Abstract
The cell cycle G2/M specific inhibitor cytolethal distending toxin (CDT) from Actinobacillus actinomycetemcomitans is composed of CdtA, CdtB, and CdtC coded on the cdtA, cdtB, and cdtC genes that are tandem on the chromosomal cdt locus. A. actinomycetemcomitans CdtA has the lipid binding consensus domain, the so-called “lipobox”, at the N-terminal signal sequence. Using Escherichia coli carrying plasmid pTK3022, we show that the 16th residue, cysteine, of CdtA bound [3H]palmitate or [3H]glycerol. Further, posttranslational processing of the signal peptide, CdtA, was inhibited using globomycin, an inhibitor of lipoprotein-specific signal peptidase II. Fractionation and immunoblotting show the lipid-modified CdtA is present in the outer membrane. Immunoprecipitation and the pull-down assay of the CDT complex from E. coli carrying a plasmid containing cdtABC demonstrated that the CDT complex in the periplasm is composed of CdtA, CdtB, and CdtC and that the CDT complex in culture supernatant is an N-terminally truncated (36 to 43 amino acids) form of CdtA (CdtA′), CdtB, and CdtC. This suggests that CDT is present as a complex both in the periplasm and the supernatant where CdtA undergoes posttranslation processing to CdtA′ in the process of biogenesis and secretion of CDT holotoxin into the culture supernatant. Site-directed mutagenesis of the 16th cysteine residue to glycine in CdtA altered localization of CdtA in the cell and reduced the amount of CDT activity in the culture supernatant. This suggests that CDT forms a complex inside the periplasm for lipid modification where posttranslational processing of CdtA plays an important role for the efficient production of CDT holotoxin into the culture supernatant.
Cytolethal distending toxin (CDT) is a cell cycle G2/M-specific inhibitor produced by several pathogenic bacteria including Campylobacter spp. (16, 38), Escherichia coli (17, 27, 28, 32), Shigella dysenteriae (25), Haemophilus ducreyi (6, 9), Helicobacter hepaticus (4, 40), Salmonella enterica serovar Typhi (13), and Actinobacillus actinomycetemcomitans (20, 36, 37). CDT-poisoned eukaryotic cells show cell cycle arrest and subsequent cellular distension followed by cell death (16, 17, 23, 27, 29). Except for S. enterica serovar Typhi, CDT is coded on the cdtA, cdtB, and cdtC genes that are located in tandem at the chromosomal cdt locus; and the expression of the three components CdtA, CdtB, and CdtC is necessary for full toxicity (2, 19, 30, 35). CDT is suggested to be a unique tripartite AB toxin in which CdtB is the active A subunit, and CdtA and CdtC constitute the heterodimeric B subunit. Recently, the crystal structure of H. ducreyi CDT confirms this structure (21). The structure CDT holotoxin consists of CdtA, CdtB, and CdtC forming a ternary complex in a 1:1:1 stoichiometry. CdtA and CdtC form ricin-like lectin domains that may play a role in recognizing the cellular receptor that delivers the CdtB subunit into the target cells. CdtB shares conserved residues with the active sites of phosphodiesterase, DNase I and sphingomyelinase, and is structurally similar to the DNase I fold. Despite the absence of direct evidence that CdtB acts as DNase on chromosomal DNA in vivo, accumulating circumstantial evidence indicates that CdtB induces DNA damage inside the target nucleus (11, 18). DNA damage by CdtB may activate the checkpoint control, phosphatidylinositol 3 kinase protein ataxia telangiectasia mutated, that is responsible for activating the phosphorylation of the downstream checkpoint protein kinase Chk2 (1, 8). Activated Chk2 phosphorylates the phosphatase, Cdc25, and promotes its binding to the 14-3-3 protein and subsequent sequestration in the cytoplasm (5, 10). Cdc25C is then unable to dephosphorylate and activate the nuclear complex Cdk1-cyclin B which is the universal mitosis inducer in eukaryotes (7, 12, 33). Consequently, Cdk1 is maintained in the inactive tyrosine-phosphorylated state, and the cells exposed to CDT remain arrested in the G2 phase of the cell cycle (29).
In spite of the accumulation of knowledge on the effect of CDT on target cells, less information is available on biogenesis of CDT holotoxin. A. actinomycetemcomitans CdtA, CdtB, and CdtC are translated as approximately 25-, 32-, and 21-kDa proteins, respectively, and are presumably exported across the cytoplasmic membrane with the cleavage of signal sequences using signal peptidases. We have previously shown that A. actinomycetemcomitans CdtA possesses a putative lipid modification motif (37) but have not characterized CdtA for the lipid modification. Since genetic manipulation of A. actinomycetemcomitans is difficult, we characterized CdtA using E. coli carrying the A. actinomycetemcomitans cdtABC genes to extrapolate the biogenesis of CDT holotoxin in A. actinomycetemcomitans. Here we show that membrane-associated CdtA is a lipoprotein. In the periplasm, CDT is a complex composed of CdtA, CdtB, and CdtC, whereas CDT in the culture supernatant is a complex composed of N-terminally truncated CdtA (CdtA′), CdtB, and CdtC. This suggests that CdtA undergoes lipid modification during the export process and subsequent N-terminal processing after forming a complex with CdtB and CdtC in the periplasm. We suggest that lipid modification of CdtA is important for the export of A. actinomycetemcomitans CDT holotoxin into the culture supernatant.
MATERIALS AND METHODS
Bacterial strains and growth media.
Bacterial strains and plasmids used in this study are shown in Table 1. E. coli was grown aerobically in Luria-Bertani (LB) medium or on LB agar plates. A. actinomycetemcomitans was grown in Trypticase soy broth (Becton Dickinson Microbiology Systems, Cockeysville, MD) supplemented with 1% (wt/vol) yeast extract in a 5% CO2 atmosphere. Ampicillin (50 μg/ml) or kanamycin (50 μg/ml) was added when necessary.
TABLE 1.
