Abstract
Gallinacins in poultry are functional equivalents of mammalian beta-defensins, which constitute an integral component of the innate immune system. Salmonella enterica serovar Enteritidis is a gram-negative bacterium that negatively affects both human and animal health. To analyze the association of genetic variations of the gallinacin genes with the phenotypic response to S. enterica serovar Enteritidis, an F1 population of chickens was created by crossing four outbred broiler sires to dams of two highly inbred lines. The F1 chicks were evaluated for bacterial colonization after pathogenic S. enterica serovar Enteritidis inoculation and for circulating antibody levels after inoculation with S. enterica serovar Enteritidis bacterin vaccine. Five candidate genes were studied, including gallinacins 2, 3, 4, 5, and 7. Gene fragments were sequenced from the founder individuals of the resource population, and a mean of 13.2 single-nucleotide polymorphisms (SNP) per kilobase was identified. One allele-defining SNP per gene was utilized to test for statistical associations of sire alleles with progeny response to S. enterica serovar Enteritidis. Among the five gallinacin genes evaluated, the Gal3 and Gal7 SNPs in broiler sires were found to be associated with antibody production after S. enterica serovar Enteritidis vaccination. Utilization of these SNPs as molecular markers for the response to S. enterica serovar Enteritidis may result in the enhancement of the immune response in poultry.
The beta-defensin family, termed gallinacins in poultry, plays a critical role in innate host defense. Gallinacins are cysteine-rich antimicrobial peptides characterized by six cysteine residues, which form three pairs of disulfide bridges (32). These are relatively small antimicrobial peptides, typically less than 100 amino acids in size, which possess a broad range of antimicrobial activity (32). In chickens, gallinacins 1 to 13 have been mapped within an 86-kb region of chromosome 3q3.5-q3.7 (40). Each gallinacin gene possesses the same genomic structure of four short exons that are separated by three introns of various lengths (40).
Gallinacins 1 to 13 are abundant in cells that are involved in the innate immune response against microbial infections (4, 40). These peptides exhibit a wide range of antimicrobial activity against both gram-positive and gram-negative bacteria (8). The tissue distribution of the defensin-like peptides in species ranging from chickens to mammals suggests that gene duplication was followed rapidly by diversification of function (4). Gal1 and Gal2 are expressed in the lung and bone marrow (41), whereas Gal3 is expressed in bone marrow, tongue, trachea, and bursa of Fabricius (41). Gal4-7 exhibit expression mainly in bone marrow cells and cells of the respiratory tract (40). In contrast, Gal8-13 are not expressed in bone marrow but are expressed preferentially in liver, kidney, testicle, ovary, and male and female reproductive tract tissues (40). The tissue-specific expression patterns of the gallinacin genes allows for grouping of the genes into two separate and distinct groups (Gal1-7 and Gal8-13). The expression of the gallinacin genes in tissues ranging from bone marrow to bursa of Fabricius to liver illustrates the important role of the gallinacin genes as a bridge between the innate and adaptive immune responses in chickens (41).
A major safety aspect of food animal production and processing is the avoidance of preharvest and postharvest pathogenic contamination of the food chain. Salmonella enterica serovar Enteritidis belongs to a genus of gram-negative, non-spore-forming, usually motile, facultative anaerobic bacilli of the family Enterobacteriaceae and is the most common cause of food poisoning cases in the United States (26).
Concerns have been raised over the possible emergence of antibiotic-resistant bacteria and possible consumption of antibiotic residues by humans due to the subtherapeutic use of antibiotics to control bacterial diseases such as S. enterica serovar Enteritidis (36). Selective breeding programs allow for allelic selection of genes that may confer an increased antimicrobial ability without the use of antibiotics (5, 38). Through the improvement of the chicken's innate immune system by molecular genetics, the dependence on antibiotics to control S. enterica serovar Enteritidis could decrease while continuing to provide greater protection against bacterial infections.
The candidate gene theory states that a significant proportion of the variation in any given population is comprised of major candidate genes associated with that trait, and it is possible to identify those genes (27). Research has been conducted on numerous candidate genes that affect the response to S. enterica serovar Enteritidis by the host cells, including the genes encoding major histocompatibility complex class I (MHC-I) and MHC-II, natural resistance-associated macrophage protein 1 (NRAMP1), tenascin C, transforming growth factor β2 (TGF-β2), TGF-β3, immunoglobulin L, inducible nitric oxide synthase, Toll-like receptor 4, and MD-2 (10, 16, 18, 20, 22, 24, 25). The gallinacin genes are eminently suitable for analysis as candidate genes based upon relevant tissue expression, genomic organization in the chicken genome, and their roles in the innate immune response.
The objective of this study was to identify and analyze new candidate genes for their association with the response to Salmonella in poultry. Specifically, gallinacins 2, 3, 4, 5, and 7 were selected for study, based upon gene size (relatively small intronic regions) and their roles in the host response to intracellular bacteria (40).
MATERIALS AND METHODS
Experimental animals.
