Abstract
Background
Many different sexual isolation and sexual selection statistics have been proposed in the past. However, there is no available software that implements all these statistical estimators and their corresponding tests for the study of mating behaviour.
Results
JMATING is an easy-to-use program developed in Java for the analysis of mating frequency data to study sexual selection and sexual isolation effects from laboratory experiments as well as descriptive studies accomplished in the wild. The software allows the re-organization of the data previous to the analysis, the estimation of the most important estimators, and a battery of complementary statistical tests.
Conclusion
JMATING is the first complete and versatile software for the analyses of mating frequency data. It is available at http://www.uvigo.es/webs/c03/webc03/XENETICA/XB2/JMsoft.htm and requires the Java runtime environment.
Background
Mating behaviour is likely to be one of the most important biological processes contributing to speciation in animals [1], sexual isolation being the most obvious evolutionary strategy to impede the production of unfit hybrids when two species meet in the wild [2,3]. Studies on sexual isolation have been typically accomplished in laboratory conditions using one of four possible experimental designs: no choice, male choice, female choice and multiple choice [reviewed in [4-6]]. For simplicity we will refer always to the multiple choice design, where males and females of two or more qualitative mating types are placed in a mating chamber and mating pairs are identified. The former designs can be studied using the same estimators and methods when they use a similar number of mating attempts for every combination of mating pairs [6]. In a multiple choice experiment the mating behaviour can be disentangled into sexual isolation and selection effects [7]. This statistical partitioning has an evolutionary justification: sexual selection can change gene frequencies in populations, while sexual isolation might be directly involved in speciation [8]. In addition to these classical laboratory experiments, there have been a few attempts to study these evolutionary processes directly in the wild [9-13] or to use maximum likelihood methods to infer the causes contributing to the former effects [14-18].
One of the most appropriate statistics to estimate sexual selection effects is the cross-product estimator (W), which represents the maximum likelihood fitness estimator of one class relative to another [4,7,19]. Sexual isolation estimators try to measure the relative importance of homotypic mating pairs (those between individuals with the same type) in relation to the heterotypic ones (between different types). However, there has been less agreement about the best estimator for sexual isolation effects [reviewed in [4,5,7,20]]. Recently, the statistical properties of all known sexual isolation estimators have been compared [21], revealing that three estimators should be preferentially used: IPSI, Yule's V and YA.
Complementary pairwise sexual selection and sexual isolation estimators have also been proposed to study mating behaviour in mating frequency data [7]. The PSI, PSS and PTI coefficients, calculated for each combination of mating types in a multiple choice design, represent the sexual isolation, sexual selection and total deviations of each pair combination from the expectations under random mating. In addition, the PSS coefficient is an additive decomposition of the cross-product estimator, thus incorporating its advantages [7]. The PSI and PSS coefficients have been used to distinguish between biological mechanisms acting in nature [12].
The statistical significance for both sexual selection and sexual isolation effects has been assessed with Chi-square or G (likelihood ratio) tests [22,23]. Theoretical resampling variances for some of these statistics have been already described [21]. Additionally, bootstrapping has been also proposed for IPSI and pairwise estimators, giving identical results to those from parametric inference when experimental replication was available [6].
Although one MSDOS program exists that calculates some of the above statistics [6,11], we do not know of any WINDOWS program that includes a comprehensive representation of estimators and statistical tests for the study of sexual selection and sexual isolation. JMATING has been developed to fill this gap.
Implementation
JMATING is a program written in Java. The java virtual machine (JVM) is needed to run the program, and can be freely downloaded [24]. Once a JVM is properly installed, the program should be able to run in different platforms like Windows, Linux or MacOS X. The user can input mating frequency data manually or from a text file in a specific format [see examples in Additional file 1]. At any time, the data loaded into the program can be saved to a file. There is no limit for the number of species or specimen types but more than 100 mating types will delay significantly the computation time especially for bootstrapping. The data table can be edited by the user and the statistics recomputed with the new data. The results will always appear in an editable panel, which content can be saved to a file. Data can be integer or real numbers, e.g. 1.5 observed matings. There are two reasons for this. First, during the analysis of hermaphrodite data, heterotypic pair counts are typically divided by 2 [25]. Second, the bootstrap test can not check the PTI or PSI statistics if there are zero observed mating pairs in a particular cell. However, the user can change the bootstrap settings given the program the possibility to change zeros by 0.5 or 1 during bootstrapping, as suggested by Coyne et al. [6]. The default number of bootstrap iterations is 10,000, while the maximum is 100,000.