Strains used in this study
| Strain | Plasmid | Characteristics or description | Reference or source |
|---|---|---|---|
| A. actinomycetemcomitans Y4 | Standard strain | ||
| E. coli | |||
| XL1 blue | pTK3022 | cdtABC on pUC19 (SmaI-EcoRI) | 37 |
| M15 | pQEcdtABC | cdtABC on pQE60 (NcoI-BglII) | 22 |
| XL1 blue | pUCcdtA (C16G)BC | cdtA (C16G)BC on pUC19 (SmaI-EcoRI) | This study |
| XL2 blue | pMWcdtABC | cdtABC on pMW219 (EcoRI-EcoRI) | This study |
| XL2 blue | pMWcdtA (C16G)BC | cdtA (C16G)BC on pMW219 (EcoRI-EcoRI) | This study |
HeLa cell culture.
HeLa cells (ATCC CCL2) were cultured in Dulbecco's modified Eagle medium (Nissui, Tokyo, Japan) supplemented with 10% fetal calf serum at 37°C in a 5% CO2-95% air atmosphere.
Assay for CDT activity.
The test samples were prepared from the culture supernatant and the cell lysate of E. coli carrying pMWcdtABC or pMWcdtA(C16G)BC (where 16th cysteine is changed to glycine in cdtA). Exponentially growing cells at an optical density at 660 nm (OD660) of ca. 0.5 were harvested using centrifugation at 5,000 × g for 10 min, and the culture supernatant was recovered. Cell lysates were prepared from the harvested cells using the method described below. The protein concentration of the culture supernatant and cell lysate were measured using the Bio-Rad protein assay kit and adjusted, respectively, by adding LB to culture supernatant or by adding phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.5 mM KH2PO4 at pH 7.3) to the cell lysate. HeLa cells were plated on a 96-well plate (Falcon; Becton Dickenson) at a concentration of 2 × 103 cells per 100-μl well 1 day before the experiment. A total of 100 μl of filter-sterilized (0.22-μm-pore-size filter) sample containing 0.5 μg of total protein from the culture supernatant or 100 μg of total protein from the cell lysate was inoculated into the HeLa cell monolayer in the first well of the 96-well plate. After the sample and medium were mixed well, a half-aliquot (100 μl) of sample-medium mixture was added to the next well and serially diluted 1:2 likewise through 12 dilutions. Morphological change was observed by phase-contrast microscopy (Nikon DIAPHOT 300) from day 1 to day 3. Cell distension was defined as more than five times the expansion in size compared to that of control cells. Cytodistending activity (total activity) was titrated using the endpoint as the highest twofold dilution of toxic material giving 50% transformed cells (CD50) after 72 h of incubation. Specific activity was defined as the CD50/mg of the total protein.
Preparation of crude CDT.
CDT holotoxin was obtained from A. actinomycetemcomitans Y4 or E. coli carrying the cdtABC genes on the plasmid pTK3022 or pQEcdtABC (22, 37). CDT expression in E. coli was induced by the addition of 1 mM isopropyl-d-thiogalactopyranoside (IPTG) for 4 h at an OD660 of 0.5 to 0.7. Crude CDT from A. actinomycetemcomitans or the recombinant E. coli was prepared in several fractions. (i) For the culture supernatant, cells exponentially growing in culture medium were inoculated into 3 liters of fresh medium and incubated with continuous agitation using a rotary shaker for 4 h at 37°C until the cells reached the stationary phase. The culture was centrifuged at 5,000 × g for 30 min at 4°C. The concentrated culture filtrate was prepared using 80% saturated ammonium sulfate precipitation of the culture supernatant followed by dialysis with PBS. The dialyzed sample was filter sterilized through a membrane filter (pore size, 0.22 μm; Millipore). (ii) For the periplasm fraction, cells recovered from culture by centrifugation were washed twice with PBS and pelleted by centrifugation. The pellet was suspended in 0.2 M Tris-HCl (pH 8.0) containing 1 M sucrose, 1 mM EDTA (pH 8.0), 0.5 mM benzamidine, and 0.02 mg/ml soybean trypsin inhibitor and gently mixed for 1 h on ice. The suspension was then centrifuged twice at 9,700 × g for 20 min at 4°C. The supernatant was diluted 10-fold with PBS. (iii) For the total cell lysate fraction, harvested cells were washed with PBS twice and resuspended in the same buffer at ca. 10 times the volume of the wet cell pellet. Cells were disrupted using an ultrasonic disruptor (UD-200; TOMY, Tokyo, Japan) for 20 s three times at an output level of four. Unbroken cells were removed by centrifugation at 5,000 × g for 5 min. The supernatant was used as the cell lysate.
Immunoaffinity purification of CDT holotoxin.
Crude CDT for immunoaffinity purification was prepared using an 80% saturated ammonium sulfate precipitation of the culture supernatant of E. coli carrying pTK3022. The precipitate after dialysis with wash buffer (0.2 M NaHCO3, 0.5 M NaCl, pH 8.0), the crude CDT, was applied to an affinity column where anti-CdtA antibody was coupled to CNBr-activated Sepharose 4B using the procedure described by the manufacturer (Amersham). The CDT complex was eluted using elution buffer (0.2 M glycine-HCl, 0.2 M NaCl, pH 2.3) following immediate neutralization with 1/10 volume of 1 M Tris-HCl, pH 8.0.
Immunoprecipitation and the pull-down assay.