The first filial (F1) generation of the Iowa Salmonella response resource population (ISRRP) of chickens was utilized. The F1 generation was produced in five hatches through the crossing of four males of an outbred broiler breeder male line (14) with dams from three highly inbred dam lines with inbreeding coefficients of 99%: one Fayoumi and two MHC-congenic Leghorn lines (G-B1 and G-B2) (42). By using genetically distant parental lines, the likelihood of detecting molecular genetic polymorphisms was increased. In addition, the highly inbred dams contributed the same allele to all offspring, thereby allowing analysis of the sire allele effects in the F1 generation.
Salmonella pathogenic challenge and quantification of bacterial load.
The F1 chicks (n = 194) from three hatches were inoculated intraesophageally (inoculation of 1 × 104 CFU/chick) at 1 day of age with pathogenic S. enterica serovar Enteritidis phage type 13a (14). Half of the birds were euthanized at 6 days of age, and the remaining birds were euthanized at 7 days of age. The S. enterica serovar Enteritidis culture and quantification procedures were previously described (13).
Salmonella vaccination and antibody measurement.
Chicks (n = 314) from two hatches were injected at 10 days of age with 0.2 ml of commercial bacterin S. enterica serovar Enteritidis vaccine (Biommune, Lenexa, KS) for antibody response evaluation at 21 days of age. Vaccination and enzyme-linked immunosorbent assay procedures used to quantify S. enterica serovar Enteritidis vaccine antibody levels were previously described (12, 15).
DNA isolation, PCR, and sequencing.
Genomic DNA was prepared from chicken erythrocytes by using a PUREGENE DNA purification kit (Gentra Systems, Minneapolis, MN). To characterize each gene, a pair of primers (Table 1) was developed using Primer3 (28), based on the published genomic DNA sequence found in the GenBank database.
TABLE 1.
Primer sequence and PCR-RFLP assay conditions for genotyping SNPs of Gal2-HpyCH4IV, Gal3-Aval, Gal4-AluI, Gal5-HinfI, and Gal7-MlyI
| Gene (GenBank accession no.) | Primer sequences (forward/reverse) | PCR product size (bp) | PCR digested sizes (bp) | Annealing Temp (°C)/time (s)a | Position (SNP)b | Restriction enzyme |
|---|---|---|---|---|---|---|
| Gal2 (AY621317) | 5′-GGCACAAAGGGTAAAGTATGG-3′ | 583 | 388 + 195 | 55.1/30 | 196 (T/C) | HpyCH4IV |
| 5′-GAGGGGTCTTCTTGCTGCTGA-3′ | ||||||
| Gal3 (AY621318) | 5′-GCACCACAAGAAGCCCAGGAA-3′ | 664 | 443 + 221 | 57.3/30 | 222 (T/C) | AvaI |
| 5′-AACTCCAGCCCTTACCACTCA-3′ | ||||||
| Gal4 (AY621319) | 5′-TGGGGATCTTAGAGGTCTTTT-3′ | 600 | 416 + 184 | 51.0/30 | 188 (A/G) | AluI |
| 5′-TTTTCCACAGATATTGCTTTT-3′ | ||||||
| Gal5 (AY621320) | 5′-CTCCCAGCAAGAAAGGAACCTG-3′ | 623 | 402 + 133 + 79 | 59.0/30 | 80 (C/A) | HinfI |
| 5′-CACAGTCCTGGGGTAATCCTCG-3′ | ||||||
| Gal7 (AY621322) | 5′-CTCAGTCGGGAGATAACCATTC-3′ | 785 | 605 + 180 | 56.1/30 | 606 (G/A) | MlyI |
| 5′-GGAGTGCCAGAGAAGCCATTTG-3′ |
PCR annealing temperature and time for primers.
SNP location within the PCR fragment.
PCRs were performed using 25-μl reaction mixture volumes that contained 25 ng of chicken genomic DNA, 0.8 μM of each primer, 200 μM of each deoxynucleoside triphosphate, 1 unit of Taq DNA polymerase (Promega Corporation, Madison, WI), 2.5 μl of 10× PCR buffer, and 1.5 mM MgCl2. The following cycling conditions were used: an initial denaturation step at 94°C for 3 min, followed by 39 cycles at 93°C for 45 s, at the optimum annealing temperature for 30 s (Table 1), and at 72°C for 1 min and a final extension step at 72°C for 10 min.
The PCR products were purified using ExoSAP-IT (Amersham Bioscience, Pittsburgh, PA) (35). An ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) was used for direct sequencing using nucleotide-specific dye terminators. PCR products were sequenced at the Iowa State University DNA Sequencing and Synthesis Facility.
Polymorphisms and restriction fragment length polymorphism (RFLP) assays.
One genomic DNA sample of broiler, Leghorn G-B1 and G-B2, and Fayoumi chickens was sequenced in both directions (total of eight sequences) for characterization of gene polymorphisms. Sequencher software (version 4.2; Gene Codes Corporation, Ann Arbor, MI) was used for sequence assembly and identification of polymorphisms. Sires that were heterozygous for the identified single-nucleotide polymorphisms (SNP) were utilized.
