Abstract
OBJECTIVE
To define the term probiotics, to indicate how to identify products that have been proven beneficial, and to assess the quality of evidence regarding probiotics.
QUALITY OF EVIDENCE
A few level I studies support the effectiveness of specific probiotics for certain diagnoses. For most so-called probiotics, however, weak or no evidence supports their effectiveness.
MAIN MESSAGE
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Level I evidence supports use of VSL#3 for maintaining remission of inflammatory colitis. Probiotics for treating vaginal infections, Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14, have level I evidence of effectiveness, but are not available in Canada. Specific probiotics taken for certain indications improve health and have few side effects.
CONCLUSION
Limited but good evidence supports the role of certain probiotics in medical practice. Because consumer pressure will undoubtedly stimulate further interest in probiotics, family doctors need to be informed about them so they can advise their patients appropriately.
Abstract
OBJECTIF
Définir le terme probiotique, indiquer comment identifier les produits dont les effets bénéfiques ont été démontrés et évaluer la qualité des preuves concernant ces agents.
QUALITÉ DES PREUVES
L’efficacité de probiotiques spécifiques dans certaines conditions est appuyée par quelques études de niveau I. La majorité des soi-disant probiotiques, toutefois, ont peu ou pas de preuves d’efficacité.
PRINCIPAL MESSAGE
Les probiotiques sont des micro-organismes vivants qui, en doses adéquates, sont bénéfiques pour la santé de l’hôte. L’utilisation de VSL#3 pour garder en rémission une colite inflammatoire repose sur des preuves de niveau I. L’efficacité des probiotiques Lactobacillus rhamnosus GR-1 et Lactobacillus reuteri RC-14 utilisés pour traiter les infections vaginales repose sur des preuves de niveau I, mais ces agents ne sont pas disponibles au Canada. Certains probiotiques spécifiques utilisés pour des indications particulières améliorent la santé et ont peu d’effets secondaires.
CONCLUSION
Il existe des preuves limitées mais de bonne qualité à l’effet que les probiotiques ont un rôle à jouer dans la pratique médicale. Les consommateurs voudront nécessairement en savoir davantage sur ces produits et le médecin de famille doit donc se renseigner pour mieux conseiller ses patients à leur sujet.
EDITOR’S KEY POINTS.
Probiotics, live microorgansms that confer a benefit to the host when given in sufficient quantities, are becoming widely known. Patients increasingly choose to use probiotics and to ask their family physicians about them.
A few specific probiotics have level I evidence of effectiveness, and they appear to be quite safe. They have been shown to be useful in preventing and treating diarrhea and urogenital infections.
Unfortunately, most probiotics that have been proven effective are unavailable in Canada. They are sold in the United States and Europe. Family physicians recommending probiotics must be aware that only specific brands have proven effects and that packaging must be intact to preserve viability.
POINTS DE REPÈRE DU RÉDACTEUR.
Les probiotiques, des micro-organismes vivants qui en doses suffisantes ont des effets bénéfiques pour l’hôte, sont de plus en plus connus. Un nombre croissant de patients qui décident d’en prendre demandent l’avis de leur médecin de famille.
L’efficacité de quelques probiotiques spécifiques repose sur des preuves de niveau I et leur usage semble plutôt sécuritaire. On a démontré qu’ils sont efficaces pour prévenir et traiter les diarrhées et les infections urogénitales.
Malheureusement, la plupart des probiotiques d’efficacité prouvée ne sont pas disponibles au Canada. On peut les acheter aux États-Unis et en Europe. Le médecin de famille qui recommande des probiotiques doit savoir que seules certaines marques spécifiques se sont montrées efficaces et que l’empaquetage doit être intact pour préserver la viabilité du micro-organisme.
The term probiotics is defined by a United Nations and World Health Organization Expert Panel as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.”1 The general population’s growing interest in natural remedies, including probiotics; the increased scientific and clinical validity of certain probiotic products; and the pending arrival of some of these probiotics in Canada make it important that family physicians understand what probiotics are2 so they can make reliable recommendations to patients.
