Abstract
OBJECTIVE
To review the epidemiology and disease manifestations of West Nile virus (WNV) in North America and to describe the current status of therapeutic approaches and vaccines for treating or preventing viral illness.
QUALITY OF EVIDENCE
Since 1999, research initiatives investigating the ecology, epidemiology, and biology of WNV have increased substantially. These studies provide a foundation for understanding current activity and predicting future activity and for describing the effect of WNV on human health.
MAIN MESSAGE
West Nile virus is transmitted to humans primarily through bites from infected mosquitoes. Most people infected have no symptoms; a few have clinical manifestations ranging from febrile illness to neurologic syndromes and possibly death. Risk of serious disease increases with age, and substantial long-term morbidity has been observed in patients who develop severe neurologic illness. No specific antiviral therapy or vaccine currently exists.
CONCLUSION
West Nile virus has established itself in North America and has become an important public health concern. Decreasing risk of virus-associated illness requires seasonal preventive and control measures.
Abstract
OBJECTIF
Faire le point sur l’épidémiologie et les manifestations cliniques du virus du Nil occidental (VNO) en Amérique du Nord et sur les données actuelles concernant les vaccins et traitements utilisés pour prévenir et traiter cette infection virale.
QUALITÉ DES PREUVES
Depuis 1999, le nombre d’études sur l’écologie, l’épidémiologie et la biologie du VNO a augmenté considérablement. Ces études nous ont permis de mieux comprendre l’activité actuelle de l’infection, de prévoir son activité future et de décrire l’effet du VNO sur la santé humaine.
PRINCIPAL MESSAGE
Le VNO se transmet à l’homme par les piqûres de moustiques infectés. La plupart des personnes infectées ne présentent pas de symptômes; quelques-unes auront des manifestations variant d’un simple accès fébrile à des syndromes neurologiques parfois létaux. Le risque d’une maladie grave augmente avec l’âge et en cas d’atteintes neurologiques sévères, certains patients ont présenté une morbidité à long terme. Il n’existe présentement aucun vaccin ni traitement antiviral spécifique.
CONCLUSION
Le VNO est bien installé en Amérique du Nord et il représente maintenant un important problème de santé public. Des mesures de contrôle et de prévention saisonnières seront nécessaires pour réduire le risque de maladies associées au virus.
EDITOR’S KEY POINTS.
Since 1999, West Nile virus has established itself firmly in North America; 15000 cases were documented in the United States and Canada in 2002 and 2003. In Canada, 1839 cases were identified between January 2001 and March 2005; 36 deaths were attributed to West Nile virus.
About 80% of people infected are asymptomatic; 95% of the rest have symptoms of only mild viremia. Very few (<1%) go on to develop symptoms of encephalitis or flaccid paralysis. Risk of serious disease increases with age and in immunocompromised people.
Vaccines are currently under development, but targeting the vulnerable population is challenging. Personal protection against mosquitoes and reducing mosquito populations remain the main protection strategies.
POINTS DE REPÈRE DU RÉDACTEUR.
Apparu en 1999, le virus du Nil occidental est maintenant solidement établi en Amérique du Nord; en 2002 et 2003, 15000 cas ont été diagnostiqués aux États-Unis et au Canada. Au Canada, 1839 cas ont été identifiés entre janvier 2001 et mars 2005 et 36 décès ont été attribués à ce virus.
Environ 80% des personnes infectées demeurent asymptomatiques; parmi les autres, 95% présenteront les symptômes d’une virémie bénigne. Très peu (<1%) développeront des symptômes d’encéphalite ou de paralysie flasque. Le risque de présenter une forme grave augmente avec l’âge et chez les immunodéprimés.
Des vaccins sont en préparation, mais il sera difficile de cibler la population vulnérable. Les principales mesures préventives demeurent la protection contre les piqûres et la réduction de la population de moustiques.
West Nile virus (WNV) is an arthropod-borne virus (arbovirus) belonging to the genus Flavivirus, family Flaviviridae.1 The virus is maintained in nature through a bird-mosquito-bird transmission cycle, but mosquitoes can transmit the virus to nonamplifying hosts, such as horses and humans, which do not develop high levels of viremia.2-4 In temperate climates, risk of human infection with WNV rises during midsummer to late summer when the number of infected mosquitoes that feed on humans increases.
The virus was first isolated in 1937 from the blood of a febrile patient in the West Nile province of Uganda.5 Since arriving in North America in 1999, WNV has spread throughout the United States and Canada and into Mexico and the Caribbean.6,7 The virus has emerged as a globally important pathogen with far-reaching implications for public health.