Results and discussion
Available estimators of sexual selection and isolation effects
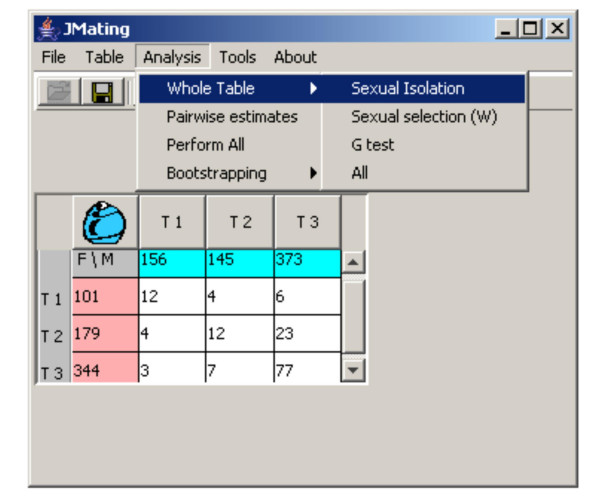
The analyses implemented in JMATING focus on mating frequency data. The program admits complex tables with up to an indeterminate number of mating types (only limited by the computer memory and speed) and different choice designs: multiple choice, male choice, female choice or no choice designs. We provide two example data sets [Additional file 1]: Example 1 is from a descriptive study made in the wild [12], where two morphs of Littorina saxatilis (RB and SU) meet and hybridize on some micro-habitats from the rocky shore (Figure 1); Example 2 was obtained from a multiple choice experiment in the laboratory using two species of Drosophila (Table 2a in [6]).
Figure 1.
Example 1 of multiple choice data obtained in a field study where the frequency of three morphs and their corresponding mating pairs were recorded [12]. Coloured marginal cells represent the frequency of the three mating types (T1, T2 and T3) in males (blue) or females (pink) sampled. The numbers within the cells in the table represent the observed mating pairs for each particular combination of mating types and sexes.
We will use Example 1 to show some features of the analyses available in JMATING (Figure 1). Coloured rows and columns represent the number of specimens of the different types (mating types) used in the experiment. In Example 1 they represent morph frequencies sampled in the wild. For convenience rows always correspond to females (pink) and columns to males (blue). Numbers within the non-coloured cells represent the different mating pairs sampled in the wild (or obtained during the choice experiment, as in Example 2). Two different kinds of analyses are available: global and pairwise estimates.
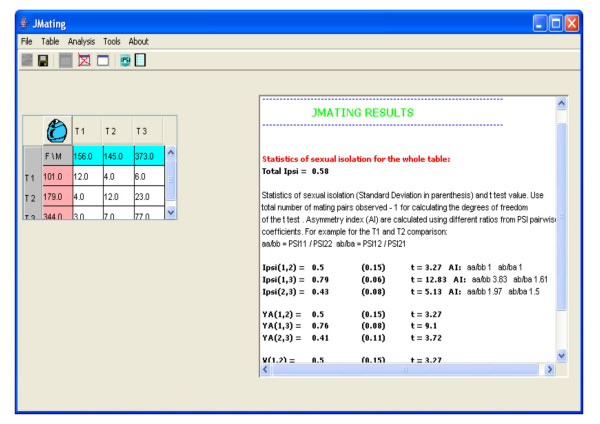
The global analysis provides the best available estimators of sexual isolation (IPSI, Yule's V and YA) [7] and sexual selection (W) [4]. The sexual isolation estimators can only be calculated, in principle, for each pair combination of types (in the example: T1 versus T2, T1 versus T3 and T2 versus T3; see Figure 2). The theoretical resampling standard deviation for each estimator is also provided following the formulae given by different authors [21]. The analysis also includes the calculation of the asymmetry of the deviations from random mating in homotypic and heterotypic pairs. For example, for the combination of T1 and T3, this index would be PSI11/PSI33 and PSI13/PSI31 for homotypic and heterotypic pairs, respectively (PSI estimates the deviations from random mating for each pair type; see below) [7]. Sexual selection estimates (W) are given for each mate type, in males and females separately, relative to the type with the highest sexual fitness [4] (Figure 2).