Restriction enzyme sites for each gene were identified by using NEBcutter version 2.0 (New England Biolabs, Ipswich, MA). The DNA was digested overnight at 37°C. The digested PCR-RFLP products were separated by electrophoresis through a 2.5% agarose gel. Ethidium bromide staining was utilized for DNA visualization.
Statistical analysis.
The association between the sire allele of the F1 chicks of the heterozygous sire families and the S. enterica serovar Enteritidis bacterial count was determined through a linear mixed model using the JMP program (29). Both the spleen and cecum bacterial count were transformed to their natural logarithms to achieve a normal distribution of the dependent variables. Model 1 was used to analyze the combined heterozygous sire families for each candidate gene:
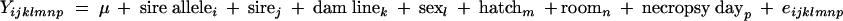 |
(1) |
where Yijklmnp is defined as the response variable from each individual F1 bird (natural logarithms of spleen or cecal bacterial count). Sire and dam line were taken as fixed effects, while hatch, room, and necropsy day were considered random effects. Sex, hatch, room, and necropsy day were all found to be not significant and were excluded from the final analysis.
A linear mixed model was used to estimate the association between the S. enterica serovar Enteritidis vaccine antibody level and the candidate gene genotype of the F1 chicks of the heterozygous sire families. Model 2 was used to analyze the combined heterozygous sire families:
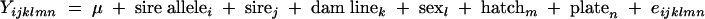 |
(2) |
where Yijklmn is defined as response variables from each F1 bird, 1 − S/N for antibody level, where S is the optical density at 630 nm (OD630) of the sample and N is the triplicate means of the OD630s from the negative controls. Plate effect, which varied among enzyme-linked immunosorbent assays, was considered a random effect, along with hatch. Sex and hatch were found to be not significant and were excluded from the final analysis.
RESULTS
SNP detection and rate.
In total, 3.25 kb was sequenced in two directions from each of four individual outbred sires and two representative inbred females. A total of 43 SNPs were identified within the sequenced regions. This equates to an SNP rate of 13.2 SNPs/kb, much higher than the previously reported 5 SNPs/kb across the entire chicken genome (39). All SNPs that were selected for analysis were intronic, except for Gal5, which has a nonsynonymous SNP resulting in an amino acid change of proline to threonine. Intronic SNPs, while not the causal mutations, can provide excellent markers for genetic selection for an increased immune response to S. enterica serovar Enteritidis.
Sequence variation and PCR-RFLP analysis.
SNPs were identified within gallinacins 2 to 5 and 7. Restriction enzyme-gene combinations of Gal2-HpyCh4IV, Gal3-AvaI, Gal4-AluI, Gal5-HinfI, and Gal7-MlyI (Table 1) were used in PCR-RFLP tests to monitor the inheritance of heterozygous sire alleles in the F1 population. Sire 8338 was not heterozygous for any of the SNPs analyzed.
An SNP in the gallinacin 2 gene was found to be within an intronic region and consisted of a T-to-C substitution at position 196 of the 583-bp PCR product (GenBank accession no. AY621317). Only one sire (sire 8170) was heterozygous at this position. PCR-RFLP digestion of the DNA of F1 offspring of this sire with the enzyme HpyCh4IV resulted in a fragment of either 583 bp for the allele lacking the HpyCh4IV restriction site or 388 and 195 bp for the allele with the restriction site.
For gallinacin 3, a 664-bp product (GenBank accession no. AY621318) that contained an intronic T-to-C substitution at position 222 was amplified. Two sires (sires 8170 and 8291) were heterozygous, and cutting with the restriction enzyme AvaI produced fragment sizes of 443 and 221 bp.
For gallinacin 4, a 600-bp product (GenBank accession no. AY621319) had an A-to-G substitution SNP within an intron. One broiler sire (sire 8296) was heterozygous at this site. The AluI restriction enzyme produced fragment sizes of 416 and 184 bp.
Primers designed from gallinacin 5 genomic DNA (GenBank accession no. AY621320) amplified a 623-bp fragment of the gene (Table 1) with an SNP of C to A within the exonic sequence. The nonsynonymous SNP produced an amino acid change from proline to threonine. Two broiler sires (sires 8291 and 8296) were identified as heterozygous for the SNP. The PCR-RFLP of F1 offspring produced products of 402, 133, and 79 bp from digestion with HinfI.
A 785-bp product amplified from gallinacin 7 (Table 1) genomic DNA (GenBank accession no. AY621322), had an A-to-G SNP at position 606. Two sires (sires 8170 and 8291) were heterozygous for the SNP by digestion with the MlyI restriction enzyme.
Association of the gallinacin genes with the S. enterica serovar Enteritidis response.
In the analysis utilizing models 1 and 2 for association with the response to S. enterica serovar Enteritidis, the following variables were not significant: sex, hatch, room, necropsy day, and dam line. Nonsignificant variables were excluded from the models for the final analysis.