Immunoprecipitation using antiserum against the CDT components and pull-down assay were performed using the culture supernatant or the periplasmic fraction. For immunoprecipitation, ammonium sulfate precipitation of 3 liters of culture supernatant from E. coli carrying pTK3022 was prepared as described above. The precipitate was dissolved in 20 ml of PBS and dialyzed for more than 4 h against 2 liters of PBS at 4°C. The dialyzed sample was concentrated to ca. 1 ml using an Amicon Centriprep YM-10 (Millipore) and subjected to pretreatment with sodium dodecyl sulfate (SDS) or not treated. The periplasmic fraction extracted from 500 ml of E. coli culture carrying pTK3022 was prepared as described above and concentrated to ca. 1 ml using an Amicon Centriprep YM-10 (Millipore) and subjected to pretreatment with SDS or not treated. For SDS treatment, 1% SDS was added to the crude preparation of either the culture supernatant or cell lysate, diluted 10-fold with PBS, and incubated with 2 μl of rabbit anti-CdtA, -CdtB, or -CdtC serum in a 1-ml sample for 1 h at 4°C (37). Twenty microliters of protein-A Sepharose beads (Amersham) was added and incubated further for 1 h. Beads were pelleted by centrifugation at 10,000 × g for 5 min, washed three times in PBS, resuspended in 70 μl of sample buffer containing 2% SDS, 10% β-mercaptoethanol, 0.01% bromophenol blue, 20% glycerol, 120 mM Tris HCl (pH 6.8), and boiled for 5 min. Beads were again pelleted by centrifugation, and the supernatant was analyzed using SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting. Antisera against CdtA, CdtB, and CdtC were prepared as described by Sugai et al. (37). For the pull-down assay, culture supernatant and the periplasmic fraction of E. coli carrying pQEcdtABC were prepared as described above except that pretreatment with SDS was performed. A total of 500 μl of Ni-chelated agarose beads (QIAGEN) was added to the sample solution and gently shaken for 1 h. The beads were recovered by centrifugation at 5,000 × g for 5 min and washed twice with 10 ml of wash buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 20 mM imidazole). The sample was eluted with 2 ml of elution buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 250 mM imidazole) by centrifugation at 5,000 × g for 5 min. The supernatant was used as the pull-down sample after dialysis against PBS and concentration using a Centricon 10 concentrator (Millipore, Bedford, MA).
Radioisotope labeling.
Recombinant CdtA was radiolabeled with [3H]glycerol (specific activity of 370 to 740 GBq/mmol; ARC, Tokyo,Japan) or [3H]palmitate (specific activity of 1.11 to 2.22 TBq/mmol; ARC, Tokyo, Japan) (24). Briefly, CDT was expressed in 100-ml cultures of E. coli carrying pTK3022 or pUCcdtA(C16G)BC by adding 1 mM IPTG at an OD660 of ca. 0.5. At the same time, 148 KBq/ml (4 μCi/ml) of radio isotope was added to the medium, and the culture was incubated for an additional 4 h (OD660 of ca. 0.9). After incubation and radiolabeling, harvested cells were given a total membrane preparation as described elsewhere (24). The prepared membrane was dissolved in 100 μl of PBS containing 1% SDS, and a 1-μl aliquot was withdrawn to measure the radioactivity to determine the amount of total membrane using a liquid LSC5100 scintillation counter (AloKa) in 5 ml of the scintillation cocktail Scintisol EX-H (DOJINDO, Kumamoto, Japan). After the radioactivities of the membranes were adjusted to ca. 250,000 to 300,000 cpm/100 μl of the [3H]palmitate-labeled sample and 150,000 to 200,000 cpm/100 μl of the [3H]glycerol-labeled sample, the samples (100 μl) were diluted 10 times with PBS, and immunoprecipitation with anti-CdtA serum was performed. After immunoprecipitation (described above), all immunoprecipitated samples from 100-ml cultures were electrophoresed by SDS-PAGE, and the radiolabeled proteins were visualized using fluorography with an amplifier (Amersham).
Outer and inner membrane separation.
E. coli outer and inner membrane separation was performed using a modification of the method described by Osborn (26). Briefly, E. coli cells carrying pTK3022 or pUCcdtA(C16G)BC were harvested at an OD660 of ca. 0.9 after induction with 1 mM IPTG for 4 h. Harvested cells were resuspended in 9 ml of 0.75 M sucrose and 10 mM Tris-HCl at pH 7.8. The cell wall peptidoglycan was digested with 90 μl of 10 mg/ml lysozyme (Sigma) for 2 min on ice. Spheroplasts were prepared by gradually adding 18 ml of 1.5 mM EDTA followed by ultrasonic disruption (UD-200; TOMY, Tokyo, Japan). Unbroken cells were removed by centrifugation at 5,000 × g for 5 min. Crude membranes (cell membrane fraction) were prepared using ultracentrifugation at 100,000 × g for 35 min. Crude membranes were resuspended in 0.5 ml of 25% sucrose and 5 mM EDTA. They were placed on a 5% step gradient consisting of sucrose at 30% (2.1 ml), 35% (2.1 ml), 40% (2.1 ml), 45% (2.1 ml), 50% (2.1 ml), and 55% (0.5 ml) in 5 mM EDTA. The step gradient was ultracentrifuged at 28,000 rpm for 20 h using a Beckman SW28.1 rotor. After centrifugation, fractionation (0.5 ml) was performed from the bottom of the tube under gravity. The protein concentration of each fraction was measured at the OD595 using the Bio-Rad Protein Assay kit. Ten microliters of each fraction was diluted into 800 μl with H2O and mixed with 200 μl of the dye reagent provided in the kit. After incubation at room temperature for 5 min, the absorbance was measured at 595 nm using an Amersham Ultraspec 100 Pro. Twenty microliters of each fraction was subjected to immunoblotting for the detection of CDT or Braun's lipoprotein. Braun's lipoprotein was used as the marker of the outer membrane fractions by immunoblotting with an antiserum provided by H. C. Wu, Uniformed Services University of the Health Sciences, Bethesda, Md. (14). NADH oxidase was assayed in each fraction as the marker for the inner membrane fraction using a method described elsewhere (26).
DNA techniques and plasmid construction.
Routine DNA manipulations were performed using standard procedures. All restriction enzymes, T4 DNA ligase, and DNA polymerase were from Roche, Tokyo, Japan, or New England BioLabs, Inc., Beverley, Mass. Other materials and chemicals used were from commercial sources.
Site-directed mutagenesis of the 16th cysteine residue to glycine coded in the cdtA gene of pTK3022 was carried out using the overlap extension method (31). The primers used were 5′-TTAGTGGCTGGTTCGTCA-3′ and 5′-TGACGAACCAGCCACTAA-3′ (boldface letters indicate mutagenic oligonucleotides to alter the target sequence). The mutated DNA containing cdtA(C16G)BC was subcloned into pUC19. In some experiments, the DNA fragment containing cdtABC in pTK3022 or the mutated DNA fragment containing cdtA(C16G)BC was cloned into pMW219 (Nippon gene, Osaka, Japan), which is a plasmid with a low copy number in E. coli (3).
Other procedures.
