Heme proteins carry out a phenomenal range of biological processes with a common prosthetic group and a relatively small variety of axial ligands. In this paper, we report the effects on the molecular and electronic structure when the imidazole ligand is deprotonated in five-coordinate iron(II) [Fe(Por)(2-MeHIm)] derivatives. 1 Although imidazole- and imidazolate-ligated iron(II) porphyrinates both exhibit an S = 2 (quintet) state, the coordination group geometries are distinctly different with axial and equatorial bond distance differences and large changes in the displacement of iron from the porphyrin plane. The Mössbauer spectra obtained in strong magnetic fields clearly indicate a difference between the two classes in the d orbital that is doubly occupied. This change in the d-electron configuration is clearly consistent with all observed differing features and may provide control of reactivity for different classes of heme proteins.
We have recently reported the synthesis, molecular and electronic structure characterization of several five-coordinate imidazole-ligated iron(II) porphyrinates.2-4 The structural features showed a strong consistency. The displacement of the iron atom from the four nitrogen atom plane is effectively constant at 0.36 Å, although there is a range of core doming due to core conformation change.5 The axial and equatorial Fe-N bond distances of six derivatives vary over a very narrow range. Moreover, the Mössbauer spectra of nine different derivatives in small and strong applied magnetic field were found to have quadrupole splittings that are strongly temperature dependent and with a negative value of the quadrupole splitting. Values of the quadrupole splitting at 4.2 K ranged from -1.93 to -2.44 44 mm/s.
We have now prepared and characterized two new fivecoordinate iron(II) complexes in which the axial ligand is 2-methylimidazolate, i.e., the deprotonated ligand. Deprotonation leads to a series of remarkable differences in properties of the new derivatives compared to the neutral imidazole complexes. The structure of the anion in [K(222)][Fe(OEP)(2-MeIm-)] is shown in Figure 1. An illustration of the full molecule is given in Figure S1. The structure of [K(222)][Fe(TPP)(2-MeIm-)] has also been determined (Figure S2). The metrical features of the imidazole-vs. the imidazolate-ligated complexes are compared in Figure 2.6
Figure 1.
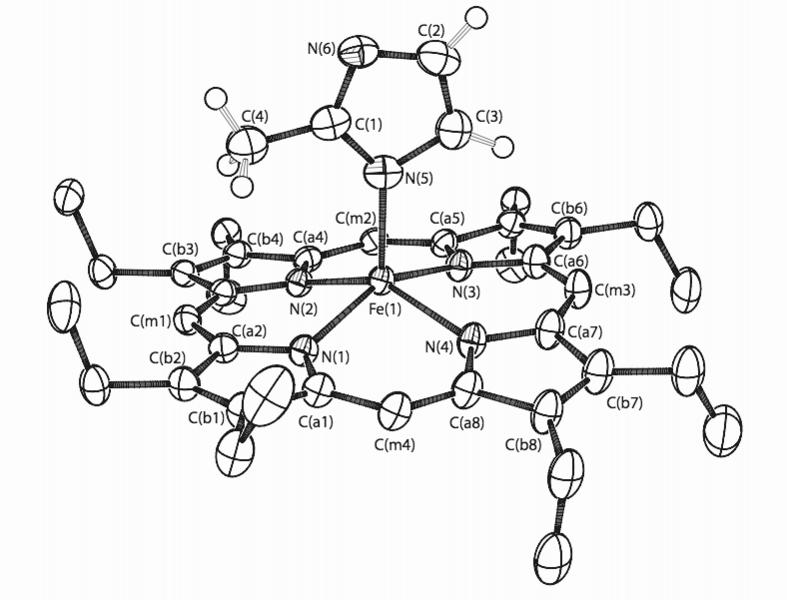
ORTEP diagram of [K(222)][Fe(OEP)(2-MeIm−)] illustrating 50% probability ellipsoids. The atom labeling scheme for the porphyrin core is shown. Only the imidazolate hydrogen atoms are shown.