Quality of evidence
Using PubMED and MEDLINE and the search words “West Nile virus,” “arbovirus,” and “Flavivirus,” we identified more than 900 articles dealing with clinical and basic microbiologic aspects of WNV. Most of these have been published during the last 5 years. We chose articles that provided detailed information on newly documented clinical aspects of viral disease and recent diagnostic developments. Epidemiologic information was obtained from several recent publications, but the most current data were obtained from Centres for Disease Control and Public Health Agency of Canada websites. Currently, there are few treatment options, and their effectiveness requires further study.
Epidemiology
In 2002 and 2003, WNV was responsible for two or more of the largest arboviral epidemics ever observed in the western hemisphere; more than 15 000 symptomatic infections were documented in the United States and Canada.6,8-10 About 6000 of these cases were diagnosed as meningitis or encephalitis, making these outbreaks the largest WNV meningoencephalitis epidemics ever recorded. Between January 2001 and March 14, 2005, 1839 cases of WNV-associated illnesses and 36 deaths were reported in Canada (virus activity was reported in seven provinces).
The 2003 Canadian epidemic of WNV disease was the largest ever documented; most cases occurred in the Prairie Provinces (Manitoba, Saskatchewan, Alberta); 848 cases were in Saskatchewan alone.10 In 2004, cases were reported in Quebec, Ontario, Manitoba, Saskatchewan, and Alberta, but the number of human cases (26) was substantially lower than the year before, possibly due to a combination of climatic and ecologic factors.10
Transmission and course of infection
Transmission of WNV occurs primarily through bites of infected mosquitoes. Less common modes of transmission include infected blood, tissues, and organs; needle-stick or sharps injuries; and transmission through the placenta or breast milk.11 A genomic test for detecting WNV in blood donations was introduced in 2003 to screen donors in Canada and the United States; more than 1000 viremic donors were identified.12-15 Implementation of this program might have saved many lives.
In humans, incubation ranges from 2 to 15 days before onset of illness16; prolonged incubation periods (up to 21 days) have been observed in patients following organ transplantation.17 Initial replication of WNV probably takes place in dendritic skin cells that migrate to lymph nodes where a second round of viral amplification occurs. The virus then enters the bloodstream. The infectious period can begin up to 6 or 7 days before onset of clinical illness, but ends soon after symptoms start. How the virus travels to organs and tissues has been described.18 A study involving patients with various types of cancers inoculated with the Egypt isolate of WNV found the virus was most frequently isolated in lungs, spleens, and lymph nodes; less frequently in hearts, small intestines, and spinal cords; and rarely in livers, kidneys, skeletal muscles, large intestines, pancreata, and brains.
Clinical features of infection and diagnosis
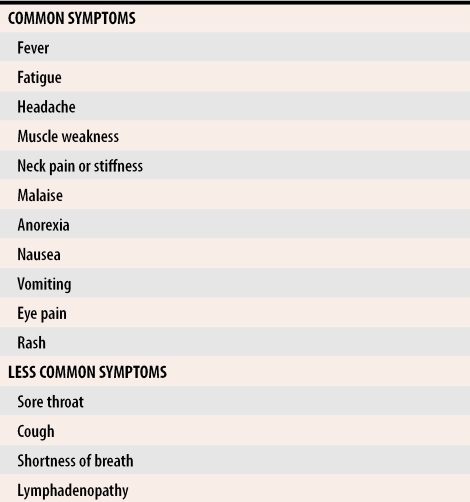
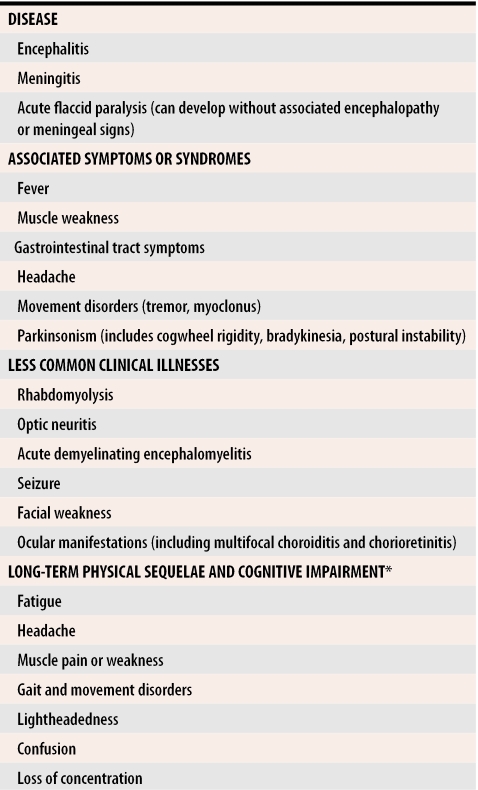
About 80% of WNV infections are asymptomatic, but some patients have symptoms ranging from mild febrile illness (>95% of patients) to meningitis or encephalitis (<1% of patients).19,20 People infected with WNV could experience fever, headache, and other nonspecific symptoms that typically last for several days (Table 1, Table 2). Patients can also have a variety of other signs and symptoms including nausea, vomiting, macular-papular rash, chills, abdominal pain, muscle weakness, photophobia, conjunctivitis, movement disorders, parkinsonism, confusion, and slurred speech. For some patients, a febrile prodrome is immediately followed by encephalitis. More severe neurologic manifestations, such as a syndrome resembling poliomyelitis and acute flaccid paralysis, have been seen.20
Table 1.
