Abstract
The homeostatic system that sets the copy number, and corrects over-replication and under-replication, seems to be different for chromosomes and plasmids in bacteria. Whereas plasmid replication is random in time, chromosome replication is tightly coordinated with the cell cycle such that all origins are initiated synchronously at the same cell mass per origin once per cell cycle. In this review, we propose that despite their apparent differences, the copy-number control of the Escherichia coli chromosome is similar to that of plasmids. The basic mechanism that is shared by both systems is negative-feedback control of the availability of a protein or RNA positive initiator. Superimposed on this basic mechanism are at least three systems that secure the synchronous initiation of multiple origins; however, these mechanisms are not essential for maintaining the copy number.
Keywords: chromosome replication, copy-number control, DnaA, Escherichia coli, synchronous replication
Introduction
During the exponential growth of bacteria, such as Escherichia coli, the single chromosome is replicated from a unique origin of replication (oriC) with the same generation time as the cell mass, which gives rise to a true steady state. Replication is initiated at a defined stage (that varies with the generation time) during the cell cycle. Replication control is exerted at the initiation of replication.
In addition to the chromosome, bacteria often contain other DNA molecules, called plasmids, which replicate in harmony with their hosts. During exponential growth, plasmids are present in defined copy numbers (the number of copies per cell or mass, or chromosome equivalent). They are independent replicons and control their own replication. Replication control is also, in this case, exerted at the level of initiation of replication from an origin of replication. Hence, plasmids are able to both measure their concentration (copy number) and adjust the replication frequency accordingly to maintain a controlled copy number. The systems that control the copy number are well known for some plasmids.
Studies of chromosome and plasmid replication have given the impression that their respective control systems are different. Therefore, there has been little communication between the two research fields. In this review, we compare the replication control of well-known plasmids and the chromosome, and propose that the two systems are basically similar.
The cell cycle and chromosome replication
The cell cycle is defined by events that occur only once per generation: chromosome duplication and segregation, and cell division. Analogous to that of eukaryotes, the E. coli cell cycle is divided into three phases: before chromosome replication (B), the duration of chromosome replication (C) and the time from the completion of chromosome duplication to complete cell division (D). For the E. coli B/r strain, the C and D periods last for about 40 and 20 min, respectively, at 37 °C and remain constant over a wide range of generation times from 25 to more than 180 min (Cooper & Helmstetter, 1968; Helmstetter, 1996). Generation times that are shorter than the C+D periods are accommodated by overlapping replication cycles such that each cell receives 2, 4 or 8 origins at birth (Fig 1). All origins in a cell fire synchronously (Skarstad et al, 1986) at a fixed cell size per origin (initiation mass) that is independent of the growth rate (Bipatnath et al, 1998; Donachie, 1968).
Figure 1.
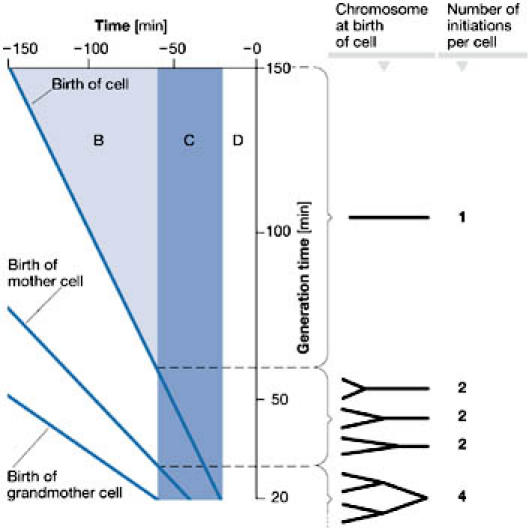
Overlapping replication cycles in Escherichia coli during rapid growth. The time of initiation of the cell cycle at different generation times, in which cell division takes place at time zero, is shown.At generation times greater than 60 min (C, D), there is a period (B) without replication in the young cells; the cells are born with a non-replicating chromosome (shown as a horizontal line).At generation times between 60 and 30 min,DNA replication starts in the mother cell and the cells are born with a partly replicated chromosome containing two origins of replication (oriCs; shown as Y-shaped structures). Finally, at generation times less than 30 min,DNA replication starts in the grandmother cell and the cells are born with chromosomes in which there are two consecutive replication cycles (shown as a structure with three branch points); the initiation of replication takes place at four oriCs during each cell cycle.