SDS-PAGE and Western blotting were carried out as described previously (37). Immunodetection was carried out using Renaissance 4CN Plus (Dupont-NEN). The N terminus was sequenced using a model 49X Procise (Applied Biosystems) after the Coomassie-stained bands were removed and transferred to a polyvinylidene difluoride membrane. Electrospray ionization mass spectrometry was performed using an Applied Biosystems/MDS-Sciex mass analysis following the manufacture's instructions.
RESULTS
CdtA is a lipoprotein.
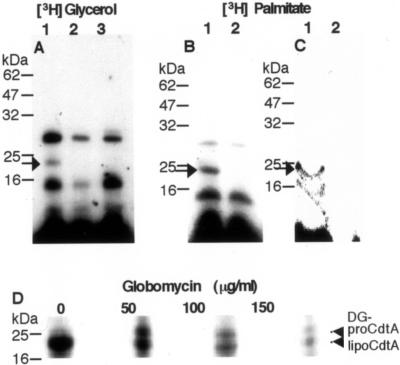
The bacterial lipoproteins were found to be a covalently linked lipid to the cysteine residue at the N terminus. These lipoproteins presumably share a common biosynthetic pathway to generate N-acyl-diacylglycerylcysteine after cleavage of the N-terminal signal peptide, which carries a consensus sequence, the so-called “lipobox” (-Leu-X-Y-Cys-) (39). We previously showed that A. actinomycetemcomitans CdtA possesses the lipid binding consensus lipobox (-Leu-Val-Ala-Cys-) in the N-terminal signal sequence, suggesting that A. actinomycetemcomitans CdtA is a lipoprotein (37). To determine if the lipobox motif is present in the signal sequence of CDT in other species, we examined published amino acid sequences of CDT homologues deposited in the database. As shown in Table 2, possible lipobox sequences were found in the signal sequence of either CdtA or CdtC in all investigated species. This suggests that the lipobox signature is ubiquitous in either CdtA or CdtC among a variety of CDT-producing bacteria. We then determined if A. actinomycetemcomitans CdtA is a lipoprotein in vivo. E. coli carrying pTK3022 was incubated with [3H]palmitate or [3H]glycerol to metabolically label proteins covalently linked with lipid, and the membrane fraction was immunoprecipitated using anti-CdtA serum. In addition, a plasmid encoding cdtABC, whose 16th cysteine residue of CdtA was mutated to glycine, was constructed, and the transformant E. coli carrying pUCcdtA(C16G)BC was treated with the same procedures. As shown in Fig. 1A and B, a radiolabeled band with a molecular mass of 23 kDa (indicated by an arrow) was observed in the total membrane of E. coli carrying pTK3022, whereas this band was missing in E. coli carrying the cysteine-deficient pUCcdtA(C16G)BC. This suggests that the lipid is covalently linked to the 16th cysteine of CdtA. To further demonstrate that CdtA is a lipoprotein, we examined the accumulation of a precursor form of CdtA in cells treated using globomycin, a specific inhibitor of the signal peptidase II (14, 15). Globomycin-treated bacteria accumulate the glyceride-modified precursors of lipoproteins (14). E. coli carrying pUCcdtABC was incubated with [3H]palmitate in the presence or absence of globomycin, and the membrane fraction was probed for CdtA using immunoprecipitation. As shown in Fig. 1D, globomycin induced the appearance of an additional radiolabeled band with a slightly higher molecular mass in SDS-PAGE gels, indicating the accumulation of the glyceride-modified precursor form of CdtA. These results clearly indicate that CdtA is a lipoprotein.
TABLE 2.
Possible lipobox consensus sequence in various CDTs
| Straina | Subunit | Possible signal sequenceb | Gene accession no. |
|---|---|---|---|
| A. actinomycetemcomitans | A | +1MKKFLPGLLLMGLVAC+17S | AB011405 |
| H. ducreyi | A | +1MKKFLPSLLLMGSVAC+17S | U53215 |
| Campylobacter jejuni | A | +1MQKIIVFILCCFMTFFLYAC+21S | U51121 |
| H. hepaticus | A | +1MRLLFFLLITLLFAAC+17S | AF163667 |
| E. coli | |||
| CDT I | A | +1MDKKLIAFLCTLIITGC+18S | U03293 |
| C | +1MKTVIVFFVLLLTGC+16A | ||
| CDT II | A | +1MANKRTPIFIAGILIPILLNGC+23S | U04208 |
| C | +1MKKLAIVFTMLLIAGC+17S | ||
| CDT III | A | +1MTNKCTSILIVGILIPILLNGC+23S | U89305 |
| C | +1MKRLIIIVTMLLIAGC+17S |
For E. coli, three types of CDTs were analyzed.
The lipobox is in boldface italics.
FIG. 1.
Lipid modification of CdtA. Bacterial cells were radiolabeled with either [3H]glycerol or [3H]palmitate. The cell membrane and the periplasmic fractions were solubilized with 1% SDS-containing buffer. A 1:10 dilution of the SDS preparation with PBS was made, and the CdtA was immunoprecipitated using anti-CdtA serum and purified with protein A-Sepharose. The immunoprecipitated protein was separated by SDS-PAGE followed by fluorography. (A) [3H]glycerol-labeled membrane fraction. (B) [3H]palmitate-labeled membrane.(C) [3H]palmitate-labeled periplasmic fraction. Lanes 1, E. coli carrying pTK3022; lanes 2, E. coli carrying pUCcdtA(C16G)BC; lane 3, E. coli carrying vector only (only in panel A). Arrow indicates the lipid-modified CdtA. (D) Effect of globomycin on the processing of CdtA. Various concentrations of globomycin, a signal peptidase II-specific inhibitor, was added to the culture 30 min before labeling with [3H]palmitate. After membrane preparation, the radiolabeled bands of lipid-modified prolipoprotein (diacylglyceryl [DG]-proCdtA), where the signal sequence remains uncleaved, and mature lipoprotein (lipoCdtA) were resolved by SDS-PAGE followed by fluorography.
Lipid-modified CdtA localizes in the outer membrane.