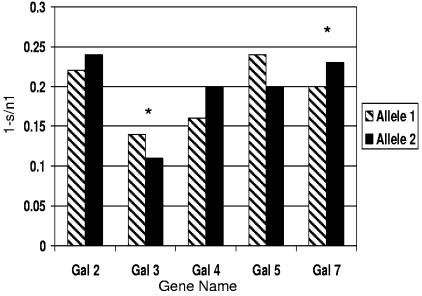
The F1 progeny of sires that were heterozygous for each gallinacin gene were genotyped for the corresponding SNP. The results for the association analysis of gallinacin genes and S. enterica serovar Enteritidis response for the F1 progeny are summarized in Table 2. Gallinacin 2 had only a moderate association (P < 0.10) between the Gal2-HpyCh4IV sire allele and progeny cecum bacterial load (Table 2). There was no association between the SNP and either spleen bacterial load or S. enterica serovar Enteritidis vaccine antibody response. The Gal3-AvaI sire allele was associated (P < 0.03) with S. enterica serovar Enteritidis vaccine antibody response in the F1 progeny (Fig. 1). Gallinacin 5 showed a moderate association (P < 0.11) between the Gal5-HinfI SNP and the antibody response to a commercial S. enterica serovar Enteritidis vaccine. Although the gallinacin 7 SNP did not show an association with bacterial load in either the spleen or cecum, the Gal7-MlyI sire allele was significantly associated with the antibody response to S. enterica serovar Enteritidis vaccine (P < 0.02) (Fig. 1). The SNPs analyzed accounted for an estimated 4.1% of the phenotypic variation in vaccine antibody response. Taken together, this is the first report, to our knowledge, of associations between the defensin genes gallinacin 3 and 7 and the response to S. enterica serovar Enteritidis in chickens.
TABLE 2.
Associations between sire Gal2, Gal3, Gal4, Gal5, and Gal7 gene polymorphisms and progeny Salmonella serovar Enteritidis response
| Gene |
P value (no. of F1 progeny)a
|
||
|---|---|---|---|
| Postchallenge bacterial load
|
Vaccine antibody response | ||
| Cecum | Spleen | ||
| Gal2 | 0.10 (65) | 0.26 (65) | 0.66 (74) |
| Gal3 | 0.57 (116) | 0.12 (114) | 0.03 (84) |
| Gal4 | 0.24 (63) | 0.45 (63) | 0.79 (26) |
| Gal5 | 0.45 (126) | 0.13 (127) | 0.11 (68) |
| Gal7 | 0.72 (90) | 0.19 (88) | 0.02 (79) |
No. of F1 progeny, number of phenotype F1 progeny from sires heterozygous for SNPs evaluated for this gene.
FIG. 1.
Vaccine antibody level by sire gallinacin allele. Bars indicate gallinacin genes 2 to 5 and 7. 1−s/n1 represents the vaccine response to S. enterica serovar Enteritidis challenge measured where s is the OD630 of the sample and n1 is the triplicate means of the OD630s of the negative controls. Allele 1 represents the allele required for restriction endonuclease digestion. * indicates differences in vaccine antibody levels at a P value of ≤0.05. Gal3 has a higher antibody response with allele 1, while Gal7 produces a higher response with allele 2.
DISCUSSION
With rising concerns over the biosafety of food products, the ability to modulate the immune response of food animals through genetic selection has become an essential tool (17). Through the use of genetic approaches to enhance the innate immune system, it may be possible to reduce S. enterica serovar Enteritidis infection of poultry, thereby increasing vaccine efficiency and reducing the dependence on antibiotics.
The five candidate genes selected for investigation in this study encode antimicrobial peptides that may play an integral role in the innate immune response to gram-negative bacteria. Because of the lack of a superoxide ion and myleoperoxidase in avian heterophils, birds rely more upon nonoxidative defense molecules that include lysozymes, cationic proteins, and peptides such as gallinacins (7). Antimicrobial action is initiated, in principle, by the binding of the peptide to the bacterial membrane through electrostatic interactions (32). Upon release, antimicrobial peptides such as gallinacins permeate the membrane of bacteria, coinciding with the inhibition of RNA, DNA, and protein synthesis (4). Along with their integral role in innate immunity, gallinacins 2 to 5 and 7 were of particular interest for analysis because of their physical proximity in the genome. The gallinacin genes are clustered within an 86-kb distance on the 3q3.5-q3.7 chromosome (40). The location of molecular markers within this cluster could be useful for marker-assisted genetic selection and positional cloning work. The heritability of the S. enterica serovar Enteritidis spleen carrier state was estimated to be 0.10 to 0.32 (6), and the heritability in the cecum was 0.06 to 0.20 (2), indicating that there is a genetic basis to the carrier phenotype.
Previous studies of the same resource population used in the current study showed associations of spleen bacterial burden with the genes encoding MD-2, caspase-1, NRAMP1, inhibitor of apoptosis protein 1, MHC-I, and prosaposin (18, 21, 24). Significant associations with bacterial load in the chicken cecum were identified for genes encoding tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TGF-β4, caspase-1, and CD-28 (21, 24, 25). Other studies have previously reported associations of genes encoding caspase-1, CD-28, IgL, and TRAIL and the antibody response to a commercially available S. enterica serovar Enteritidis vaccine (21, 24, 25). Kramer et al. (16) previously showed that the associations of these genes with the antibody response to S. enterica serovar Enteritidis were present in unrelated populations of meat-type chickens (outbred broilers and Dutch Landrace lines); thus, the gene trait associations that were identified in the F1 populations were robust in various genetic backgrounds, so the identified SNPs are able to be widely used for marker-assisted selection.