Clinical features of West Nile fever
Table 2.
West Nile virus neurologic disease
*Can last 12 to 18 months and might be more severe with increasing age.
Previous characterizations of West Nile febrile illness have generally described it as a mild, acute syndrome lasting 3 to 6 days, but West Nile fever can be a serious disease that takes several months to resolve.21 For patients who develop severe neurologic illness, recovery can take a long time (Table 2).22,23 Some patients experience serious long-term sequelae that include physical symptoms, such as muscle weakness, fatigue, and headache, and effects on cognitive function including confusion, depression, and memory loss. Recovery can take more than a year; in extreme cases, lingering effects might last a lifetime.22 Sequelae from acute flaccid paralysis are lifelong.
Risk of neurologic disease increases with age and underlying medical conditions; diabetes and heart disease, for example, can increase risk.7,23 Transplant recipients appear more likely to be severely ill upon exposure to WNV, probably because of immunosuppression.24
People of all ages can get WNV-associated illness.11 Animal studies suggest that genetic factors influence severity of disease.25 Incidence of viral meningitis in temperate climates is highest in the summer and fall, and outbreaks of enterovirus infection in younger people overlap with increased risk of WNV infection. Although incidence of neurologic disease is low, WNV should be included in the differential diagnosis of children who develop aseptic meningitis or encephalitis during times of mosquito activity.
The front-line laboratory diagnostic assay for WNV infection tests serum (and cerebrospinal fluid if a patient exhibits neurologic disease) for presence of WNV-reactive immunoglobulin-M (IgM) antibody using either in-house or commercial enzyme-linked immunosorbent assays (ELISA).16,26 If results of IgM ELISA are positive, it might be necessary to evaluate cross-reactivity with other flaviviruses by performing a viral neutralization assay to document cases. A second serum sample obtained 10 to 15 days after the first is helpful in confirming WNV infection by demonstrating a fourfold rise in specific neutralizing antibody titre. The IgM antibody to WNV can persist for more than a year, which might cause confusion when those with compatible illness who reside in places that experienced epidemics the previous year are tested.27 Demonstration of seroconversion might be needed to identify patients who test positive, but were exposed during the previous season.
Laboratories can also test cerebrospinal fluid for presence of WNV nucleic acid, and although sensitivity is low (about 50%), positive results confirm WNV infection of the central nervous system.28 Serum samples taken early during the acute phase of infection (usually less than 1 week after symptom onset) can be negative by serology but positive on a nucleic acid detection test. Combining IgM ELISA and WNV nucleic acid detection tests might be warranted to ensure the most sensitive testing. Sometimes, WNV can be cultured from cerebrospinal fluid and blood, but the sensitivity of viral culture is usually extremely poor, so culturing is not recommended.29
Treatment
To date, the only treatments for WNV infection are supportive. Ribavirin and interferon-alpha-2b inhibit replication of the virus in vitro, but no controlled clinical trials using either agent have taken place.30 Some human case reports indicate that treatment with intravenous immunoglobulin (IVIG) could help recovery from infection31 but, because the precise timing of infection is usually unknown and most people do not go to their doctors before severe illness, administering antibodies is unlikely to be useful as a therapy. As a prophylaxis, IVIG could prove useful for those at high risk of infection due to needle-stick exposure. Hamsters who had been given immunoglobulin 24 hours before infection were completely protected from infection.32 This observation indicates that passive immunization might be effective for short-term, immediate exposures in people at high risk. Before IVIG can be used as therapy, controlled clinical trials should be carried out to better determine the dose, timing, and efficacy of the procedure.