A bacterium or product containing bacteria is not a probiotic unless the bacteria have been shown to be viable at time of use in sufficient quantity to confer a physiologic health benefit. The organisms themselves must be speciated using appropriate molecular methods and given a designation, such as Lactobacillus rhamnosus GR-1, so that peer-reviewed studies of specific organisms can be followed in PubMed to document the efficacy or lack thereof of the probiotic in defined patient populations. Therefore, Lactobacillus acidophilus is not a probiotic, but L acidophilus NCFM is a probiotic because it has demonstrated some benefit for people who are lactose intolerant.3
Quality of evidence
A PubMed search was conducted using the terms “probiotics,” “Lactobacillus,” and “human clinical trials.” The website of the Food and Agriculture Organization of the United Nations was consulted, as were the websites of various commercial probiotic producers. Randomized, placebo-controlled trials (level I evidence) have established the effectiveness of probiotic strains for treatment of diarrhea, maintenance of remission of inflammatory bowel disease, and prevention of urogenital infections.
How do probiotics work?
The concept of treatment with probiotics comes from a belief that modern humans do not consume or replenish the beneficial microbes in their bodies and that they can do so by taking probiotics.4 Of course, simply eating more bacteria will not in itself guarantee good health. Most probiotic products are foods containing lactobacilli or bifidobacteria, genera with no known virulence that commonly inhabit the healthy gut and vagina. These genera have been used for more than a century in fermented foods.
Historically, ingested probiotic strains were believed to adhere to the gut wall, to block pathogen adhesion and growth,5 and also to give a nonspecific boost to immunity.6 Current thinking suggests that probiotics have other functions, including producing anti-infectives, such as hydrogen peroxide and bacteriocins7; cell signals that strengthen host-cell mucus barriers against pathogen invasion8; and other signals that prevent virulent factors, such as toxins, from being released.
It was once believed that probiotic strains needed to be resistant to acid and bile. If they were not, strains of Lactobacillus delbruekii subsp. bulgaricus and Streptococcus thermophilus in yogurt often would not survive well in the gut. Delivery systems, such as gel matrix coatings on the bacteria themselves and enterocoated capsules, allow bacteria to get past the stomach and to hydrate the small intestine. For example, Lactobacillus strains GR-1 and RC-14 can function in the gut, survive passage, and be excreted in feces.9,10
Treatment of diarrhea
Level I evidence shows that the probiotic strains L rhamnosus GG and Lactobacillus reuteri DSM 12246 (not available in Canada) can reduce the risk of diarrhea in children. A study of 204 undernourished children 6 to 24 months old in Peru showed that once-daily intake of L rhamnosus GG 6 days a week for 15 months resulted in significantly fewer episodes of diarrhea per child per year (5.21 in the treatment group compared with 6.02 in the placebo group, P = .028).11 A prospective, double-blind, randomized, placebo-controlled study of 118 infants (aged 3 to 4 months) showed that consumption of a milk-based formula with or without 1 x 107 colony-forming units/g each of Bifidobacterium lactis BB12 and S thermophilus for 210 ± 127 days resulted in less frequent reports of colic or irritability (P < .001) and less frequent antibiotic use (P < .001).12
Level I evidence exists for probiotic treatment of diarrhea. After 4 to 6 hours of oral rehydration, 140 children aged 1 to 3 months randomly assigned to receive milk with placebo or with L rhamnosus GG had shorter bouts of diarrhea when they were given the probiotics. Duration of diarrhea was reduced from a mean of 3 days to 2.4 days (P = .03).13 In a randomized, placebo-controlled study of 40 patients (6 to 36 months old) with acute diarrhea (75% rotavirus) treatment with L reuteri DSM 12246 for up to 5 days resulted in reduced duration of watery stools (1.6 days in the treatment group compared with 2.9 days in the placebo group, P = .07).14
A systematic review of published, randomized, double-blind, placebo-controlled trials of probiotics for treatment or prevention of acute diarrhea, defined as more than three loose or watery stools in 24 hours in infants and children, showed that probiotics significantly reduced risk of diarrhea lasting more than 3 days.15 A subsequent meta-analysis of 18 eligible studies indicated that coadministration of probiotics and standard rehydration therapy reduced duration of acute diarrhea by approximately 1 day (random-effects pooled estimate -0.8 days [-1.1 to -0.6], P < .001).16
In Canada, if we estimate that 1 million cases of gastroenteritis occur each year, the potential for preventing it or improving treatment with probiotics is worthy of consideration, but only if suitable probiotic products are made available here. The Walkerton, Ont, tragedy highlighted the dangers of gastroenteritis. While a vaccine might reduce Escherichia coli O157 shedding 30% (versus 78% with placebo) 2 days after injection17 in some cows, it is unlikely to address the sporadic nature of shedding or carcass contamination.18,19 Meanwhile, E coli O157:H7 was twice as likely to be detected in control animals’ hides as in those of animals receiving L acidophilus NPC 747.20,21 Probiotic supplementation significantly decreased the number of hides testing positive for E coli O157:H7 (P < .05). Also, adding L acidophilus NP 51 and Propionibacterium freudenreichii to feed for 7 days resulted in cattle being 57% less likely to shed E coli O157 in their feces than controls were (P < .01).22 Although family physicians have no control over livestock practices, they should have some say in approaches to disease prevention and public health.