The first recognized event during the initiation of replication is the binding to oriC of about 20 molecules of the rate-limiting initiator protein DnaA, the active form of which is DnaA–ATP (Sekimizu et al, 1988). The expression of DnaA is autoregulated, with the protein functioning as its own repressor (Messer, 2002; Sekimizu et al, 1988; Skarstad & Boye, 1994). In our view, this is the most important step in the copy-number control of the E. coli chromosome. At least three systems are responsible for the coordinated initiation.
The first system is sequestration. The hemimethylated GATC sites in the origin, oriC, and the dnaA promoter are sequestered by the SeqA protein and are not methylated until about one-third of the generation time has passed after initiation (Campbell & Kleckner, 1990). Hence, new initiations cannot occur and new DnaA is not synthesized during this period.
The second system is the titration of DnaA. At least 300 DnaA-binding sites (DnaA boxes) are spread over the entire chromosome (Messer, 2002) with a special high-affinity cluster of DnaA boxes (DnaA titration or datA site) located about 470 kb clockwise of oriC (Kitagawa et al, 1996). This makes the whole chromosome into a titration sink for free DnaA protein.
The third system is the regulatory inactivation of DnaA (RIDA). The homologous to DnaA (Hda) protein catalyses the hydrolysis of the active DnaA–ATP to the inactive DnaA–ADP through a replication-dependent reaction (Camara et al, 2003; Kato & Katayama, 2001).
Together, these systems limit the amount of time when DnaA–ATP is available for initiating replication from oriC to once per generation, resulting in an eclipse of about two-thirds of a generation time during which oriC cannot reinitiate (Olsson et al, 2002). This defines the minimum inter-replication time, whereas the average is, of course, exactly one generation. Inactivation of any one of the controls leads to the asynchronous initiation of replication and reduced eclipse.
In conclusion, the control of initiation of chromosome replication in E. coli is characterized by two properties: first, it occurs at a defined initiation mass (that is, it couples replication to growth of the population); and second, multiple initiations are synchronized. The thesis of this review is that the key parameter is the initiation mass, and that the synchrony is an addition that contributes little to the copy-number control—it just upholds the ‘once and only once' (Boye et al, 2000) initiation from each oriC. Below, we compare the replication control of the chromosome with that of plasmids.
Plasmid replication
Plasmids are extrachromosomal replicons that live in harmony with their host bacteria. During steady-state growth, they are present in defined copy numbers, which vary from a few to hundreds of copies per cell. The copy-number control of plasmids operates by either limiting the supply of initiation factors (RNA or protein) or inactivating the initiator through dimerization and iteron binding (Das et al, 2005; Nordström, 1990). For most plasmids, these negative-control systems set the level of synthesis of a rate-limiting initiator protein (Rep). Fig 2A shows the basic replicon of plasmid R1 in which the copy number is determined by an antisense RNA. In a bimolecular reaction with the upstream region of the mRNA of the repA gene, this antisense RNA regulates the rate of synthesis of the RepA protein (Nordström, 2005; Nordström & Wagner, 1994). The copy number is essentially determined by the ratio between the rate constants for the synthesis of the repA mRNA and the antisense RNA (Nordström & Wagner, 1994). This control system corrects deviations from the controlled copy number (Fig 3). For plasmid R1, the rate of replication per plasmid copy is inversely proportional to the copy number (Nordstöm et al, 1984).
Figure 2.