Vaccines
Vaccines for WNV vaccine are in various stages of development and testing.33 An experimental recombinant WNV vaccine was constructed by inserting the premembrane (prM) and envelope (E) genes from the New York 1999 virus into an infectious clone of the yellow fever 17D vaccine virus. This hybrid elicits a strong and potentially long-lasting humoral immune response in hamsters, and additional trials involving non-human primates have promising results.34 Other vaccine candidates include recombinant DNA vaccines expressing the prM and E or capsid proteins and a recombinant E protein subunit preparation.33 Several of these vaccines might be effective, but the benefits and risks of vaccination remain to be determined. Due to the low incidence of disease in humans and the sporadic nature of most outbreaks, it could be difficult to select human populations for vaccination and to assess the practical aspects of a human vaccine. The most appropriate initial use for vaccines might be for immunizing elderly people in high-risk areas.
Prevention
Although various modes of transmission have been identified, the major risk factor for exposure is being bitten by an infected mosquito. In many areas, coordinated mosquito-control programs have been put in place as part of preventive measures against WNV.35 Personal protective measures should still be emphasized as a strategy for reducing risk. People should apply insect repellent to their skin, wear protective clothing when exposed to mosquitoes, and minimize outdoor activities during peak mosquito-feeding times.
The most effective repellent for use on the skin against mosquitoes is N,N-diethyl-m-toluamide (DEET)36; DEET or permethrin also can be applied directly to clothing to repel mosquitoes. The Canadian Paediatric Society recommends use of formulations no greater than 10% DEET on children older than 2 years and advises against using DEET on infants younger than 6 months. Although DEET is absorbed into the system through the skin and has been shown to cross the placenta, studies of both animals and humans indicate that DEET can be used during pregnancy without adversely affecting fetuses.37
Conclusion
In Canada and the United States, WNV has been responsible for large outbreaks of febrile and neurologic disease. Although people of all ages can be affected, elderly and immunocompromised people are most at risk of serious illness. No specific treatments currently exist for WNV disease, so continued monitoring and surveillance of the virus is warranted since measures to prevent and control it are critical for decreasing risk of infection. The long-term effect of this virus on public health in North America is unknown; future epidemics and spread of the virus remain distinct possibilities.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Biography
Dr Drebot is Chief of the Viral Zoonoses section in the National Microbiology Laboratory at the Public Health Agency of Canada in Winnipeg, Man. Dr Artsob is Director of the Zoonotic Diseases and Special Pathogens Program in the National Microbiology Laboratory. Both Dr Drebot and Dr Artsob are cross-appointed as Adjunct Professors in the Department of Medical Microbiology at the University of Manitoba.
Footnotes
Competing interests: None declared
References
- 1.Mackenzie JS, Barrett ADT, Deubel V. The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese encephalitis and West Nile viruses. New York, NY: Springer-Verlag; 2002. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 2.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turrell MJ, Sardelis MR, O’Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese encephalitis and West Nile viruses. New York, NY: Springer-Verlag; 2002. pp. 241–252. [DOI] [PubMed] [Google Scholar]
- 4.Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, et al. Experimental infection of horses with West Nile virus. Emerg Infect Dis. 2002;8:380–386. doi: 10.3201/eid0804.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Tropic Med. 1940;20:471–492. [Google Scholar]
- 6.Drebot MA, Lindsay R, Barker I, Buck PA, Fearon M, Hunter F, et al. West Nile virus surveillance and diagnostics: a Canadian perspective. Can J Infect Dis. 2003;14:105–114. doi: 10.1155/2003/575341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granwehr BP, Lillibridge KM, Higgs S, Mason PW, Aronson JF, Campbell GA, et al. West Nile virus: where are we now? Lancet Infect Dis. 2004;4:547–556. doi: 10.1016/S1473-3099(04)01128-4. [DOI] [PubMed] [Google Scholar]
- 8.O’Leary DR, Marfin AA, Montgomery SP, Kipp AM, Lehman JA, Biggerstaff BJ, et al. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004;4:61–70. doi: 10.1089/153036604773083004. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. West Nile virus activity—United States, November 20-25, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1160. [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada. West Nile virus surveillance information. Ottawa, Ont: Public Health Agency of Canada; 2005. [cited 2005 June 9]. Available at: http://www.phac-aspc.gc.ca/wnv-vwn/index.html. [Google Scholar]