Figure 2.
Sexual isolation global analysis for Example 1 (see text).
Additionally, the program estimates the global sexual isolation using a modification of the IPSI estimator: the deviations from random mating in homotypic (∑(PSIii)) and heterotypic (∑(PSIij)) pairs are weighted by the number of mating clases.
being n the number of different mating types used in the experiment and PSIjj and PSIij are the sexual isolation estimates for homotypic and heterotypic pair combinations, respectively. To our knowledge, this is the first time that such estimator is proposed and has the advantage to present an overall estimation of sexual isolation when multiple mating types are being used.
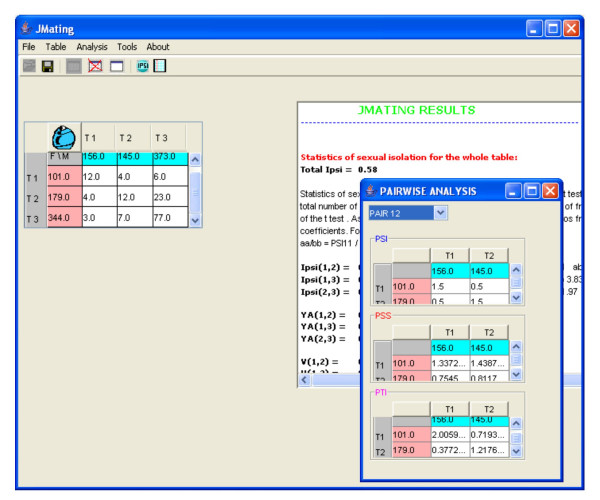
The program also gives the pairwise estimates of total (PTI), sexual isolation (PSI) and sexual selection (PSS) effects from mating frequency data [described in [7]] (Figure 2). The PSI coefficients are the sexual isolation effects for each pair. The PSS coefficients represent the sexual fitness of each pair, and are an additive decomposition of the cross product estimator (W). The PTI coefficients, obtained from the product of PSI and PSS coefficients, represent the combined sexual selection and sexual isolation effects. All these coefficients can be calculated for the whole data set (all mating types) or comparing exclusively data from a given pair of mating types (Figure 3).
Figure 3.
Example of the pairwise analysis for the mating types 1 and 2. It calculates the statistics PTI, PSI and PSS as if only data from mating types 1 (T1) and 2 (T2) were available.
Available statistical tests
Three different types of statistical tests can be accomplished with JMATING. First, a non-parametric G test is available to check for the whole data set if the sexual isolation and sexual selection effects (or both taken together) are significant. The G test has additive properties (Sokal and Rohlf, 1996), and thus it can be decomposed additively into the sexual selection (GS) and sexual isolation (GI) components. This decomposition was developed for laboratory experiments, like Example 2, but it can be used for wild data (like Example 1) if the estimates of the morphs in the population are based on large sample sizes (> 30 for each trait and sex). The program gives the value of GS and GI, and their degrees of freedom, as well as their combined effects (GT = GS + GI), and they can be compared with a χ2 distribution with their corresponding degrees of freedom. Second, JMATING also gives the theoretical sampling distribution for IPSI, YA and V indexes, allowing the use of a t-test for classical parametric inference [26]. This approach, however, has a high false positive rate [21]. Third, JMATING also provides the bootstrap probability for rejecting the null hypothesis. This alternative is rather conservative [21], and so that we recommend not to use multitest corrections for the bootstrap probability values obtained with our program unless a great number of tests (> 10) are being performed.