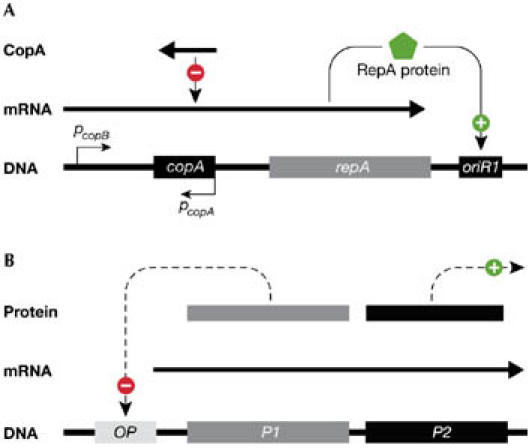
Examples of replication-contrrol systems. (A) Simplified version of the basic replicon of plasmid R1. The replicon contains the following elements: an origin of replication, oriR1; the structural gene, repA,which encodes the initiator protein RepA that binds to oriR1; CopA,which negatively regulates the translation of the repA mRNA; and the constitutive PcopA and PcopB promoters. (B) The autorepressor model proposed by Sompayrac & Maaløe (1973). The two genes (P1 and P2) in the regulatory circuit form an operon (OP, operator and promoter). The P1 product is an autorepressor of the operon and the product of P2 is the initiator protein of replication.
Figure 3.
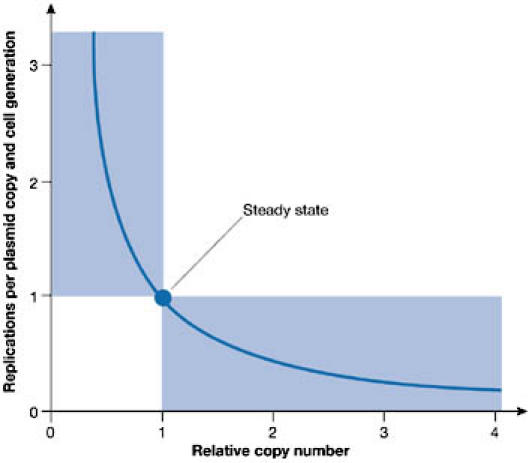
Kinetics of control of plasmid replication (Nordström et al, 1984). Control curves lie within the shaded areas. The control curve of plasmid R1 (blue line) follows an inverse proportionality between the frequency of replication (replications per plasmid copy and cell generation) and the relative copy number (Nordström et al, 1984; Nordström & Wagner, 1994).
Plasmid replication is random both in time—spread over the entire cell cycle (Bogan et al, 2001; Gustafsson et al, 1978)—and with respect to which copy is replicated at each instance (Table 1; Gustafsson et al, 1978). Hence, during one cell cycle, some plasmid copies do not replicate at all, whereas others replicate two or more times; however, the average for the population is one replication per plasmid copy and cell generation.
Table 1.
Comparison of key characteristics of replication of the Escherichia coli chromosome and plasmids
| Characteristics | Synchronous chromosome | Plasmids | Asynchronous chromosome |
|---|---|---|---|
| Replication during the cell cycle | Fixed time | Random | Randomthe |
| Replication of an origin during one cell generation | Only once | Random | Random |
| Fixed copy numbers | Yes (see text) | Yes | Yes (see text) |
| Corrections of copy-number deviations | Probably yes (see text) | Yes | Probably yes (see text) |
In conclusion, control of plasmid replication is characterized by the control of the average copy number, and the correction of deviations from this controlled copy number.
Comparison of plasmid and chromosome replication
It is clear that there are distinct differences between plasmids and chromosomes (Table 1). As mentioned above, initiation of replication of the chromosome takes place at a cell size that is defined by the initiation mass (cell mass at initiation per oriC); this might imply that the chromosome also measures its copy number. However, the introduction of minichromosomes (plasmids that replicate from oriC) affects neither the timing of initiation nor the initiation mass (Helmstetter & Leonard, 1987), which might cause a problem with control models as discussed by Donachie & Blakely (2003).
Furthermore, the copy number of minichromosomes varies markedly in the population, and the variation increases during growth of the population due to the uneven distribution of the minichromosomes in the daughter cells (Løbner-Olesen, 1999). This study showed that the content of oriC is not measured, but did not exclude the possibility that the copy number of the chromosome is measured indirectly. Below we re-address this question, and propose that the E. coli chromosome measures as well as corrects deviations in its copy number.