Inflammatory bowel disease and irritable bowel syndrome
A product called VSL#3 (Seaford Pharma, Toronto, Ont) containing more than 1010 viable bacteria (eight strains) in dried sachet form has level I evidence of effectiveness in ameliorating pouchitis and Crohn’s disease.23,24 In a recent randomized, placebo-controlled study, 36 patients with inflammation and infection in a rectal pouch, in whom remission was induced by 4 weeks of treatment with combined metronidazole and ciprofloxacin, were randomized to receive 6 g of VSL#3 or placebo once daily for 1 year or until relapse.25 Seventeen patients (85%) taking VSL#3 were still in remission 1 year later, as was one patient (6%) taking placebo (P < .0001). The inflammatory bowel disease questionnaire score remained high in the VSL#3 group (P = .3), but decreased in the placebo group (P = .0005).
Evidence is lacking for other strains, such as L rhamnosus GG. A 45-patient study failed to show that this probiotic prevented endoscopically observed recurrence of Crohn’s disease or reduced severity of recurrent lesions.26
Evidence is also lacking for the efficacy of probiotics for irritable bowel syndrome (IBS). Twice-daily use of VSL#3 was not particularly effective in one study of gastrointestinal transit and symptoms.27 Lactobacillus plantarum 299V was not shown to be effective in a double-blind, placebo-controlled, cross-over, 4-week trial in 12 previously untreated patients with IBS,28 nor was L rhamnosus GG in another study of 25 patients.29
Urogenital infections
Family practitioners see many women with non–sexually transmitted urogenital infections (urinary tract infections [UTIs], bacterial vaginosis, yeast vaginitis, and group B streptococcal colonization). Unfortunately, there are no good treatments available in Canada. An Internet search (using the search terms “probiotics AND urinary OR vaginal”) showed that women received misinformation about the cause and treatment of these ailments and were recommended unproven products. For example, http://www.natren.com incorrectly states that hygiene, bubble baths, and underwear are associated with increased risk of UTI.30 The website recommends treatment of UTI with “2 capsules each of L acidophilus and Bifidobacterium bifidum (or 1 teaspoon each of powder), along with 1 teaspoon L delbruekii subsp. bulgaricus powder mixed in 6 to 8 ounces unchilled filtered water, twice daily, for 14 days.” No known efficacy studies support this recommendation. Symptomatic UTIs require antibiotic therapy.
Other examples can be found at http://www.uaslabs.com (or in material published by the owner), http://www.customprobiotics.com, and http://www.khadergroup.com where statements lead readers to believe that the products are probiotics and are proven to be beneficial for urogenital infections. These statements cannot be confirmed by the literature.
Fermalac (Rosell-Lallemand, Montreal, Que) is advertised at the http://www.only-in-canada.com website where its lactobacilli content is referred to as “the Xena warrior princesses of the vagina,” with a claim that it prevents yeast infections. The http://www.berrytechnologies.ca website claims that Fermalac will “stop recurrent bacterial or yeast infections from returning after an antibiotic or antifungal treatment.” This product is supported by one peer-reviewed Czech study on prevention of colpitis (inflammation of the vagina) during pregnancy,31 so the evidence is level II.