Figure 2.
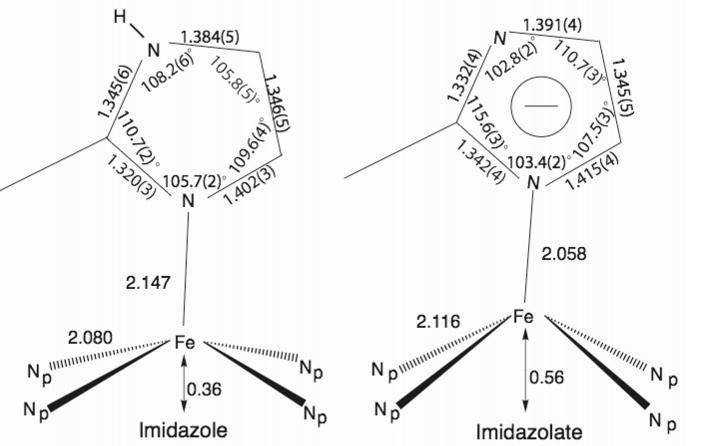
Formal diagrams displaying the average coordination group parameters in four imidazole-ligated [Fe(Por)(L)] complexes (left) and the analogous 2-methylimidazolate species (right).
Some structural differences shown in Figure 2 are expected and readily accounted for. First, the differences in the bond angles and distances in the five-membered rings are as expected for increased bond delocalization for the imidazolate ring. Thus, bond distances and angles involving the two nitrogen atoms are similar in imidazolate and different in imidazole. Second, the imidazolate ligand is known7 to be a stronger field ligand than imidazole and is also expected to be a better s donor; that these effects lead to an ∼0.09 Å axial Fe-N bond distance shortening, favoring imidazolate, is sensible. The reasons for the differences in the iron equatorial parameters are less obvious. Why should the displacement of iron(II) be much larger (0.20 Å) when imidazolate is the axial ligand than when imidazole is the axial ligand since the iron has a high-spin state in both? Concomitantly, why should the equatorial Fe-NP distances be almost 0.04 Å longer in the imidazolate system? Otherwise put, why does the size of the high-spin iron(II) ion appear to be larger in the imidazolate complexes?
An analysis of electronic structure in the two systems by the application of Mössbauer spectroscopy, especially in strong magnetic fields, furnishes the answers. The isomer shift for the imidazolate complexes is very large, about 1.00 mm/s at 4.2 K, consistent with a high-spin iron(II) state, and similar to that observed for the imidazole complexes. The two imidazolate complexes both display a quadrupole doublet with a modest temperature dependence over the range of 4.2 to 298 K (about 15% change), whereas the temperature variation observed for the imidazole complexes is much larger (up to ∼55% change).
The magnitude of the quadrupole splitting value is substantially larger for the imidazolates at 3.60-3.71 mm/s (4.2 K). Most telling, the experimentally determined sign of the quadrupole splitting for the imidazolates is positive, not negative as seen for the imidazoles2 and strongly implying that the d electron configuration has changed upon deprotonation of imidazole. An illustration of the fit to the high field (9T) spectrum for [Fe(OEP)(2-MeIm-)] is given in Figure 3; information on all fits are found in the SI. In addition to the positive sign of the quadrupole splitting, a positive value of the zero field splitting constant, and significant rhombicity were seen to be needed for good fits to the data.
Figure 3.
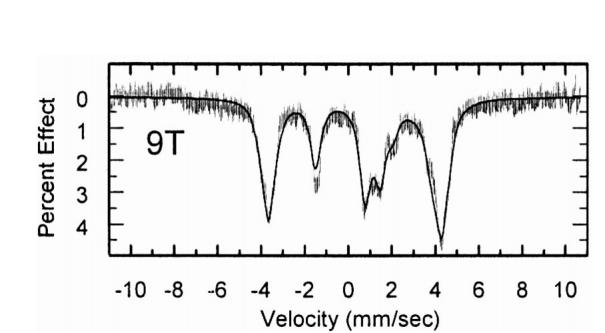
Fit of 9T spectrum for [K(222)][Fe(OEP)(2-MeIm−)]. 15% change), whereas the temperature variation observed for the imidazole complexes is much larger (up to ∼55% change).