Models for control of replication
Several models have been proposed to explain the control of replication. The classical replicon theory (Jacob et al, 1963) suggested positive control. The Pritchard group proposed the extremely influential inhibitor-dilution model (Pritchard et al, 1969), which introduced negative-feedback control of replication. In the autorepressor model there is an operon for the synthesis of a rate-limiting initiator protein (Fig 2B; Sompayrac & Maaløe, 1973). The operon contains a gene that autoregulates transcription of the operon. This ensures that the correct amount of the initiator protein is formed during each cell cycle, and that deviations in the concentration of the operon/replicon are corrected. Elaborate mathematical models have been proposed for the control of replication of the E. coli chromosome (Browning et al, 2004; Hansen et al, 1991a; Mahaffy & Zyskind, 1989). Donachie & Blakely (2003) suggested that the ratio between DnaA–ATP and DnaA–ADP, and not their amounts and/or concentrations, determines the timing of initiation.
During steady-state growth, all components of a population are present in constant proportions. However, the concentrations of origins are never measured in the plasmid systems. The key component of the plasmid R1 system is the antisense RNA, copA. As CopA is formed constitutively and is unstable (with a half-life of about 90 s), its concentration is always proportional to the plasmid concentration. The control system keeps the concentration of CopA constant and corrects fluctuations. Hence, the control of replication is indirect. Similarly, the other models and real systems use indirect measures of the concentration of the replicons as shown in Table 1, and act by regulating the availability of a rate-limiting initiator protein. This is analogous to the initiation of replication of the chromosome controlled by the DnaA protein, as is implicit in the models of Hansen et al (1991a) and Browning et al (2004), and was specifically stressed by Herrick et al (1996). The synthesis of DnaA is autoregulated (Messer & Weigel, 1997), which makes the system similar to the Sompayrac–Maaløe model with DnaA combining autorepressor and initiator functions.
Minichromosomes have posed a problem for the construction of copy-number-control models (see, for example, Donachie & Blakely, 2003). However, as stated above (and in Table 2), the concentration of origins has never been measured in any system. E. coli minichromosomes do not carry the dnaA gene and would not be able to replicate in the absence of a DnaA donor. The control of replication of minichromosomes was recently reviewed by Dasgupta & Løbner-Olesen (2004).
Table 2.
Substances that are measured by different replication-control systems
| Model or system | Measured control substance |
|---|---|
| Pritchard inhibitor-dilution model | Putative inhibitor |
| Sompayrac–Maaløe autorepressor | Autorepressormodel |
| Plasmid λdv | Cro/Tof protein |
| Plasmids with antisense-RNA control | Antisense RNA |
| Atlung–Hansen inhibitor-titration | Initiator protein DnaAmodel |
| Escherichia coli chromosome | Initiator protein and autorepressor DnaA–ATP |
Asynchronous replication and over-replication
As the E. coli chromosome has an elaborate system for maintaining the synchronous initiation of replication, here we compare chromosomes that have asynchronous initiation of replication with plasmids that replicate randomly.
Loss of synchrony by inactivation of, for example, sequestration gives a shortened eclipse and random replication with respect to both timing and selection (Table 1; Olsson et al, 2002). Hence, some oriCs initiate two or more times per cell generation. Although this has been described as over-initiation because some origins fire more than once per cell generation, it is merely a logical consequence of random initiation of replication and is not necessarily indicative of a higher copy number—the copy number is still maintained by the autoregulation of the dnaA gene. To a plasmidologist, over-initiation is an increase in the copy number. For plasmids, random replication leads to variations in the copy number in individual cells (Løbner-Olesen, 1999). Hence, asynchronous chromosomes replicate in the same manner as plasmids. It should therefore be stressed that the terms ‘over-initiation' and ‘asynchrony' are often used synonymously in the literature, which is unfortunate and misleading.