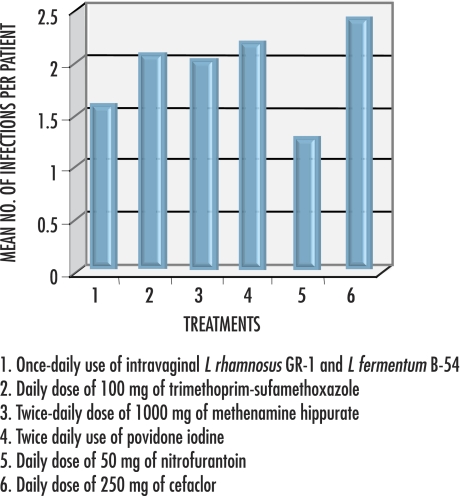
There is level I evidence of the effectiveness of L rhamnosus GR-1 and Lactobacillus reuteri (formerly fermentum) B-54 and RC-14 in restoring vaginal lactobacilli and reducing infections in more than 50% of women after daily oral use and in 79% of women after once-weekly vaginal use.10,32-34 Results of 25 women’s once-weekly vaginal use of L rhamnosus GR-1 and L fermentum B-5433 compare favourably with results from various daily antibiotic regimens and twice-daily vaginal washes35-37 for breakthrough UTI (Figure 138-41).
Figure 1. Mean number of breakthrough infections per year in women taking Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum B-54 compared with number of infections among those using various other treatments38-41.
Mean number of urinary tract infections was reduced from 6 per year with preventive treatment to less than 2.5 per year. The data suggest that weekly vaginal probiotic use compares with daily antibiotic treatment or twice-daily vaginal washes.
In a study published in 1989, 15 women taking long-term daily prophylaxis with norfloxacin had no episodes of UTI.42 Since then, escalating resistance to fluoroquinolones in some countries,43,44 increased resistance to trimethoprim-sulfamethoxazol45,46 (formerly recommended daily for up to 5 years38), decreasing rates of UTIs due to E coli,46,47 and the adverse effects of antibiotics (reported as 28% for ciprofloxacin, 34% for nitrofurantoin, and 38% for trimethoprim-sulfamethoxazole in one study48) suggest that probiotics to prevent UTIs should be further studied.
Orally administered lactobacilli reach the vagina via the anus and the perineal and vulval skin, as do pathogens, irrespective of hygiene.30 For reasons not yet understood, not all lactobacilli are able to colonize the vagina.39-41,49
Labeling and how to know what to recommend
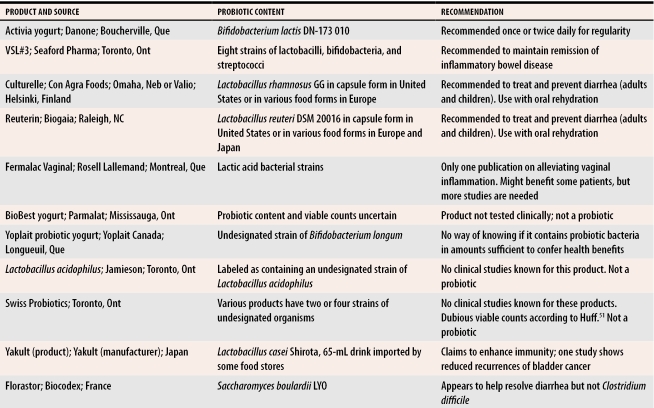
Some products in Canada claim that they contain Lactobacillus sporogenes, an organism that does not exist.50 Currently, Danone’s Activia yogurt containing B lactis DN-173 010 for regularity, VSL#3’s product for inflammatory bowel disease, and perhaps Rosell Lallemand’s Fermalac vaginal suppositories for bacterial infections, are this country’s only proven probiotics. Across the border, Culturelle containing L rhamnosus GG and Reuterin containing L reuteri SD2112 are reliable remedies for diarrhea in adults and children (Table 151). Unless a probiotic product is manufactured under the best possible conditions and packaged extremely carefully, the contents could die steadily at room temperature.
Table 1.
Products that Canadian patients might ask their physicians to comment on
Safety
The safety record of probiotics is remarkable considering that more than 20 billion doses are estimated to be used each year.52 Nevertheless, there have been a few reports of bacteremia. One retrospective analysis showed 89 cases of lactobacilli bacteremia, of which 11 might have been related to probiotic L rhamnosus GG use.53 In 82% of these cases, patients had severe or fatal comorbidity.