As outlined earlier,2 the negative sign for the quadrupole splitting value led to the conclusion that the doubly occupied d orbital for the imidazole complexes is a dπ orbital, i.e., the doubly occupied orbital is perpendicular to the heme plane.8 Following the discussion of Debrunner,11 a large positive quadrupole splitting with a small asymmetry parameter for the imidazolate derivatives is consistent only with the doubly occupied d orbital being in the heme plane, i.e., the orbital must be either dxy or dx2-y2.11
The dxz and dyz orbitals, on the other hand, have an EFG with a negative component Vzz along the heme normal. So if the doublyoccupied d orbital is, (due to low-symmetry ligand fields) a mixture of the dxz, dyz, and dxy orbitals, the sign of QS can be negative, and ? can be relatively large. In this model, when the QS is negative, the largest component of the EFG will no longer lie near the heme normal. Indeed, single crystal Mössbauer data have shown that for deoxymyoglobin, Vzz is negative and far from the heme normal, within 22° of the heme plane.12 Why the QS has a consistently smaller magnitude when it is negative does not follow immediately from the mixed nature of the ground state. Possible causes of the smaller QS include greater p donation into one of the two remaining singly-occupied hybrid orbitals, and a greater ligand contribution to the EFG with a negative Vzz.
For the imidazolates, with more dxy character, spin-orbit coupling will mix in dx2-y2 character more strongly into the doubly occupied orbital than for the imidazoles. The electrostatic repulsion of the in-plane orbital by the negative charge of the pyrrole nitrogens is thus expected to increase the magnitude of the iron atom displacement from the porphyrin plane with a concomitant increase in the value of the Fe-Np bond distances in comparison to the imidazole-ligated systems.
The (dxy)2(dxz)1(dyz)1(dz2)1(dx2-y2)1 ground state has also been assigned for [Fe(TPP)(OPh)]-13 and Na(222)[Fe(OAc)(TpivPP)].14 Indeed, we believe that the (dxy)2(dxz)1(dyz)1(dz2)1(dx2-y2)1 state is the usual one for high-spin iron(II) porphyrinates; the “unusually large” quadrupole split doublet signal from high-spin iron(II) should be regarded as normal.15 We believe that it is the lowsymmetry mixed-d-orbital configuration with a QS that is smaller and negative, as found for deoxymyoglobin, deoxyhemoglobin and the imidazole-ligated iron(II) porphyrinates that is the unusual electronic state for high-spin iron(II) porphyrinates. It seems likely that this electronic state is important for the biological function of these heme proteins, which is to reversibly bind the diatomic molecule O2. A doubly occupied state with a strong admixture of a dπ orbital may facilitate the spin-forbidden binding of the dioxygen molecule. Reversibility may be facilitated if the axial ligation and/or ligand binding pocket state allow the subsequent oxy derivative to return to this (dπ)2 electron configuration upon release of O2. This might explain the differences between the oxygen binding of myoglobin and reduced HRP.16 Clearly this relates to the question of how hydrogen bonding to the N-H group of a coordinated imidazole of a high-spin iron(II) porphyrinate might affect the electronic structure, a point that is under active investigation in these laboratories.
Supplementary Material
Acknowledgments
Acknowledgment. We thank the National Institutes of Health for support of this research under Grant GM-380401.