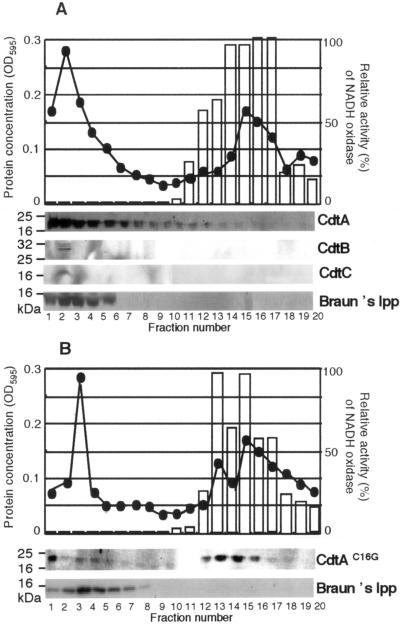
Since one of the important features of a lipoprotein is membrane localization due to lipid insertion in the membrane lipid bilayer, we next attempted to determine if lipid-modified CdtA localized in the inner or outer membrane. We used an E. coli membrane fractionation method to separate E. coli carrying pTK3022 membrane by spheroplast formation and subsequent sucrose density gradient ultracentrifugation (26). As shown in Fig. 2A, membrane fractionation revealed authentic fractionation patterns and generated two protein peaks as expected. Presence of Braun's lipoprotein in the first peak demonstrated that the first peak corresponds to the outer membrane. The second peak was NADH oxidase positive, showing it to be the inner membrane. Immunoblotting shows that CdtA was present only in the outer membrane fraction. This result agrees with the “+2 rule” of the lipobox serine residue which is located next to the 16th cysteine residue in the outer membrane (34). At the same time, we prepared the membrane fractions from E. coli carrying pUCcdtA(C16G)BC. The mutated CdtA(C16G) was found mainly in the inner membrane fractions, suggesting that the 16th cysteine residue is important for localization in the outer membrane (Fig. 2B). Since the three CDT components are shown to form a tripartite complex in a stoichiometry of 1:1:1 in the culture supernatant (19, 22), we attempted to determine if the membrane-bound CdtA was associated with either CdtB and/or CdtC. Immunoblots of the membrane fractions of E. coli carrying pTK3022 show CdtB and CdtC in the outer membrane (Fig. 2A).
FIG. 2.
CdtA in the outer membrane. Outer and inner membrane separation of E. coli carrying pTK3022 (A) or pUCcdtA(C16G)BC (B) was performed after spheroplast isolation using sucrose density gradient separation. After fractionation (0.5 ml/tube) from the bottom of the tube, each fraction was assayed for protein concentration (line) using the Bio-Rad protein assay kit. The OD595 value represents relative protein concentrations. NADH oxidase activity (bars) shows the inner membrane fractions. Immunoblots of each fraction are shown using anti-CdtA, anti-CdtB, anti-CdtC, or anti-Braun's lipoprotein (Braun's lpp) serum. The anti-Braun's lipoprotein serum shows the outer membrane fractions.
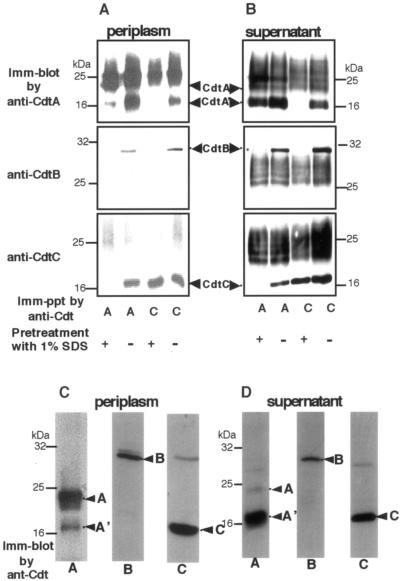
CDT complexes in the periplasm consist of CdtA, CdtB, and CdtC.
We next determined if the CDT components were present as a complex in the periplasmic space. Immunoprecipitation and Western blotting of the crude preparation of periplasm were performed. The crude preparation of the periplasm was either not treated or treated with SDS (final concentration of 1%), diluted 10 times, and incubated with anti-CDT serum. The immune complex was recovered using protein A-Sepharose 4B beads and analyzed using immunoblotting. In the SDS-treated sample, anti-CdtA serum immunoprecipitated CdtA but not CdtB or CdtC. Conversely, in the sample without SDS treatment, anti-CdtA serum immunoprecipitated not only CdtA but also CdtB and CdtC. An extra band with a molecular mass of 18 to 19 kDa reacting with anti-CdtA serum was observed. This band was named CdtA′. Anti-CdtC serum also immunoprecipitated all three components in the absence of pretreatment with 1% SDS. Because the bands smeared at ca. 25 kDa (possibly the immunoglobulin light chain) and obscured the CdtA bands in the immunoprecipitated samples, we prepared a crude fraction of the periplasm of E. coli carrying pQEcdtABC and attempted to pull down the associated proteins with His6-tagged CdtC using Ni-beads. Figure 3C shows the pull-down of CdtA, CdtA′, CdtB, and CdtC from the periplasm. This indicates that CDT is present as a complex in the periplasm, and the complex consists of CdtA, CdtB, and CdtC with a very small amount of CdtA′. In order to determine if CdtA in the periplasm is a lipid-modified form, E. coli carrying pTK3022 was incubated with [3H]palmitate, and the periplasmic fraction was isolated. The fraction was subjected to immunoprecipitation using anti-CdtA serum. The precipitate was separated by SDS-PAGE followed by fluorography. As shown in Fig. 1C, a faint radiolabeled band corresponding to 23 kDa was observed. This indicates that at least part of the CdtA in the complex in periplasm is in the lipid-modified form.
FIG. 3.
Immunoprecipitation and the pull-down assay for the CDT holotoxin. Crude CDT was prepared from either the periplasmic space (A) or the culture supernatant (B) of E. coli carrying pTK3022. CDT components were immunoprecipitated with anti-CdtA or anti-CdtC in the presence or absence of SDS pretreatment at a concentration of 1%. Immunoprecipitated samples were analyzed using SDS-PAGE and immunoblotted using rabbit anti-CdtA, anti-CdtB, or anti-CdtC. The smeared bands at ca. 25 kDa may be rabbit immunoglobulin light chains. Similarly, crude CDT was prepared from either periplasmic space (C) or culture supernatant (D) of E. coli carrying pQEcdtABC. CDT components were pulled down with Ni-chelated beads using His6-tagged CdtC from E. coli carrying pQEcdtABC. Pull-down samples were subjected to SDS-PAGE and immunoblotting using rabbit anti-CdtA, anti-CdtB, or anti-CdtC.