Physicians are presented with a dilemma in giving seriously ill hospital patients (with pancreatitis or liver transplants and those undergoing abdominal surgery) probiotics. Studies show that such patients have benefited from daily intake of L plantarum 299.54-56 They had fewer infectious complications of surgery. Animal studies showed less severe intra-abdominal infection with Lactobacillus R2LC treatment.57 Some patients with short bowel syndrome58 and leukemia59 might be at risk of bacteremia from probiotics, yet L reuteri SD2112 has been safely taken by HIV and AIDS patients.60 Probiotics are generally regarded as safe, but physicians should monitor their use in high-risk patients.61
Conclusion
The increasing availability of probiotic products makes it important that family physicians understand what to look for when making recommendations.51 While products are available in Canada only for regularity, inflammatory bowel disease, and vaginal inflammation, patients gain access to products from other countries and seek advice about them from their local physicians. Examining the label for strain speciation and designation and shelf-life and learning about clinically proven strains of probiotics will help physicians feel comfortable recommending suitable probiotic supplements.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Acknowledgments
Research undertaken in our Centre is supported by the Ontario Challenge Fund and the Natural Sciences and Engineering Research Council of Canada. Dr Reid is Secretary of the International Scientific Association for Probiotics and Prebiotics.
Biography
Dr Reid and Dr Hammond are researchers in the Canadian Research and Development Centre for Probiotics at the Lawson Health Research Institute in London, Ont. Dr Reid is a Professor in the Departments of Microbiology and Immunology and Surgery at the University of Western Ontario in London. Dr Hammond is a family practitioner and an Assistant Professor in the Department of Family Medicine at the University of Western Ontario.
Footnotes
Competing interests: Dr Reid owns strains Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri (formerly fermentum) B-54 and RC-14.
References
- 1.Food and Agriculture Organization of the United Nations and World Health Organization. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Geneva, Switz: Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report; 2001. [cited 2005 September 13]. Available at: ftp://ftp.fao.org/docrep/fao/meeting/009/y6398e.pdf. [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. Geneva, Switz: Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report; 2002. [cited 2005 September 13]. Available at: http://www.fao.org/es/ESN/food/food_probio_en.stm. [Google Scholar]
- 3.Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001;84(2):319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 4.Mann NJ. Paleolithic nutrition: what can we learn from the past? Asia Pac J Clin Nutr. 2004;13(Suppl):17. [Google Scholar]
- 5.Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties which may influence bacterial interference in the urinary tract. J Urol. 1987;138:330–335. doi: 10.1016/s0022-5347(17)43137-5. [DOI] [PubMed] [Google Scholar]
- 6.Perdigon G, Alvarez S, Rachid M, Aguero G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995;78(7):1597–1606. doi: 10.3168/jds.S0022-0302(95)76784-4. [DOI] [PubMed] [Google Scholar]
- 7.Ocana VS, Elena Nader-Macias M. Production of antimicrobial substances by lactic acid bacteria II: screening bacteriocin-producing strains with probiotic purposes and characterization of a Lactobacillus bacteriocin. Methods Mol Biol. 2004;268:347–353. doi: 10.1385/1-59259-766-1:347. [DOI] [PubMed] [Google Scholar]
- 8.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardiner G, Heinemann C, Baroja ML, Bruce AW, Beuerman D, Madrenas J, et al. Oral administration of the probiotic combination Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 for human intestinal applications. Int Dairy J. 2002;12:191–196. [Google Scholar]
- 10.Morelli L, Zonenenschain D, Del Piano M, Cognein P. Utilization of the intestinal tract as a delivery system for urogenital probiotics. J Clin Gastroenterol. 2004;38(6 Suppl):107–110. doi: 10.1097/01.mcg.0000128938.32835.98. [DOI] [PubMed] [Google Scholar]