- 11.Centers for Disease Control. Provisional surveillance summary of the West Nile virus epidemic—United States, January–November 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1129–1133. [PubMed] [Google Scholar]
- 12.Sibbald B. Canada will check donor blood for West Nile virus if test available. CMAJ. 2003;168:207. [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. Update: West Nile virus screening of blood donations and transfusion-associated transmission—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;53:281–284. [PubMed] [Google Scholar]
- 14.Health Canada. Guidance document: measures to prevent West Nile virus transmission through cells, tissues and organs for transplantation and assisted reproduction 2004. Ottawa, Ont: Health Canada; 2004. [cited 2005 June 9]. Available at: http://www.hc-sc.gc.ca/hpfb-dgpsa/bgtd-dpbtg/wnv2004_ctoguidance_e.html. [Google Scholar]
- 15.Centers for Disease Control, Division of Vector-borne Infectious Diseases. West Nile virus. Atlanta, Ga: Centers for Disease Control; 2004. [cited 2005 June]. Available at: http://www.cdc.gov/ncidod/dvbid/westnile/index.htm. [Google Scholar]
- 16.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137(3):173–179. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 18.Southam CM. Induced virus infections in man by the Egypt isolates of West Nile virus. Am J Tropic Med Hyg. 1954;3:19–50. doi: 10.4269/ajtmh.1954.3.19. [DOI] [PubMed] [Google Scholar]
- 19.Petersen LR, Marfin AA, Gubler DJ. West Nile virus. JAMA. 2003;290:524–528. doi: 10.1001/jama.290.4.524. [DOI] [PubMed] [Google Scholar]
- 20.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, et al. Neurologic manifestations and outcome of West Nile virus infection [published erratum appears in JAMA 2003;290(10):1318]. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 21.Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, et al. Clinical characteristics and functional outcomes of West Nile fever. Ann Intern Med. 2004;141:360–365. doi: 10.7326/0003-4819-141-5-200409070-00010. [DOI] [PubMed] [Google Scholar]
- 22.Klee AL, Maidin B, Edwin B, Poshni I, Mostashari F, Fine A, et al. Long term prognosis for clinical West Nile virus infection. Emerg Infect Dis. 2004;10:1405–1411. doi: 10.3201/eid1008.030879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B, et al. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ. 2003;168:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar D, Drebot MA, Wong SJ, Lim G, Artsob H, Buck P, et al. A seroprevalence study of west nile virus infection in solid organ transplant recipients. Am J Transplant. 2004;4:1883–1888. doi: 10.1111/j.1600-6143.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 25.Perelygin AA, Scherbik SV, Zhulin IB, Stockman BM, Li Y, Brinton MA, et al. Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci. 2002;99:9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus and other flavivirus-specific antibodies. J Clin Microbiol. 2004;42:4641–4648. doi: 10.1128/JCM.42.10.4641-4648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, et al. Rapid detection of West Nile virus from human clinical specimens, field collected mosquitoes, and avian samples by a TaqMan reverse-transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Slater B, Rudd R, Parchuri N, Hull R, Dupuis M, et al. First isolation of West Nile virus from a patient with encephalitis in the United States. Emerg Infect Dis. 2002;8:1367–1371. doi: 10.3201/eid0812.020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–108. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal AG, Petersen LR. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis. 2003;188:1–4. doi: 10.1086/376871. [DOI] [PubMed] [Google Scholar]
- 32.Tesh RB, Travassos da Rosa AP, Guzman H, Araujo TP, Xiao SY, Monath TP, et al. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall RA, Khromykh AA. West Nile virus vaccines. Expert Opin Biol Ther. 2004;4:1295–1305. doi: 10.1517/14712598.4.8.1295. [DOI] [PubMed] [Google Scholar]
- 34.Monath TP, Arroyo J, Miller C, Guirakhoo F. West Nile virus vaccine. Curr Drug Targets Infect Disord. 2001;1:37–50. doi: 10.2174/1568005013343254. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro H, Micucci S. Pesticide use for West Nile virus. CMAJ. 2003;168:1427–1430. [PMC free article] [PubMed] [Google Scholar]
- 36.Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 37.Koren G, Matsui D, Bailey B. DEET-based insect repellents: safety implications for children and pregnant and lactating women [published erratum appears in CMAJ 2003;169:283]. CMAJ. 2003;169:209–212. [PMC free article] [PubMed] [Google Scholar]