CdtA in the complex goes through another processing step to form the mature form.
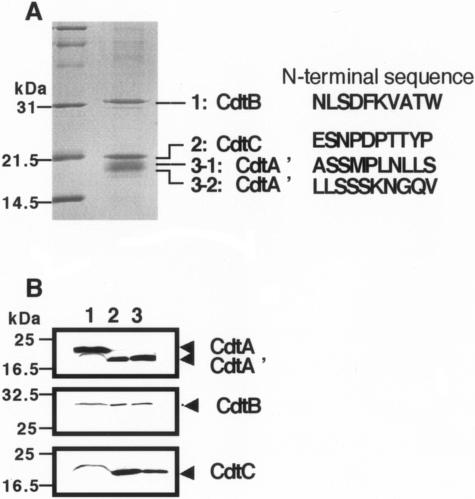
The immunoprecipitation and pull-down experiments were also performed using the culture media supernatant. As shown in Fig. 3B and D, Western blot analysis demonstrated a difference in the ratio of CdtA and CdtA′ in the immunoprecipitated or pull-down sample between the culture supernatant and the periplasmic fraction. CDT holotoxin in culture supernatant contained almost solely CdtA′. To determine the molecular nature of the CdtA′, a component of CDT complex principally found in culture supernatant, we prepared a Sepharose 6B (Amersham) conjugated with affinity-purified anti-CdtA antibody and purified CDT holotoxin from the culture supernatant of E. coli carrying pTK3022. The immunoaffinity-purified CDT holotoxin recovered from the column showed a specific activity of 1.13 × 106 CD50/mg. As shown in Fig. 4, SDS-PAGE analysis of the eluted fraction demonstrated the presence of three major bands stained with Coomassie brilliant blue. The calculated molecular mass of the proteins from the PAGE were 30, 20, and 18 to 19 kDa. The N-terminal amino acid sequence of each protein band was determined using a PROCITE gas phase protein sequencer. The amino acid sequences of the 30-kDa (Fig. 4A, 1) and 20-kDa (Fig. 4A, 2) protein were NLSDFKVATW and ESNPDPTTYP, respectively. Two amino acid sequences, ASSMPLNLLS (Fig. 4A, 3-1) and LLSSSKNGQV (Fig. 4A, 3-2), were obtained from the broader band of 18 to 19 kDa. Comparison with the deduced amino acid sequences shows that the 30-kDa protein corresponds to CdtB and that the 20-kDa band corresponds to CdtC without the predicted signal peptides (Fig. 4A). The broader band of 18 to 19 kDa was a composite of the N-terminally processed fragments of the lipid-modified CdtA. The immunopurified complex was further analyzed using electrospray ionization mass spectrometry. Four major peaks were obtained with masses of 18,912.4, 18,211.6, 28,886.6, and 18,410.8 daltons. This was in agreement with the calculated molecular masses of CdtA′-1 at 18,915, CdtA′-2 at 18,215, CdtB at 28,880, and CdtC at 18,622 daltons from the predicted amino acid sequence of the mature CDT components. These results indicate that CdtA′3-1 and CdtA′3-2 are N-terminally truncated forms beginning at the 52nd alanine and 59th leucine, respectively.
FIG. 4.
Immunoaffinity purification of the CDT complex. Crude CDT was prepared using 80% saturated ammonium sulfate precipitation of the culture supernatant of E. coli carrying pTK3022. After dialysis with wash buffer (0.2 M NaHCO3, 0.5 M NaCl, pH 8), crude CDT was applied to an affinity column, where anti-CdtA antibody was coupled to CNBr-activated Sepharose 4B. The CDT complex was eluted with elution buffer (0.2 M glycine-HCl, 0.2 M NaCl, pH 2.3) followed by immediate neutralization with a 1/10 volume of 1 M Tris-HCl, pH 8.0. The complex was analyzed using SDS-PAGE with Coomassie brilliant blue staining (A). The left lane contains molecular size markers. Detected N-terminal amino acid sequences of each Coomassie-stained band are shown at the right in panel A. (B) Immunoaffinity purification of CDT complex from A. actinomycetemcomitans Y4 culture supernatant. Crude CDT was prepared by 80% saturated ammonium sulfate precipitation of the culture supernatant of A. actinomycetemcomitans Y4. Immunoaffinity purification using an anti-CdtA affinity column and immunoblotting was performed as described above. Lane 1, A. actinomycetemcomitans Y4 total cell lysate; lane 2, immunopurified sample from A. actinomycetemcomitans Y4 culture supernatant; lane 3, immunopurified sample from culture supernatant of E. coli carrying pTK3022.
The above results show that A. actinomycetemcomitans CDT is present as a complex of CdtA or CdtA′, CdtB, and CdtC in the periplasm and a complex of CdtA′, CdtB, and CdtC in the culture supernatant of E. coli carrying pTK3022. We therefore characterized the constituents of the CDT complex in A. actinomycetemcomitans. As shown in Fig. 4B, immunoprecipitated CDT from A. actinomycetemcomitans cell lysate consists of CdtA, CdtB, and CdtC, whereas that from A. actinomycetemcomitans culture supernatant principally was CdtA′, CdtB, and CdtC. These data strongly suggest that similar processing of CdtA takes place not only in E. coli but also in A. actinomycetemcomitans.
Lipid modification of CdtA is important for secretion of the CDT complex.
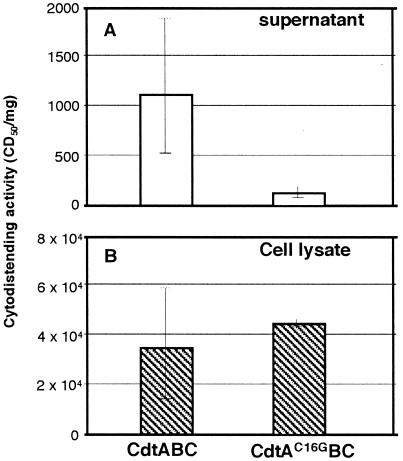
We next determined the biological importance of the lipid-modification of CdtA. We used a low-copy-number plasmid, pMW219, to express the CDT holotoxin to mimic the expression of CdtABC genes in A. actinomycetemcomitans. We compared CDT activities in the culture supernatant and in the sonic lysate of E. coli carrying pMWcdtABC and in E. coli carrying pMWcdtA(C16G)BC. As shown in Fig. 5, the CDT activity in the culture supernatant of E. coli carrying pMWcdtA(C16G)BC was significantly lower compared to the strain producing the wild-type CDT, whereas this significant change was not observed in the CDT activity in the cell lysate.