Leveque et al. (19) conducted a linkage study between a previously identified candidate gene (gene encoding Toll-like receptor 4) and susceptibility to Salmonella enterica serovar Typhimurium in chicken. Associations identified in the current F1 cross utilized in this study supported evidence presented previously by Leveque et al. that explained some of the phenotypic variances in Salmonella resistance (19). Tilquin et al. (33) used a genome scan for quantitative trait loci (QTL) affecting the carrier state of birds for Salmonella serovar Typhimurium and Salmonella serovar Enteritidis and identified two genomewise QTL on chromosome 1 and chromosome 2 along with four other possible QTL. One of the identified QTL (chromosome 1) overlapped regions of the genome containing a candidate gene (IAP1) that was previously identified using the F1 cross for associations with S. enterica serovar Enteritidis bacterial burden. Cumulatively, these studies support the observations made previously by Wigley et al. (37) regarding the existence of Salmonella-resistant and -susceptible lines with dominant genetic factors that are not linked to sex. These studies also reinforce the ability to identify candidate genes in the current F1 population.
Most of the genes analyzed through candidate gene analysis were responsible for a small amount of the phenotypic variation, typically 4 to 8% (16). This is not unexpected, because Salmonella resistance is known to be a polygenic trait. Only SNPs that are heterozygous in the outbred sires and homozygous in the inbred dams were able to be utilized for analysis in the F1 generation of the ISRRP population. For each gene analyzed, therefore, the number of heterozygous sires and genotyped offspring varied, which may be one reason why the gene SNP-trait associations are not identical among all genes in the gallinacin cluster, even though the genes are in close proximity. Interestingly, the SNPs in gallinacins 3 and 7 were not in complete linkage disequilibrium with each other, yet each gene's SNP, when separately tested, was significantly associated with vaccine antibody production.
The gallinacin genes have been identified as important members of the innate immune response of poultry to bacterial infections (23, 32, 41). Gallinacins react to the components of the bacterial outer membrane, such as lipopolysaccharides from gram-negative bacteria (11, 30). The reaction to membrane components induces the production of proinflammatory cytokines such as tumor necrosis factor alpha, interleukin-1β, and interleukin-6. The expression level of Gal3 in the trachea increases significantly upon infection with Haemophilus paragallinarum (41).
In the current study, chicks were administered S. enterica serovar Enteritidis intraesophageally to model the natural route of exposure through the gastrointestinal tract. In our study, moderate associations between Gal2 and Gal5 genes (P values of <0.10 and <0.13, respectively) and S. enterica serovar Enteritidis bacterial load in either the spleen or the cecum were detected, whereas Gal3 and Gal7 showed significant associations (P values of <0.03 and <0.02, respectively) with vaccine antibody response to S. enterica serovar Enteritidis bacterin (Fig. 1).
The observation of a significant association between S. enterica serovar Enteritidis vaccine antibody and the gallinacin genes, while there was only moderate association between the genes and S. enterica serovar Enteritidis bacterial burden, closely follows what is known about immune protection by beta-defensins. Bar-Shira et al. previously hypothesized that innate effector mechanisms such as defensins enable immune protection during the first week after hatching until functional maturation of the adaptive immune system occurs (1). They showed that mRNA levels of Gal1 and Gal2 decreased relative to the day of hatching throughout the first week of life and then increased again during the second week. The samples taken from the ISRRP birds were collected at 7 and 8 days of age, thereby falling within the low expression range of the gallinacin genes.
Although only a moderate association of gallinacin gene polymorphisms with the bacterial load of the challenged birds was observed at 1 week postchallenge, gallinacins could be responsible for recognizing the bacteria in the initial stages of infection and inducing other cells of the immune system, such as dendritic cells, to mature and respond to the infection at later ages (1, 4). Defensins may also increase resistance by enhancing the recruitment of macrophages, granulocytes, and lymphocytes to the infected tissues (1, 34). The gallinacins that are expressed in the tongue, trachea, bone marrow, and bursa of Fabricius (40) may activate the immune response to combat bacterial infection of the bird. An antibody response would be essential, as it has previously been shown that S. enterica serovar Enteritidis effectively avoids or suppresses the activation of T cells (31). Based upon the antibody response to an S. enterica serovar Enteritidis vaccine, it appears that the gallinacin genes help in the transition from an innate immune response to an adaptive response in the newly hatched birds. The increased antibody levels suggest that gallinacin genes may be more beneficial to birds exposed to S. enterica serovar Enteritidis later in life.