Minichromosomes can become established in wild-type bacteria, whereas they are severely incompatible with deoxyadenosine methyltransferase (dam) mutants; this is another plasmid-like feature (Løbner-Olesen & von Freiesleben, 1996).
DnaA sets the chromosome copy number
The DnaA protein is not only the key element in the initiation of replication of the E. coli chromosome, but is also responsible for linking this initiation of replication to cell growth. Hansen et al (1991b) reported that the amount of DnaA per oriC is about 200 molecules at growth rates ranging from 0.6 to 2.3 generations per hour. As the initiation mass is constant over this range of growth rates, they concluded that DnaA determines the initiation mass.
However, in contrast to plasmids, for which increased production of the initiation protein can lead to runaway replication (Nordström & Uhlin, 1992), large increases in the DnaA concentration lead to only marginal changes in the DNA concentration (Atlung et al, 1987; Churchward et al, 1983). Atlung & Hansen (1993) extended these studies by measuring the concentration of oriC in addition to the total amount of chromosomal DNA. At DnaA concentrations above the normal level, there was a proportional increase in oriC/mass up to at least 50%, whereas the DNA/mass increased only slightly and then levelled off. This was because many initiations were abortive and replication never reached the terminus, or because the rate of fork movement was reduced. Atlung & Hansen (1993) concluded that the initiation mass was determined by the availability of DnaA (see also Herrick et al, 1996).
Negative control of chromosome replication
The copy number of most plasmids is determined by the rate of synthesis of a rate-limiting initiator protein (Rep). Deviations from the copy number are corrected by the presence of a negative loop.
The dnaA promoter is autoregulated (Messer & Weigel, 1997), which allows deviations in copy number of the chromosome to be corrected. Hansen et al (1987) inserted a lambda phage carrying a dnaA–lacZ fusion into λatt and introduced plasmids carrying different numbers of dnaA boxes into the strain; this caused a marked increase in dnaA expression. Hence, there is a strong potential for de-repression of the dnaA promoter, and the concentration of DnaA–ATP is within the range that gives effective control of expression of the dnaA gene.
There do not seem to be any direct experiments showing that copy numbers are corrected. However, the autoregulation of the dnaA gene strongly suggests that copy-number deviations are corrected. Such experiments have been performed with plasmids in shifts between different copy-number levels (Gustafsson & Nordström, 1980).
Effect of the synchrony systems on initiation mass
Strains with asynchronous initiation of replication (for example dam mutants) grow at steady states; hence, the three synchrony systems are not vital for the functioning of the cells (Boye & Løbner-Olesen, 1990; Kato & Katayama, 2001; Kitagawa et al, 1998; Olsson et al, 2003; von Freiesleben et al, 1994); the main effect seems to be that cell division is disturbed, which results in broader cell-size distributions. Hence, the systems that maintain synchrony are not important for the maintenance of copy number, although the copy number of the chromosome might be different in some of the asynchronous mutants compared with the wild type. The systems might function to secure better co-ordination between replication and cell division.
A newly replicated oriC cannot take part in a new initiation during an eclipse period of about two-thirds of a generation time (Olsson et al, 2002). The eclipse is caused by sequestration (which makes a newly replicated oriC unavailable for initiation and, presumably, DnaA binding for one-third of a generation time), titration of DnaA by the datA locus, and the RIDA system. Sequestration prohibits the synthesis of DnaA, and DnaA titration and RIDA prolong the period of relatively low concentrations of free DnaA–ATP. Inactivation of any of these functions therefore leads to asynchronous initiation.
Inactivation of the dam gene reduces the length of the eclipse to about one-third of a generation time (Olsson et al, 2002); therefore, the period during which the concentration of DnaA–ATP is extremely low is shortened compared with the wild type, because the dnaA promoter is not sequestered and is active throughout. This causes asynchronous replication.
