI anchor in the mature protein, yet Babb35A4 clearly binds to live merozoites (10). Based on this, we suggest that the epitope recognized by Babb35A4 either is located between aa 300 and aa 315 or is within aa 200 to 315 of the HVR but requires a contribution from the aa-300-to-330 region or the GPI anchor for proper conformation. Both the aa-300-to-330 region and the GPI anchor are hydrophobic. Interestingly, a recent study of another babesial surface protein, Bd37 of Babesia divergens (5), demonstrated that immune protection generated by recombinant Bd37 was optimal only when hydrophobic sequences were included in the immunogen, suggesting that epitopes inducing protection are either encoded by the hydrophobic sequence or altered by its presence.
Hyperimmune serum produced against full-length rMSA-1 can inhibit invasion of erythrocytes by merozoites (12). The HVR of MSA-1 clearly induces surface-reactive antibodies. To determine if these antibodies can inhibit invasion, monoclonal antibodies Babb35A4 and 23/10.41 were used in in vitro invasion-blocking assays essentially as described previously (17, 23). In these experiments, 5 × 105 merozoites were incubated in triplicate with 100, 30, 10, 3, and 1 μg/ml of monoclonal antibodies in 96-well plates. A 100-μg/ml concentration of isotype-matched monoclonal antibody Ana22B1 against Anaplasma marginale major surface protein 1a was used as a negative control, and anti-rMSA-1 hyperimmune bovine serum from calf B681 (11) was used as a positive control for inhibition. An equal volume of 5% bovine erythrocytes in culture medium (100 μl) was added to each well, and the plates were incubated at 37°C in 5% O2 and 5% CO2 for 72 h. The percentage of parasitized erythrocytes was determined after 24 and 48 h by microscopic examination of 4,000 erythrocytes in Giemsa-stained smears prepared from each well. Statistical analysis software (NCSS 2000, Kaysville, UT) was used to analyze the data using analysis of variance and a two-sample t test for differences among all the treatment groups.
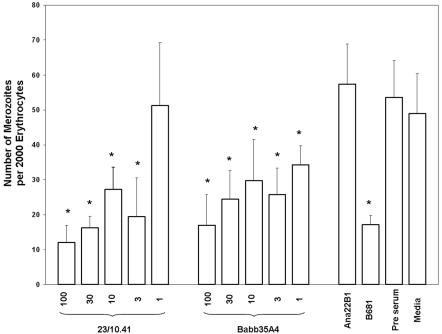
Both MSA-1 HVR-reactive monoclonal antibodies significantly inhibited merozoite infectivity (Fig. 4). Significant but decreasing percentages of inhibition occurred at monoclonal antibody concentrations of 100, 30, 10, and 3 μg/ml (P < 0.05) for both MAbs, while Babb35A4, but not 23/10.41, was able to inhibit infectivity at 1 μg/ml (P = 0.03). Reduction of infectivity ranged from 70 to 40% for Babb35A4 and from 79 to 52% for 23/10.41. Control hyperimmune bovine serum against rMSA-1 reduced infectivity by 68%. These results are similar to those of previous experiments using bovine serum against recombinant full-length MSA-1 (71%) (12) and slightly higher than the reductions in invasion obtained using bovine serum against recombinant MSA-2a (46 and 38%), MSA-2b (38 and 34%), or MSA-2c (26 and 25%) (16). While decreasing inhibition occurs when the antibody concentration is lowered, complete or near-complete blocking of merozoite infectivity could not be achieved, in agreement with the results of previous studies using hyperimmune serum (12). The reason for this is unclear, but it is unlikely to be due to expression of allelic variants of MSA-1, since msa-1 is a single-copy gene (23) and does not appear to vary over long periods of time in culture (T. McElwain, unpublished observation). Collectively, however, the data do suggest that extensive sequence variation in the hypervariable region may be a result of immune selection to avoid merozoite neutralization in vivo. How, when, and where this occurs is under investigation.
FIG. 4.
Monoclonal-antibody-mediated inhibition of merozoite infectivity. The total number of merozoites within 2,000 erythrocytes was determined at 48 h after incubation of merozoites with decreasing concentrations of monoclonal antibodies from 100 to 1 μg/ml, as indicated. M199 medium alone and 100 μg/ml of monoclonal antibody Ana22B1 against A. marginale MSP1a were used as negative controls. Bovine serum from hyperimmunized calf B681 was used as a positive control for inhibition. Error bars indicate standard deviations of the results from triplicate cultures. Data from each treatment group were compared using analysis of variance, and a two-sample t test was used for differences among all treatment groups. *, P < 0.05.
The ability of surface-reactive monoclonal antibodies against the HVR to inhibit merozoite invasion in vitro indicates that the HVR is important in the invasion process. Although sequence conservation in the HVR is extremely low among strains and isolates, predictive algorithms indicate a conserved structure despite these sequence differences (14). MSA-1 has been postulated to be involved in the initial, low affinity interaction with the erythrocyte membrane as a component of the surface coat. If this is so, structural conservation may be more important for the function of the HVR than a strictly conserved amino acid sequence. The semiconservation of proline-rich motifs despite extensive sequence polymorphisms also supports a conserved functional role for the HVR. Interestingly, structural conservation in the HVR occurs without conservation of epitopes bound by these monoclonal antibodies (19). Thus, the ability of the parasite to conserve function while escaping antibody-mediated immunity appears to be key to its survival.
Acknowledgments
The skillful technical assistance of Bev Hunter and Deb Alperin is acknowledged. Additionally, we thank Guy Palmer for helpful discussions and critical review of the manuscript.