- 11.Oberhelman RA, Gilman RH, Sheen P, Taylor DN, Black RE, Cabrera L, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr. 1999;134(1):15–20. doi: 10.1016/s0022-3476(99)70366-5. [DOI] [PubMed] [Google Scholar]
- 12.Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79(2):261–267. doi: 10.1093/ajcn/79.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997;24:399–404. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(Suppl 2):17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 16.Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig Dis Sci. 2002;47(11):2625–2634. doi: 10.1023/a:1020501202369. [DOI] [PubMed] [Google Scholar]
- 17.Potter AA, Klashinsky S, Li Y, Frey E, Townsend H, Rogan D, et al. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine. 2004;22(3-4):362–369. doi: 10.1016/j.vaccine.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Ogden ID, MacRae M, Strachan NJ. Is the prevalence and shedding concentrations of E. coli O157 in beef cattle in Scotland seasonal? FEMS Microbiol Lett. 2004;233(2):297–300. doi: 10.1016/j.femsle.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Edrington TS, Hume ME, Looper ML, Schultz CL, Fitzgerald AC, Callaway TR, et al. Variation in the faecal shedding of Salmonella and E. coli O157:H7 in lactating dairy cattle and examination of Salmonella genotypes using pulsed-field gel electrophoresis. Lett Appl Microbiol. 2004;38(5):366–372. doi: 10.1111/j.1472-765X.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- 20.Brashears MM, Galyean ML, Loneragan GH, Mann JE, Killinger-Mann K. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J Food Prot. 2003;66(5):748–754. doi: 10.4315/0362-028x-66.5.748. [DOI] [PubMed] [Google Scholar]
- 21.Elam NA, Gleghorn JF, Rivera JD, Galyean ML, Defoor PJ, Brashears MM, et al. Effects of live cultures of Lactobacillus acidophilus (strains NP45 and NP51) and Propionibacterium freudenreichii on performance, carcass, and intestinal characteristics, and Escherichia coli strain O157 shedding of finishing beef steers. J Animal Sci. 2003;81(11):2686–2698. doi: 10.2527/2003.81112686x. [DOI] [PubMed] [Google Scholar]
- 22.Younts-Dahl SM, Galyean ML, Loneragan GH, Elam NA, Brashears MM. Dietary supplementation with Lactobacillus- and Propionibacterium-based direct-fed microbials and prevalence of Escherichia coli O157 in beef feedlot cattle and on hides at harvest. J Food Prot. 2004;67(5):889–893. doi: 10.4315/0362-028x-67.5.889. [DOI] [PubMed] [Google Scholar]
- 23.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 24.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 25.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51(3):405–409. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47(11):2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan MA, O’Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. pp. 294–301. [DOI] [PubMed]
- 30.Krieger JN. Urinary tract infections: what’s new? J Urol. 2002;168:2351–2358. doi: 10.1016/S0022-5347(05)64145-6. [DOI] [PubMed] [Google Scholar]
- 31.Chlebecek J, Reich I. [Fermalac Vaginal (Rougier Inc.) in the prevention of colpitis in pregnancy]. Cesk Gynekol. 1993;58(5):237–238. [PubMed] [Google Scholar]
- 32.Reid G, Millsap K, Bruce AW. Implantation of Lactobacillus casei var rhamnosus into the vagina. Lancet. 1994;344:1229. doi: 10.1016/s0140-6736(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 33.Reid G, Bruce AW, Taylor M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol Ther. 1995;23:32–45. [Google Scholar]
- 34.Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 35.Brumfitt W, Hamilton-Miller JM. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years’ experience. J Antimicrob Chemother. 1998;42:363–371. doi: 10.1093/jac/42.3.363. [DOI] [PubMed] [Google Scholar]
- 36.Brumfitt W, Hamilton-Miller JM, Gargan RA, Cooper J, Smith GW. Long-term prophylaxis of urinary infections in women: comparative trial of trimethoprim, methenamine hippurate and topical povidone-iodine. J Urol. 1983;130:1110–1114. doi: 10.1016/s0022-5347(17)51709-7. [DOI] [PubMed] [Google Scholar]
- 37.Brumfitt W, Hamilton-Miller JM, Walker S, Roberts D. Cefaclor as a prophylactic agent for recurrent urinary infections: a comparative trial with macrocrystalline nitrofurantoin. Drugs Exp Clin Res. 1992;18:239–244. [PubMed] [Google Scholar]
- 38.Ronald AR. Urinary tract infections: the efficacy of trimethoprim/sulfamethoxazole. Clin Ther. 1980;3(3):176–189. [PubMed] [Google Scholar]