The F1 resource population of the present study was created by crossing highly inbred dams from diverse breeds to commercially outbred sires. One advantage of this cross was the consistent contribution of identical alleles by each inbred dam line to their offspring. The cross therefore made it possible to estimate the effect of only the sire SNP allele without any ambiguity of the inherited allele. However, with the generation of this F1 resource population from a cross between divergent breeds, substantial linkage disequilibrium exists. Therefore, it is not possible to determine if the SNPs identified in the current study are causal mutations or are in linkage with a causal mutation elsewhere in the gallinacin gene cluster. In 2004, the chicken genome was sequenced and annotated (9), and along with it, an SNP map consisting of 2.8 million SNPs was created (39). This makes it possible to utilize the locations of known SNPs to fine-map a region of interest in the chicken genome. To capitalize on advancements in genome information available for poultry, an advanced intercross population has been produced from the same populations examined in the present study. In an F8 advanced intercross line, linkage disequilibrium blocks should be reduced by three- to fivefold compared to the F1 generation (3). This facilitates the fine-mapping of regions that were previously identified by SNPs that showed an association with the response to S. enterica serovar Enteritidis challenge in the F1 generation.
In summary, a new chromosomal region with effects on the response to S. enterica serovar Enteritidis in chickens was characterized in this study. Within this region, the SNPs in the gallinacin candidate genes could potentially be used in a marker-assisted selection program to enhance the response to Salmonella. Analysis of the gallinacin genes in the protective pathways of disease resistance has also opened the possibilities for therapeutic strategies using endogenous antimicrobial peptides.
Acknowledgments
This work was supported by Animal Health and State of Iowa funds; National Research Initiative grant no. 2004-35205-14234 from the USDA Cooperative State Research, Education, and Extension Service; and research grant IS-3021-98CR from BARD, the Binational Agriculture Research and Development fund.
We gratefully acknowledge the Poultry Research Center crew at Iowa State University for managing the birds and Michael Kaiser and Bill Larson for technical support.
Editor: F. C. Fang
REFERENCES
- 1.Bar-Shira, E., and A. Friedman. 9 January 2006, posting date. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. [Online.] doi: 10.1016/j.dci.2005.12.002. [DOI] [PubMed]
- 2.Berthelot, F., C. Beaumont, F. Mompart, O. Girard-Santosuosso, P. Pardon, and M. Duchet-Suchaux. 1998. Estimated heritability of the resistance to cecal carrier state of Salmonella enteritidis in chickens. Poult. Sci. 77:797-801. [DOI] [PubMed] [Google Scholar]
- 3.Darvasi, A., and M. Soller. 1995. Advanced intercross lines, an experimental population for fine genetic mapping. Genetics 141:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 5.Georges, M. 2001. Recent progress in livestock genomics and potential impact on breeding programs. Theriogenology 55:15-21. [DOI] [PubMed] [Google Scholar]
- 6.Girard-Santosuosso, O., P. Menanteau, M. Duchet-Suchaux, F. Berthelot, F. Mompart, J. Protais, P. Colin, J. F. Guillot, C. Beaumont, and F. Lantier. 1998. Variability in the resistance of four chicken lines to experimental intravenous infection with Salmonella enteritidis phage type 4. Avian Dis. 42:462-469. [PubMed] [Google Scholar]
- 7.Harmon, B. G. 1998. Avian heterophils in inflammation and disease resistance. Poult. Sci. 77:972-977. [DOI] [PubMed] [Google Scholar]
- 8.Higgs, R., D. J. Lynn, S. Gaines, J. McMahon, J. Tierney, T. James, A. T. Lloyd, G. Mulcahy, and C. O'Farrelly. 2005. The synthetic form of a novel chicken beta-defensin identified in silico is predominantly active against intestinal pathogens. Immunogenetics 57:90-98. [DOI] [PubMed] [Google Scholar]
- 9.Hillier, L. W., W. Miller, E. Birney, W. Warren, R. C. Hardison, C. P. Ponting, P. Bork, D. W. Burt, M. A. Groenen, M. E. Delany, J. B. Dodgson, A. T. Chinwalla, P. F. Cliften, S. W. Clifton, K. D. Delehaunty, C. Fronick, R. S. Fulton, T. A. Graves, C. Kremitzki, D. Layman, V. Magrini, J. D. McPherson, T. L. Miner, P. Minx, W. E. Nash, M. N. Nhan, J. O. Nelson, L. G. Oddy, C. S. Pohl, J. Randall-Maher, S. M. Smith, J. W. Wallis, S. P. Yang, M. N. Romanov, C. M. Rondelli, B. Paton, J. Smith, D. Morrice, L. Daniels, H. G. Tempest, L. Robertson, J. S. Masabanda, D. K. Griffin, A. Vignal, V. Fillon, L. Jacobbson, S. Kerje, L. Andersson, R. P. Crooijmans, J. Aerts, J. J. van der Poel, H. Ellegren, R. B. Caldwell, S. J. Hubbard, D. V. Grafham, A. M. Kierzek, S. R. McLaren, I. M. Overton, H. Arakawa, K. J. Beattie, Y. Bezzubov, P. E. Boardman, J. K. Bonfield, M. D. Croning, R. M. Davies, M. D. Francis, S. J. Humphray, C. E. Scott, R. G. Taylor, C. Tickle, W. R. Brown, J. Rogers, J. M. Buerstedde, S. A. Wilson, L. Stubbs, I. Ovcharenko, L. Gordon, S. Lucas, M. M. Miller, H. Inoko, T. Shiina, J. Kaufman, J. Salomonsen, K. Skjoedt, G. K. Wong, J. Wang, B. Liu, J. Yu, H. Yang, M. Nefedov, M. Koriabine, P. J. Dejong, L. Goodstadt, C. Webber, N. J. Dickens, I. Letunic, M. Suyama, D. Torrents, C. von Mering, E. M. Zdobnov, et al. 2004. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432:695-716. [DOI] [PubMed] [Google Scholar]