This work was supported by NIH K08 award 1 K08 A151391 and USDA SCA58-5348-2-683.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Berens, S. J., K. A. Brayton, J. B. Molloy, R. E. Bock, A. E. Lew, and T. F. McElwain. 2005. Merozoite surface antigen 2 proteins of Babesia bovis vaccine breakthrough isolates contain a unique hypervariable region composed of degenerate repeats. Infect. Immun. 73:7180-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, W. C., and G. H. Palmer. 1999. Designing blood-stage vaccines against Babesia bovis and Babesia bigemina. Parasitol. Today 15:275-281. [DOI] [PubMed] [Google Scholar]
- 3.Brown, W. C., and A. C. Rice-Ficht. 1994. Use of helper T cells to identify potential vaccine antigens of Babesia bovis. Parasitol. Today 10:145-149. [DOI] [PubMed] [Google Scholar]
- 4.Chitnis, C. E., and M. J. Blackman. 2000. Host cell invasion by malaria parasites. Parasitol. Today 16:407-408. [DOI] [PubMed] [Google Scholar]
- 5.Delbecq, S., K. Hadj-Kaddour, S. Randazzo, J. Kleuskens, T. Schetters, A. Gorenflot, and E. Precigout. 2006. Hydrophobic moieties in recombinant proteins are crucial to generate efficient saponin-based vaccine against Apicomplexan Babesia divergens. Vaccine 24:613-621. [DOI] [PubMed] [Google Scholar]
- 6.Dubremetz, J. F., N. Garcia-Reguet, V. Conseil, and M. N. Fourmaux. 1998. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 28:1007-1013. [DOI] [PubMed] [Google Scholar]
- 7.Fang, X. D., D. C. Kaslow, J. H. Adams, and L. H. Miller. 1991. Cloning of the Plasmodium vivax Duffy receptor. Mol. Biochem. Parasitol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 8.Florin-Christensen, M., C. E. Suarez, S. A. Hines, G. H. Palmer, W. C. Brown, and T. F. McElwain. 2002. The Babesia bovis merozoite surface antigen 2 locus contains four tandemly arranged and expressed genes encoding immunologically distinct proteins. Infect. Immun. 70:3566-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaur, D., D. C. Mayer, and L. H. Miller. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413-1429. [DOI] [PubMed] [Google Scholar]
- 10.Hines, S. A., T. F. McElwain, G. M. Buening, and G. H. Palmer. 1989. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol. Biochem. Parasitol. 37:1-10. [DOI] [PubMed] [Google Scholar]
- 11.Hines, S. A., G. H. Palmer, D. P. Jasmer, W. L. Goff, and T. F. McElwain. 1995. Immunization of cattle with recombinant Babesia bovis merozoite surface antigen-1. Infect. Immun. 63:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 13.Jasmer, D. P., D. W. Reduker, S. A. Hines, L. E. Perryman, and T. C. McGuire. 1992. Surface epitope localization and gene structure of a Babesia bovis 44-kilodalton variable merozoite surface antigen. Mol. Biochem. Parasitol. 55:75-83. [DOI] [PubMed] [Google Scholar]
- 14.LeRoith, T., K. A. Brayton, J. B. Molloy, R. E. Bock, S. A. Hines, A. E. Lew, and T. F. McElwain. 2005. Babesia bovis merozoite surface antigen 1 (MSA-1) sequence variation and immunologic cross-reactivity among vaccine strains and vaccine breakthrough isolates. Infect. Immun. 73:5388-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madruga, C. R., C. E. Suarez, T. F. McElwain, and G. H. Palmer. 1996. Conservation of merozoite membrane and apical complex B cell epitopes among Babesia bigemina and Babesia bovis strains isolated in Brazil. Vet. Parasitol. 61:21-30. [DOI] [PubMed] [Google Scholar]
- 16.Mosqueda, J., T. F. McElwain, and G. H. Palmer. 2002. Babesia bovis merozoite surface antigen 2 proteins are expressed on the merozoite and sporozoite surface, and specific antibodies inhibit attachment and invasion of erythrocytes. Infect. Immun. 70:6448-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosqueda, J., T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect. Immun. 70:1599-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, G. H., T. F. McElwain, L. E. Perryman, W. C. Davis, D. R. Reduker, D. P. Jasmer, V. Shkap, E. Pipano, W. L. Goff, and T. C. McGuire. 1991. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect. Immun. 59:3340-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich, S. M., M. U. Ferreira, and F. J. Ayala. 2000. The origin of antigenic diversity in Plasmodium falciparum. Parasitol. Today 16:390-396. [DOI] [PubMed] [Google Scholar]
- 21.Shkap, V., E. Pipano, T. F. McElwain, U. Herzberg, Y. Krigel, L. Fish, and G. H. Palmer. 1994. Cross-protective immunity induced by Babesia bovis clones with antigenically unrelated variable merozoite surface antigens. Vet. Immunol. Immunopathol. 41:367-374. [DOI] [PubMed] [Google Scholar]
- 22.Singh, S. K., A. P. Singh, S. Pandey, S. S. Yazdani, C. E. Chitnis, and A. Sharma. 2003. Definition of structural elements in Plasmodium vivax and P. knowlesi Duffy-binding domains necessary for erythrocyte invasion. Biochem. J. 374:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez, C. E., M. Florin-Christensen, S. A. Hines, G. H. Palmer, W. C. Brown, and T. F. McElwain. 2000. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 68:6865-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]