Footnotes
Supporting Information Available: Synthetic details. Figures S1 and S2 showing ORTEP diagrams of the full molecules. Figures S3 and S4 show the simultaneous Mössbauer fits to the 1, 5, and 9 T data. Tables S1 and S2 give full details on the fits. Tables S3-S14 give complete crystallographic details, atomic coordinates, distances and angles, anisotropic temperature factors, and fixed hydrogen atom positions for [K(222)][Fe(OEP)(2-MeIm-)] and [K(222)][Fe(TPP)(2-MeIm-)]. This information is available as a PDF file. The crystallographic information files (CIF) are also available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1). Abbreviations used in this work: Por, general porphyrin; OEP, octaethylporphyrin dianion; TPP, meso-tetraphenylporphyrin dianion; TpivPP, αααα-tetrakis(o-pivalamidophenyl)porphyrin dianion; Np, pyrrolic nitrogen; OPh, phenolate; 2-MeIm-;, 2-methylimidazolate; 2-MeHIm, 2-methylimidazole; 222, Kryptofix 222; EFG, electric field gradient.
- (2).Hu C, Roth A, Ellison MK, An J, Ellis CM, Schulz CE, Scheidt WR. J. Am. Chem. Soc. 2005;127:5675. doi: 10.1021/ja044077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ellison MK, Schulz CE, Scheidt WR. Inorg. Chem. 2002;41:2173. doi: 10.1021/ic020012g. [DOI] [PubMed] [Google Scholar]
- (4). The imidazoles of these studies were the sterically hindered imidazoles, 2-methylimidazole or 1,2-dimethylimidazole.
- (5). A convenient measure of core doming is the difference in the displacement of iron from the 24-atom porphyrin plane and from the plane of the four nitrogen atoms.
- (6).Mandon D, Ott-Woelfel F, Fisher J, Weiss R, Bill E, Trautwein AX. Inorg. C hem. 1990;29:2442. [Google Scholar]
- (7).Landrum JT, Hatano K, Scheidt WR, Reed CA. J. Am. Chem. Soc. 1980;102:6729. [Google Scholar]
- (8). We now recognize that the Mössbauer properties are better described by a hybrid orbital comprised of both dπ orrbitals, with a significant dxy contribution. Such mixing of dxy with the dp orbitals will occur in any ligand field of sufficiently low symmetry. For example, an axial potential rotated off of an octahedral symmetry axis will mix the states. Such a field would be expected for 5-coordinate hemes with the axial ligand displaced from the heme normal. A detailed analysis of the Mössbauer spectra of all high-spin iron(II) porphyrinates will be published subsequently9.
- (9).Schulz CE, Scheidt WR. work in progress.
- (10).Debrunner P. In: Iron Porphyrins. Lever ABP, Gray HB, editors. VCH Publishers Inc.; New York: 1983. Part 3. Chapter 2. [Google Scholar]
- (11). If the d-orbital choice is correct, a small value of the asymmetry parameter η is expected and observed from the fits to the high-field Mössbauer data.
- (12).Kent T, Spartalian K, Lang G, Yonetani T. Biochim. Biophys. Acta. 1977;490:331. doi: 10.1016/0005-2795(77)90008-3. [DOI] [PubMed] [Google Scholar]
- (13).Shaevitz BA, Lang G, Reed CA. Inorg. Chem. 1988;27:4607. [Google Scholar]
- (14).Bominaar EL, Ding X, Gismelseed A, Bill E, Winkler H, Trautwein AX, Nasri H, Fischer J, Weiss R. Inorg. Chem. 1992;31:1845. [Google Scholar]
- (15). Lang et al.13 concluded from a detailed study of the Mössbauer spectra of [Fe(TPP)(OPh)]-that the doubly occupied orbital in this complex was dxy; a subsequent analysis for Na(222)[Fe(OAc)(TpivPP)]14 concurred in this dxy assignment. These studies emphasized the unique character of these species, and not, as we conclude, that this electronic state is more likely the norm for high-spin iron(II) porphyrinates.
- (16).Champion PM, Chiang R, Münck E, Debrunner P, Hager LP. Biochemisty. 1975;14:4159. doi: 10.1021/bi00690a002. Differences in the Mössbauer spectra were noted but not analyzed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