JMATING resamples 10,000 times the observed values of mating pairs in order to estimate the bootstrap sampling distribution for the estimator (IPSI, YA and Yule's V). Then the program calculates the bootstrap average and standard deviation as well as the two-tail probability of getting a sexual isolation estimate significantly different from zero (equivalent to random mating). JMATING can also calculate the bootstrap mean, standard deviation of the cross-product (W) estimators, as well as its (one tail) probability of getting values significantly smaller than 1 (in our case we consider values of W larger than 1 because we use the largest W as the reference value). Notice that the frequency of mating types in the population (in blue and pink cells) are not resampled, because we assume that they are the population frequencies in the experiment. This is only true in laboratory experimental data (Example 2). However, we allow the option to change this and to resample also non-mated data in the case of field experiments (like in Example 1).
Additionally, JMATING also calculates the bootstrap mean, standard deviation and probabilities for PTI, PSI and PSS coefficients. The program resamples 10,000 times the observed frequencies for getting PTI coefficients and the observed and the expected frequencies (assuming random mating) from mated data when estimating the PSI and PSS coefficients. This procedure is somewhat more conservative than resampling exclusively the observed mating pairs [12], but it allows getting independent bootstrapping for PSI, PSS and PTI coefficients. The latter is convenient if these coefficients are going to be analysed together.
Conclusion
JMATING implements a complete analysis of sexual selection and sexual isolation effects from laboratory or field mating frequency data. The software permits a battery of complementary tests, including bootstrapping. We believe that it will be helpful for those researchers interested on the evolution of reproductive isolation and the study of sexual selection.
Availability and requirements
JMATING is freely available from http://www.uvigo.es/webs/c03/webc03/XENETICA/XB2/JMsoft.htm. The software is written in Java, thus reading the java virtual machine (JVM), which can be freely downloaded from http://java.sun.com. The JMATING software is provided with no guarantee or warranty of any kind. It may be distributed under the terms of the GNU General Public License.
Authors' contributions
A.C.-R. is the exclusive programmer of JMATING, although the algorithms and methods were implemented in collaboration with E.R.-A, which also wrote the manuscript.
Supplementary Material
The program includes one file with two examples of input data for JMating. Example 1: This example represents wild mating data for three ecotypes (mating types) that meet and mate in a particular hybrid zone [12]. Example 2: This example is a laboratory multiple choice experiment between two Drosophila species (mating types) that can meet and hybridize in the wild [6].
Acknowledgments
Acknowledgements
We want to thank to A. Caballero, S. Giokas and D. Posada for checking the program and comments on the manuscript. This work was funded by the MINISTERIO DE EDUCACIÓN Y CIENCIA (CGL2004-03920/BOS and VEM2003-20047) from Spain, and the Xunta de Galicia and the Universidade de Vigo.
Contributor Information
Antonio Carvajal-Rodriguez, Email: ac549@byu.edu.
Emilio Rolan-Alvarez, Email: rolan@uvigo.es.
References
- Coyne JA, Orr HA. Speciation. Suntherland MA: Sinauer; 2004. [Google Scholar]
- Andersson M. Sexual Selection. Princeton: Princeton University Press; 1994. [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila. Evolution. 1997;43:362–381. doi: 10.2307/2409213. [DOI] [PubMed] [Google Scholar]
- Knoppien P. Rare male mating advantage: a review. Biol Rev. 1985;60:81–117. [Google Scholar]
- Spieth HT, Ringo JM. In: Mating behaviour and sexual isolation in Drosophila in The genetics and biology of Drosophila. Ashburner M, Carson HL, Thompson JN, editor. 3c. London: Academic Press; 1983. pp. 224–270. [Google Scholar]
- Coyne JA, Elwyn S, Rolán-Alvarez E. Impact of experimentaldesign on Drosophila sexual isolation studies: direct effect and comparison to field hybridization data. Evolution. 2005;59:2588–2601. [PubMed] [Google Scholar]