Loss of sequestration results in the production of DnaA during the entire cell cycle, whereas DnaA is not produced during the first one-third of the wild-type cell cycle. However, this might be compensated by de-repression of the dnaA promoter in the wild type, due to the extremely low concentration of DnaA at the end of the sequestration period. Therefore, a similar amount of DnaA might be formed per cell cycle in a dam mutant and in the wild type. The situation is different for seqA mutants because of the change in superhelicity of the chromosome (Olsson et al, 2002).
The datA locus titrates DnaA, and its deletion increases the origin copy number by about 30% (Kitagawa et al, 1998). Hence, the rest of the chromosome binds about 70% of the DnaA. This might fit with the doubling of the initiation mass caused when a threefold increase in datA is caused by an introduced plasmid (Morigen et al, 2003).
Loss of RIDA (inactivation of the hda gene) also causes some degree of asynchrony (Camara et al, 2003). This might be due to the release of DnaA–ATP from the DnaA boxes, and its re-use in initiation of replication. Consequently, there is an increased oriC concentration in hda strains (Camara et al, 2003).
Conclusions
This review has compared the control of replication of the E. coli chromosome with that of some plasmids. The main conclusion is that the two control systems are similar.
First, the copy number of the replicons is measured indirectly by the availability of a rate-limiting initiator protein. The synthesis of this protein is controlled negatively, which enables a given copy number to be set and variations to be adjusted. For the chromosome, negative control is exerted by autoregulation of the dnaA gene by the DnaA protein. We claim that this is the main system that controls copy number. Hence, the control system is analogous to the Sompayrac–Maaløe model.
Second, plasmids replicate randomly during the entire cell cycle, whereas chromosome replication occurs at a fixed time; during rapid growth, multiple initiations occur and are highly synchronized. However, this synchrony is ensured by several systems: sequestration of oriC and the dnaA promoter, titration of DnaA, and RIDA. Loss of synchrony by mutation is not lethal to the bacteria, which means that these systems are not crucial for maintaining controlled chromosome replication. Chromosome replication in strains with asynchronous replication is similar to plasmid replication. It should also be stressed that the synchrony systems all operate by affecting either the synthesis or the fate of the DnaA protein.
Third, the copy number of some mutants with asynchronous chromosome replication is different from that of the wild type. This is due to effects on the synthesis and fate of the DnaA protein. However, the new copy number is maintained by autoregulation of the dnaA gene.
Fourth, during asynchronous—that is, random—replication, some origins fire more than once per cell cycle. This is a logical consequence of randomness, but has often been called over-initiation in the literature. For plasmidologists this is not an appropriate term, as it implies an increased copy number. This is, for example, evident from the behaviour of the dnaA46 mutant, which, at permissive temperatures, has a reduced copy number compared with the wild type but replicates its chromosome asynchronously.
The emerging picture of the homeostatic control of chromosome copy number is that the DnaA protein is the key regulator of both timing and frequency of the initiation of chromosome replication, and their coordination with cell growth. Several additional elements of control (SeqA, Dam, datA, Hda and so on) are used for fine-tuning, but are neither necessary nor sufficient for initiation. By contrast, loss or excess of the initiator activity of DnaA causes cell death. From this perspective, the copy-number control of the chromosome seems similar to that of plasmids, with superimposed mechanisms that coordinate replication with the cell cycle.

Kurt Nordström & Santanu Dasgupta
Acknowledgments
Our work was supported by the Swedish Cancer Society and the Swedish Research Council.