FIG. 5.
Lipid modification and cytodistending activity. Wild-type and mutated CDT holotoxin were produced from E. coli carrying pMWcdtABC and pMWcdtA(C16G)BC, respectively. Cells carrying both plasmids were cultured at an OD660 of 0.05 and incubated under the same conditions with vigorous shaking. Both samples were harvested at logarithmic phase (OD660 of 0.5); then the culture supernatant (A) and cell pellet were prepared. Cells were resuspended in PBS at pH 7.3 and ultrasonically disrupted (B, cell lysate). Both supernatant (0.5 μg/100 μl) and cell lysate (100 μg/100 μl) fractions were sterilized using 0.22-μm-pore-size filter and titrated for cytodistending activity (CD50/mg) on HeLa cells using the serial dilution described in Materials and Methods.
DISCUSSION
Based on cloning of the cdt genes from many microorganisms, several reports have shown that the CdtA or CdtC component possesses a putative lipid modification motif (27, 28, 37). However, no experiments have been conducted to determine the exact lipid modification of CdtA or CdtC. With A. actinomycetemcomitans, the lipobox was observed in the cdtA. In a previous study, we demonstrated that every CDT component is present in not only the periplasm but also the culture supernatant of A. actinomycetemcomitans as well as in an E. coli carrying the plasmid containing A. actinomycetemcomitans cdtABC (22). These results show that CDT was extracellularly secreted in both organisms and that the E. coli system worked well in mimicking the production of CDT in A. actinomycetemcomitans. Western analysis shows an anti-CdtA-reactive band with a molecular mass lower than CdtA in the supernatant fraction of both microorganisms. This suggests that CdtA undergoes proteolytic processing during biogenesis of the CDT holotoxin. To analyze the lipid modification using radiolabeling to characterize biogenesis of CDT holotoxin, fairly large amounts of toxin production are necessary. We therefore used E. coli carrying the cdtABC genes on a multicopy plasmid to characterize the cdt gene products and to extrapolate the biogenesis of CDT holotoxin in A. actinomycetemcomitans. This is the first report to demonstrate the incorporation of palmitate or glycerol into CdtA that indicates CdtA is a lipoprotein. Susceptibility of the signal sequence processing of CdtA to globomycin further supports this. Lipid-modified CdtA is present in either the outer membrane or the periplasm as a complex with CdtB and CdtC. However, how the lipid-modified CdtA is released into the periplasm remains to be determined. A possibility is that the assembly with CdtB and CdtC enforces the detachment of CdtA from the outer membrane. This requires confirmation. Purification of the CDT complex from the culture supernatant using anti-CdtA antibody affinity chromatography shows that CdtA′ in the complex is an N-terminally truncated form of CdtA. Together with the observation that CdtA′ was found in the immunoprecipitated sample of the periplasm fraction, this indicates that the lipid-modified CdtA undergoes further processing of the N terminus in the periplasm, and the complex with processed CdtA (CdtA′) will be secreted from the periplasm to the culture supernatant. This agrees with previous reports that A. actinomycetemcomitans CdtA exists in two forms in crude extracts at 25 and 18 kDa (35).
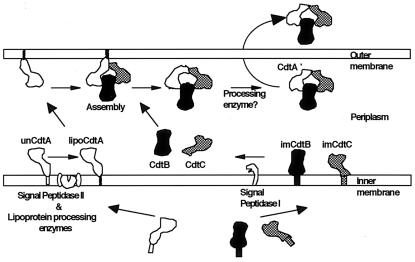
Figure 6 shows our current model for the biogenesis of the CDT complex based on the observations obtained in this study. We suggest that the unmodified CdtA, immature CdtB, and immature CdtC are secreted into the periplasmic space through a sec-dependent secretion pathway (data not shown). After translocation to the inner membrane, immature CdtB and CdtC undergo processing by signal peptidase I. Unmodified CdtA then undergoes processing by lipoprotein-specific signal peptidase II followed by lipid modification. After the amide-linked fatty acid modification at the N-terminal cysteine, lipid-modified CdtA is transported to the outer membrane where the lipid-modified CdtA, CdtB, and CdtC form a complex and subsequently are released into the periplasm. Processing of the N-terminal ca. 40 amino acids of CdtA by an unidentified protease(s) takes place in the periplasm, and the resulting mature holotoxin is secreted into the culture medium by an unknown mechanism. The significant decrease in CDT activity in the culture supernatant of E. coli carrying pMWcdtA(C16G)BC strongly suggests that the incapacity for lipid modification of CdtA affects secretion of CDT holotoxin into the culture supernatant. Membrane anchoring of lipid-modified CdtA may be important for the efficient formation of the CDT complex or for efficient secretion. Further studies regarding the in vivo kinetics of the complex formation and subsequent secretion are necessary to understand the biological importance of lipid modification of the CdtA in A. actinomycetemcomitans.
FIG. 6.
Hypothetical model of CDT complex formation and secretion. Immature CdtA, CdtB, and CdtC are translated from the cdtA, cdtB, and cdtC genes and secreted into the periplasmic space in a sec-dependent general secretion pathway using their N-terminal signal sequences. During passage through the inner membrane, the signal sequences are cleaved and immature CdtB (imCdtB) and immature CdtC (imCdtC) become mature forms using the truncating signal peptidase I. Unmodified CdtA (unCdtA) is cleaved at its signal sequence by signal peptidase II and modified with lipid. The lipid-modified CdtA (lipoCdtA) is carried to the outer membrane using the serine residue next to a lipid-modified cysteine residue (lipoCdtA). This forms a complex with CdtB and CdtC in the outer membrane and periplasmic space. The lipid moiety of CdtA may be hidden by CdtA itself or by CdtB and CdtC in the periplasm. Finally, an unknown protease(s) cleaves the N-terminal ca. 40 amino acids of the lipid-modified cysteine residue, and then the complex is secreted into the culture medium. Molecular sizes and structures of the CDT complex are arbitrarily estimated.