- 10.Hu, J., N. Bumstead, P. Barrow, G. Sebastiani, L. Olien, K. Morgan, and D. Malo. 1997. Resistance to salmonellosis in the chicken is linked to NRAMP1 and TNC. Genome Res. 7:693-704. [DOI] [PubMed] [Google Scholar]
- 11.Kagan, B. L., M. E. Selsted, T. Ganz, and R. I. Lehrer. 1990. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 87:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser, M. G., N. Lakshmanan, T. Wing, and S. J. Lamont. 2002. Salmonella enterica serovar Enteritidis burden in broiler breeder chicks genetically associated with vaccine antibody response. Avian Dis. 46:25-31. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser, M. G., and S. J. Lamont. 2001. Genetic line differences in survival and pathogen load in young layer chicks after Salmonella enterica serovar Enteritidis exposure. Poult. Sci. 80:1105-1108. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser, M. G., and S. J. Lamont. 2002. Microsatellites linked to Salmonella enterica serovar Enteritidis burden in spleen and cecal content of young F1 broiler-cross chicks. Poult. Sci. 81:657-663. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser, M. G., T. Wing, and S. J. Lamont. 1998. Effect of genetics, vaccine dosage, and postvaccination sampling interval on early antibody response to Salmonella enteritidis vaccine in broiler breeder chicks. Poult. Sci. 77:271-275. [DOI] [PubMed] [Google Scholar]
- 16.Kramer, J., M. Malek, and S. J. Lamont. 2003. Association of twelve candidate gene polymorphisms and response to challenge with Salmonella enteritidis in poultry. Anim. Genet. 34:339-348. [DOI] [PubMed] [Google Scholar]
- 17.Lamont, S. J. 1998. Impact of genetics on disease resistance. Poult. Sci. 77:1111-1118. [DOI] [PubMed] [Google Scholar]
- 18.Lamont, S. J., M. G. Kaiser, and W. Liu. 2002. Candidate genes for resistance to Salmonella enteritidis colonization in chickens as detected in a novel genetic cross. Vet. Immunol. Immunopathol. 87:423-428. [DOI] [PubMed] [Google Scholar]
- 19.Leveque, G., V. Forgetta, S. Morroll, A. L. Smith, N. Bumstead, P. Barrow, J. C. Loredo-Osti, K. Morgan, and D. Malo. 2003. Allelic variation in TLR4 is linked to susceptibility to Salmonella enterica serovar Typhimurium infection in chickens. Infect. Immun. 71:1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, W., M. G. Kaiser, and S. J. Lamont. 2003. Natural resistance-associated macrophage protein 1 gene polymorphisms and response to vaccine against or challenge with Salmonella enteritidis in young chicks. Poult. Sci. 82:259-266. [DOI] [PubMed] [Google Scholar]
- 21.Liu, W., and S. J. Lamont. 2003. Candidate gene approach: potentional association of caspase-1, inhibitor of apoptosis protein-1, and prosaposin gene polymorphisms with response to Salmonella enteritidis challenge or vaccination in young chicks. Anim. Biotechnol. 14:61-76. [DOI] [PubMed] [Google Scholar]
- 22.Liu, W., M. M. Miller, and S. J. Lamont. 2002. Association of MHC class I and class II gene polymorphisms with vaccine or challenge response to Salmonella enteritidis in young chicks. Immunogenetics 54:582-590. [DOI] [PubMed] [Google Scholar]
- 23.Lynn, D. J., R. Higgs, S. Gaines, J. Tierney, T. James, A. T. Lloyd, M. A. Fares, G. Mulcahy, and C. O'Farrelly. 2004. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics 56:170-177. [DOI] [PubMed] [Google Scholar]
- 24.Malek, M., J. R. Hasenstein, and S. J. Lamont. 2004. Analysis of chicken TLR4, CD28, MIF, MD-2, and LITAF genes in a Salmonella enteritidis resource population. Poult. Sci. 83:544-549. [DOI] [PubMed] [Google Scholar]
- 25.Malek, M., and S. J. Lamont. 2003. Association of INOS, TRAIL, TGF-beta2, TGF-beta3, and IgL genes with response to Salmonella enteritidis in poultry. Genet. Sel. Evol. 35(Suppl. 1):S99-S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigue, D. C., R. V. Tauxe, and B. Rowe. 1990. International increase in Salmonella enteritidis: a new pandemic? Epidemiol. Infect 105:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothschild, M. F., and M. Soller. 1997. Candidate gene analysis to detect genes controlling traits of economic importance in domestic livestock. Probe 8:13-20. [Google Scholar]
- 28.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 29.Sall, J., and A. Lehman. 2000. JMP start statistics: a guide to statistical and data analysis using JMP and JMP IN software. Duxbury Press, Belmont, Calif.