- Rolán-Alvarez E, Caballero A. Estimating sexual selection and sexual isolation effects from mating frequencies. Evolution. 2000;54:30–36. doi: 10.1111/j.0014-3820.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Lewontin RC, Kirk D, Crow JF. Selective mating, assortative mating, and inbreeding: definitions and implications. Eugenics Q. 1968;15:141–143. doi: 10.1080/19485565.1968.9987764. [DOI] [PubMed] [Google Scholar]
- Rolán-Alvarez E, Zapata C, Alvarez G. Multilocus heterozygosity and sexual selection in a natural population of the marine snail Littorina mariae (Gastropoda: Prosobranchia) Heredity. 1995;75:17–25. [Google Scholar]
- Johannesson K, Rolán-Alvarez E, Ekendahl A. Incipient reproductive isolation between two sympatric morphs of the intertidal snail Littorina saxatilis. Evolution. 1995;49:1180–1190. doi: 10.2307/2410443. [DOI] [PubMed] [Google Scholar]
- Rolán-Alvarez E, Erlandsson J, Johannesson K, Cruz R. Mechanisms of incomplete prezygotic reproductive isolation in an intertidal snail: testing behavioural models in wild populations. J Evol Biol. 1999;12:879–890. doi: 10.1046/j.1420-9101.1999.00086.x. [DOI] [Google Scholar]
- Cruz R, Carballo M, Conde-Padín P, Rolán-Alvarez E. Testing alternative models for sexual isolation in natural populations of Littorina saxatilis : indirect support for by-product ecological speciation? J Evol Biol. 2004;17:288–293. doi: 10.1111/j.1420-9101.2003.00689.x. [DOI] [PubMed] [Google Scholar]
- Malausa T, Bethenod M-T, Bontemps A, Bourguet D, Cornuet J-M, Ponsard S. Assortative mating in sympatric host races of theEuropean corn borer. Science. 2005;308:258–260. doi: 10.1126/science.1107577. [DOI] [PubMed] [Google Scholar]
- O'Donald P. Genetic models of sexual selection. Cambridge: Cambridge University Press;; 1980. [Google Scholar]
- Davies N, Aiello A, Mallet J, Pomiankowski A, Silberglied RE. Speciation in two neotropical butterflies: extending Haldane's rule. Proc Roy Soc Lond B. 1997;264:845–851. doi: 10.1098/rspb.1997.0118. [DOI] [Google Scholar]
- Tregenza T, Pritchard V, Butlin RK. The origins of premating reproductive isolation: testing hypothesis in the grasshopper Chorthippus parallelus. Evolution. 2000;54:1687–1698. doi: 10.1111/j.0014-3820.2000.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Jiggins CD, Naisbit RE, Coe RL, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- Naisbit RE, Jiggins CD, Mallet J. Disruptive sexualselection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc R Soc Lond B. 2001;268:1849–1854. doi: 10.1098/rspb.2001.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LM. Coefficients of Natural Selection. London: Hutchinson Univ. Library; 1971. [Google Scholar]
- Gilbert DG, Starmer WT. Statistics of sexual isolation. Evolution. 1985;39:1380–1383. doi: 10.2307/2408793. [DOI] [PubMed] [Google Scholar]
- Pérez-Figueroa A, Caballero A, Rolán-Alvarez E. Comparing the estimation properties of different statistics for measuring sexual isolation from mating frequencies. Biol J Linn Soc. 2005;85:307–318. doi: 10.1111/j.1095-8312.2005.00491.x. [DOI] [Google Scholar]
- Anderson WW, McGuire PR. Mating pattern and mating success of Drosophila pseudoobscura karyotypes in large experimental populations. Evolution. 1978;32:416–423. doi: 10.2307/2407608. [DOI] [PubMed] [Google Scholar]
- Majerus M, O'Donald P, Weir J. Evidence for preferential mating in Adalia bipunctata. Heredity. 1982;49:37–49. [Google Scholar]
- The java virtual machine (JVM) runtime environment http://java.sun.com
- Giokas S, Mylonas M, Rolán-Alvarez E. Disassociation between weak sexual isolation and genetic divergence in a hermaphrodite land snail and implications about chirality. J Evol Biol. 2006. [DOI] [PubMed]
- Sokal RR, Rohlf FJ. Biometry. New York: Freeman and co; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The program includes one file with two examples of input data for JMating. Example 1: This example represents wild mating data for three ecotypes (mating types) that meet and mate in a particular hybrid zone [12]. Example 2: This example is a laboratory multiple choice experiment between two Drosophila species (mating types) that can meet and hybridize in the wild [6].