Acknowledgments
We are grateful to Neil Ledger and Jim Nelson for their editorial assistance. We thank the Research Center for Molecular Medicine, the Radioisotope Center at the Kasumi Campus, and the Research Facility, Hiroshima University Faculty of Dentistry, for allowing us to use their facilities.
This study was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan.
Editor: V. J. DiRita
REFERENCES
- 1.Alby, F., R. Mazars, J. de Rycke, E. Guillou, V. Baldin, J. M. Darbon, and B. Ducommun. 2001. Study of the cytolethal distending toxin (CDT)-activated cell cycle checkpoint. Involvement of the CHK2 kinase. FEBS Lett. 491:261-265. [DOI] [PubMed] [Google Scholar]
- 2.Avenaud, P., M. Castroviejo, S. Claret, J. Rosenbaum, F. Megraud, and A. Menard. 2004. Expression and activity of the cytolethal distending toxin of Helicobacter hepaticus. Biochem. Biophys. Res. Commun. 318:739-745. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi, A., and F. Bernardi. 1984. Complete sequence of pSC101. Nucleic Acids Res. 12:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 5.Comayras, C., C. Tasca, S. Y. Peres, B. Ducommun, E. Oswald, and J. De Rycke. 1997. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect. Immun. 65:5088-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. J. Munson, T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes-Bratti, X., E. Chaves-Olarte, T. Lagergard, and M. Thelestam. 1999. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J. Clin. Investig. 103:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 9.Deng, K., J. L. Latimer, D. A. Lewis, and E. J. Hansen. 2001. Investigation of the interaction among the components of the cytolethal distending toxin of Haemophilus ducreyi. Biochem. Biophys. Res. Commun. 285:609-615. [DOI] [PubMed] [Google Scholar]
- 10.De Rycke, J., V. Sert, C. Comayras, and C. Tasca. 2000. Sequence of lethal events in HeLa cells exposed to the G2 blocking cytolethal distending toxin. Eur. J. Cell Biol. 79:192-201. [DOI] [PubMed] [Google Scholar]
- 11.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37:952-963. [DOI] [PubMed] [Google Scholar]
- 12.Escalas, N., N. Davezac, J. De Rycke, V. Baldin, R. Mazars, and B. Ducommun. 2000. Study of the cytolethal distending toxin-induced cell cycle arrest in HeLa cells: involvement of the CDC25 phosphatase. Exp. Cell Res. 257:206-212. [DOI] [PubMed] [Google Scholar]
- 13.Haghjoo, E., and J. E. Galan. 2004. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 101:4614-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 15.Inukai, M., M. Takeuchi, K. Shimizu, and M. Arai. 1978. Mechanism of action of globomycin. J. Antibiot. 31:1203-1205. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb. Pathog. 4:115-126. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb. Pathog. 4:103-113. [DOI] [PubMed] [Google Scholar]
- 18.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer, M. P., L. C. Bueno, E. J. Hansen, and J. M. DiRienzo. 1999. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect. Immun. 67:1227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesic, D., Y. Hsu, and C. E. Stebbins. 2004. Assembly and function of a bacterial genotoxin. Nature 429:429-433. [DOI] [PubMed] [Google Scholar]
- 22.Ohara, M., T. Hayashi, Y. Kusunoki, M. Miyauchi, T. Takata, and M. Sugai. 2004. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 cell lines. Infect. Immun. 72:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohara, M., Y. Ueno, H. Shiba, S. Nishikubo, M. Ikura, H. Kurihara, H. Komatsuzawa, and M. Sugai. 2004. Actinobacillus actinomycetemcomitans CDT induces cell cycle arrest in primary culture of human gingival fibroblast. Dent. Jpn. 40:18-22. [Google Scholar]
- 24.Ohara, M., H. C. Wu, K. Sankaran, and P. D. Rick. 1999. Identification and characterization of a new lipoprotein, NlpI, in Escherichia coli K-1. J. Bacteriol. 181:4318-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda, J., H. Kurazono, and Y. Takeda. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167-172. [DOI] [PubMed] [Google Scholar]
- 26.Osborn, M. J., J. E. Gander, E. Parisi, and J. Carson. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J. Biol. Chem. 247:3962-3972. [PubMed] [Google Scholar]
- 27.Peres, S. Y., O. Marches, F. Daigle, J. P. Nougayrede, F. Herault, C. Tasca, J. De Rycke, and E. Oswald. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095-1107. [DOI] [PubMed] [Google Scholar]
- 28.Pickett, C. L., D. L. Cottle, E. C. Pesci, and G. Bikah. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 30.Saiki, K., K. Konishi, T. Gomi, T. Nishihara, and M. Yoshikawa. 2001. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 45:497-506. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sert, V., C. Cans, C. Tasca, L. Bret-Bennis, E. Oswald, B. Ducommun, and J. De Rycke. 1999. The bacterial cytolethal distending toxin (CDT) triggers a G2 cell cycle checkpoint in mammalian cells without preliminary induction of DNA strand breaks. Oncogene 18:6296-6304. [DOI] [PubMed] [Google Scholar]
- 34.Seydel, A., P. Gounon, and A. P. Pugsley. 1999. Testing the “+2 rule” for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 34:810-821. [DOI] [PubMed] [Google Scholar]
- 35.Shenker, B. J., D. Besack, T. McKay, L. Pankoski, A. Zekavat, and D. R. Demuth. 2004. Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): evidence that the holotoxin is composed of three subunits: CdtA, CdtB, and CdtC. J. Immunol. 172:410-417. [DOI] [PubMed] [Google Scholar]
- 36.Shenker, B. J., R. H. Hoffmaster, T. L. McKay, and D. R. Demuth. 2000. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J. Immunol. 165:2612-2618. [DOI] [PubMed] [Google Scholar]
- 37.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66:5008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse, C. A., P. B. Balbo, E. C. Pesci, D. L. Cottle, P. M. Mirabito, and C. L. Pickett. 1998. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 66:1934-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 40.Young, V. B., K. A. Knox, and D. B. Schauer. 2000. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 68:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]