- 30.Satchell, D. P., T. Sheynis, Y. Shirafuji, S. Kolusheva, A. J. Ouellette, and R. Jelinek. 2003. Interactions of mouse Paneth cell alpha-defensins and alpha-defensin precursors with membranes. Prosegment inhibition of peptide association with biomimetic membranes. J. Biol. Chem. 278:13838-13846. [DOI] [PubMed] [Google Scholar]
- 31.Sheela, R. R., U. Babu, J. Mu, S. Elankumaran, D. A. Bautista, R. B. Raybourne, R. A. Heckert, and W. Song. 2003. Immune responses against Salmonella enterica serovar Enteritidis infection in virally immunosuppressed chickens. Clin. Diagn. Lab. Immunol. 10:670-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiarto, H., and P. L. Yu. 2004. Avian antimicrobial peptides: the defense role of beta-defensins. Biochem. Biophys. Res. Commun. 323:721-727. [DOI] [PubMed] [Google Scholar]
- 33.Tilquin, P., P. A. Barrow, J. Marly, F. Pitel, F. Plisson-Petit, P. Velge, A. Vignal, P. V. Baret, N. Bumstead, and C. Beaumont. 2005. A genome scan for quantitative trait loci affecting the Salmonella carrier-state in the chicken. Genet. Sel. Evol. 37:539-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welling, M. M., P. S. Hiemstra, M. T. van den Barselaar, A. Paulusma-Annema, P. H. Nibbering, E. K. Pauwels, and W. Calame. 1998. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J. Clin. Investig. 102:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werle, E., C. Schneider, M. Renner, M. Volker, and W. Fiehn. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 22:4354-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, D. G., S. Zhao, R. Sudler, S. Ayers, S. Friedman, S. Chen, P. F. McDermott, S. McDermott, D. D. Wagner, and J. Meng. 2001. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl. J. Med. 345:1147-1154. [DOI] [PubMed] [Google Scholar]
- 37.Wigley, P., S. D. Hulme, N. Bumstead, and P. A. Barrow. 2002. In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes Infect. 4:1111-1120. [DOI] [PubMed] [Google Scholar]
- 38.Windon, R. G. 1990. Selective breeding for the control of nematodiasis in sheep. Rev. Sci. Tech. 9:555-576. [DOI] [PubMed] [Google Scholar]
- 39.Wong, G., B. Liu, J. Wang, Y. Zhang, X. Yang, Z. Zhang, Q. Meng, J. Zhou, D. Li, J. Zhang, P. Ni, S. Li, L. Ran, H. Li, R. Li, H. Zheng, W. Lin, G. Li, X. Wang, W. Zhao, J. Li, C. Ye, M. Dai, J. Ruan, Y. Zhou, Y. Li, X. He, X. Huang, W. Tong, J. Chen, J. Ye, C. Chen, N. Wei, L. Dong, F. Lan, Y. Sun, Z. Yang, Y. Yu, Y. Huang, D. He, Y. Xi, D. Wei, Q. Qi, W. Li, J. Shi, M. Wang, F. Xie, X. Zhang, P. Wang, Y. Zhao, N. Li, N. Yang, W. Dong, S. Hu, C. Zeng, W. Zheng, B. Hao, L. W. Hillier, S. P. Yang, W. C. Warren, R. K. Wilson, M. Brandstrom, H. Ellegren, R. P. Crooijmans, J. J. van der Poel, H. Bovenhuis, M. A. Groenen, I. Ovcharenko, L. Gordon, L. Stubbs, S. Lucas, T. Glavina, A. Aerts, P. Kaiser, L. Rothwell, J. R. Young, S. Rogers, B. A. Walker, A. van Hateren, J. Kaufman, N. Bumstead, S. J. Lamont, H. Zhou, P. M. Hocking, D. Morrice, D. J. de Koning, A. Law, N. Bartley, D. W. Burt, H. Hunt, H.H.Cheng, U. Gunnarsson, P. Wahlberg, L. Andersson, K. Institutet, E. Kindlund, M. T. Tammi, B. Andersson, C. Webber, C. P. Ponting, et al. 2004. A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature 432:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao, Y., A. L. Hughes, J. Ando, Y. Matsuda, J. F. Cheng, D. Skinner-Noble, and G. Zhang. 2004. A genome-wide screen identifies a single beta-defensin gene cluster in the chicken: implications for the origin and evolution of mammalian defensins. BMC Genomics 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, C., T. Nguyen, L. Liu, R. E. Sacco, K. A. Brogden, and R. I. Lehrer. 2001. Gallinacin-3, an inducible epithelial beta-defensin in the chicken. Infect. Immun. 69:2684-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, H., and S. J. Lamont. 1999. Genetic characterization of biodiversity in highly inbred chicken lines by microsatellite markers. Anim. Genet. 30:256-264. [DOI] [PubMed] [Google Scholar]