References
- Atlung T, Hansen FG (1993) Three distinct chromosome replication states are induced by increasing concentrations of DnaA protein in Escherichia coli. J Bacteriol 175: 6537–6545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T, Løbner-Olesen A, Hansen FG (1987) Overproduction of DnaA protein stimulates initiation of chromosome and minichromosome replication in E. coli. Mol Gen Genet 206: 51–59 [DOI] [PubMed] [Google Scholar]
- Bipatnath M, Dennis PP, Bremer H (1998) Initiation and velocity of chromosome replication in Escherichia coli B/r and K-12. J Bacteriol 180: 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan JA, Grimwade JE, Thornton M, Zhou P, Denning GDC, Helmstetter CE (2001) P1 and NR1 plasmid replication during the cell cycle of Escherichia coli. Plasmid 45: 200–208 [DOI] [PubMed] [Google Scholar]
- Boye E, Løbner-Olesen A (1990) The role of dam methyltransferase in the control of DNA replication in E. coli. Cell 62: 981–989 [DOI] [PubMed] [Google Scholar]
- Boye E, Løbner-Olesen A, Skarstad K (2000) Limiting DNA replication to once and only once. EMBO Rep 1: 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning SH, Castellanos M, Shuler ML (2004) Robust control of initiation of prokaryotic chromosome replication: essential considerations for a minimal cell. Biotechnol Bioeng 88: 575–584 [DOI] [PubMed] [Google Scholar]
- Camara JE, Skarstad K, Crooke E (2003) Controlled initiation of chromosomal replication in Escherichia coli requires functional Hda protein. J Bacteriol 185: 3244–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Kleckner N (1990) E. coli oriC and dnaA gene promoter are sequestered from dam methyl transferase following the passage of the chromosome replication fork. Cell 62: 967–979 [DOI] [PubMed] [Google Scholar]
- Churchward G, Holmans P, Bremer H (1983) Increased expression of the dnaA gene has no effect on DNA replication in a dnaA strain of Escherichia coli. Mol Gen Genet 192: 506–508 [DOI] [PubMed] [Google Scholar]
- Cooper S, Helmstetter CE (1968) Chromosome replication and the cell division cycle of Escherichia coli B/r. J Mol Biol 31: 519–540 [DOI] [PubMed] [Google Scholar]
- Das N, Valjavec-Gratian M, Basuray AN, Fekete RA, Papp PP, Paulsson J, Chattoraj DK (2005) Multiple homeostatic mechanisms in the control of P1 plasmid replication. Proc Natl Acad Sci USA 102: 2856–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Løbner-Olesen A (2004) Host controlled plasmid replication: Escherichia coli minichromosomes. Plasmid 52: 151–168 [DOI] [PubMed] [Google Scholar]
- Donachie WD (1968) Relationship between cell size and time of initiation of DNA replication. Nature 219: 1077–1079 [DOI] [PubMed] [Google Scholar]
- Donachie WD, Blakely GW (2003) Coupling the initiation of chromosome replication to cell size in Escherichia coli. Curr Opin Microbiol 6: 146–150 [DOI] [PubMed] [Google Scholar]
- Gustafsson P, Nordström K (1980) Control of replication of plasmid R1. Kinetics of replication in shifts between different copy numbers. J Bacteriol 141: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P, Nordström K, Perram JW (1978) Selection and timing of replication of plasmids R1drd-19 and F'lac in Escherichia coli. Plasmid 1: 187–203 [DOI] [PubMed] [Google Scholar]
- Hansen FG, Koefoed S, Sørensen L, Atlung T (1987) Titration of DnaA protein by oriC DnaA-boxes increases dnaA gene expression in Escherichia coli. EMBO J 6: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FG, Atlung T, Braun RE, Wright A, Hughes P, Kohiyama M (1991) Initiator (DnaA) protein concentration as a function of growth rate in Escherichia coli and Salmonella typhimurium. J Bacteriol 173: 5194–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FG, Christensen BB, Atlung T (1991) The initiator titration model: computer simulation of chromosome and minichromosome control. Res Microbiol 142: 161–167 [DOI] [PubMed] [Google Scholar]
- Helmstetter CE (1996) Timing of synthetic activities in the cell cycle. In Escherichia coli and Salmonella typhimurium; Cellular and Molecular Biology, Neidhardt FC et al. (eds), pp 1627–1639. Washington, DC: ASM [Google Scholar]