- 39.Reid G, Burton J, Hammond JA, Bruce AW. Nucleic acid–based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli. J Med Food. 2004;7(2):223–228. doi: 10.1089/1096620041224166. [DOI] [PubMed] [Google Scholar]
- 40.Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care. 1994;12(4):239–243. doi: 10.3109/02813439409029247. [DOI] [PubMed] [Google Scholar]
- 41.Reid G. In vitro analysis of a dairy strain of Lactobacillus acidophilus NCFMTM as a possible probiotic for the urogenital tract. Int Dairy J. 2000;10:415–419. [Google Scholar]
- 42.Nicolle LE, Harding GK, Thompson M, Kennedy J, Urias B, Ronald AR. Prospective, randomized, placebo-controlled trial of norfloxacin for the prophylaxis of recurrent urinary tract infection in women. Antimicrob Agents Chemother. 1989;33(7):1032–1035. doi: 10.1128/aac.33.7.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO. SENS Project. J Antimicrob Chemother. 2003;51:69–76. doi: 10.1093/jac/dkg028. [DOI] [PubMed] [Google Scholar]
- 44.Goettsch W, van Pelt W, Nagelkerke N, Hendrix MG, Buiting AG, Petit PL, et al. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in The Netherlands. J Antimicrob Chemother. 2000;46:223–228. doi: 10.1093/jac/46.2.223. [DOI] [PubMed] [Google Scholar]
- 45.Gupta K, Stamm WE. Outcomes associated with trimethoprim/sulphamethoxazole (TMP/SMX) therapy in TMP/SMX resistant community-acquired UTI. Int J Antimicrob Agents. 2002;19(6):554–556. doi: 10.1016/s0924-8579(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 46.Reid G, Seidenfeld A. Drug resistance amongst uropathogens isolated from women in a suburban population: laboratory findings over 7 years. Can J Urol. 1997;4:432–437. [PubMed] [Google Scholar]
- 47.Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis. 2001;33:89–94. doi: 10.1086/320880. [DOI] [PubMed] [Google Scholar]
- 48.Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(Suppl A):67–75. [PubMed] [Google Scholar]
- 49.Cadieux P, Burton J, Kang CY, Gardiner G, Braunstein I, Bruce AW, et al. Lactobacillus strains and vaginal ecology. JAMA. 2002;287:1940–1941. doi: 10.1001/jama.287.15.1940. [DOI] [PubMed] [Google Scholar]
- 50.Sanders ME, Morelli L, Bush S. Lactobacillus sporogenes is not a Lactobacillus probiotic. ASM News. pp. 385–386.
- 51.Huff BA. Caveat emptor. Probiotics might not be what they seem. Can Fam Physician. 2004;50:583–587. [PMC free article] [PubMed] [Google Scholar]
- 52.Reid G. Lactobacillus safety as probiotic agents. Clin Infect Dis. 2002;35:349–350. doi: 10.1086/342477. [DOI] [PubMed] [Google Scholar]
- 53.Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis. 2004;38:62–69. doi: 10.1086/380455. [DOI] [PubMed] [Google Scholar]
- 54.Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific Lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89(9):1103–1107. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 55.Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, et al. Early enteral supply of Lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123–127. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 56.Rayes N, Hansen S, Seehofer D, Muller AR, Serke S, Bengmark S, et al. Early enteral supply of fiber and lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18(7-8):609–615. doi: 10.1016/s0899-9007(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 57.Thorlacius H, Nobaek S, Wang XD, Andersson R, Molin G, Bengmark S, et al. Lactobacilli attenuate bacteremia and endotoxemia associated with severe intra-abdominal infection. Surgery. 2003;134(3):467–473. doi: 10.1067/s0039-6060(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 58.Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr. 2004;38:457–458. doi: 10.1097/00005176-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez L, Batlle M, Oriol A, Ribera JM. [Lactobacillus spp. bacteremia in a patient with neutropenia secondary to the treatment of acute leukemia]. Med Clin (Barc) 2001;117(19):758. doi: 10.1016/s0025-7753(01)72248-8. [DOI] [PubMed] [Google Scholar]
- 60.Wolf BW, Wheeler KB, Ataya DG, Garleb KA. Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food Chem Toxicol. 1998;36(12):1085–1094. doi: 10.1016/s0278-6915(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 61.Antony SJ. Lactobacillemia: an emerging cause of infection in both the immunocompromised and the immunocompetent host. J Natl Med Assoc. 2000;92(2):83–86. [PMC free article] [PubMed] [Google Scholar]