- Helmstetter CE, Leonard AC (1987) Coordinate initiation of chromosome and minichromosome replication in Escherichia coli. J Bacteriol 169: 3489–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick J, Kohiyama M, Atlung T, Hansen FG (1996) The initiation mess? Mol Microbiol 19: 659–666 [DOI] [PubMed] [Google Scholar]
- Jacob F, Brenner S, Cuzin F (1963) On the regulation of DNA replication in bacteria. Cold Spring Harbor Symp Quant Biol 28: 329–348 [Google Scholar]
- Kato J, Katayama T (2001) Hda, a novel DnaA-related protein, regulates the replication cycle in Escherichia coli. EMBO J 20: 4253–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R, Mitsuki H, Okazaki T, Ogawa T (1996) A novel DnaA protein-binding site at 94.7 min on the Escherichia coli chromosome. Mol Microbiol 19: 1137–1147 [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Ozaki T, Moriya S, Ogawa T (1998) Negative control of replication initiation by a novel chromosomal locus exhibiting exceptional affinity for Escherichia coli DnaA protein. Genes Dev 12: 3032–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A (1999) Distribution of minichromosomes in individual Escherichia coli cells: implications for replication control. EMBO J 18: 1712–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A, von Freiesleben U (1996) Chromosomal replication incompatibility in Dam methyltransferase deficient Escherichia coli cells. EMBO J 15: 5999–6008 [PMC free article] [PubMed] [Google Scholar]
- Mahaffy JM, Zyskind JW (1989) A model for the initiation of replication in Escherichia coli. J Theor Biol 140: 453–477 [DOI] [PubMed] [Google Scholar]
- Messer W (2002) The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol Rev 26: 355–374 [DOI] [PubMed] [Google Scholar]
- Messer W, Weigel C (1997) DnaA initiator—also a transcription factor. Mol Microbiol 24: 1–6 [DOI] [PubMed] [Google Scholar]
- Morigen, Løbner-Olesen A, Skarstad K (2003) Titration of the Escherichia coli DnaA protein to excess datA sites causes destabilization of replication forks, delayed replication initiation and delayed cell division. Mol Microbiol 50: 349–362 [DOI] [PubMed] [Google Scholar]
- Nordström K (1990) Control of plasmid replication—How do DNA iterons set the replication frequency? Cell 63: 1121–1124 [DOI] [PubMed] [Google Scholar]
- Nordström K (2006) Plasmid R1—Replication and its control. Plasmid 55: 1–26 [DOI] [PubMed] [Google Scholar]
- Nordström K, Uhlin BE (1992) Runaway-replication plasmids as tools to produce large quantities of proteins from cloned genes in bacteria. Biotechnology 10: 661–666 [DOI] [PubMed] [Google Scholar]
- Nordström K, Wagner EGH (1994) Kinetic aspects of control of plasmid replication by antisense RNA. Trends Biochem Sci 19: 294–300 [DOI] [PubMed] [Google Scholar]
- Nordström K, Molin S, Light J (1984) Control of replication of bacterial plasmids: genetics, molecular biology, and physiology of the plasmid R1 system. Plasmid 12: 71–90 [DOI] [PubMed] [Google Scholar]
- Olsson J, Dasgupta S, Berg OG, Nordström K (2002) Eclipse period without sequestration in Escherichia coli. Mol Microbiol 44: 1429–1440 [DOI] [PubMed] [Google Scholar]
- Olsson JA, Nordström K, Hjort K, Dasgupta S (2003) Eclipse–synchrony relationship in Escherichia coli strains with mutations affecting sequestration, initiation of replication and superhelicity of the bacterial chromosome. J Mol Biol 334: 919–931 [DOI] [PubMed] [Google Scholar]
- Pritchard RH, Barth PT, Collins J (1969) Control of DNA synthesis in bacteria. Symp Soc Gen Microbiol 19: 263–297 [Google Scholar]
- Sekimizu K, Bramhill D, Kornberg A (1988) Sequential early stages in the in vitro initiation of replication at the origin of the Escherichia coli chromosome. J Biol Chem 263: 7124–7130 [PubMed] [Google Scholar]
- Skarstad K, Boye E (1994) The initiator protein DnaA: evolution, properties and function. Biochim Biophys Acta 1217: 111–130 [DOI] [PubMed] [Google Scholar]
- Skarstad K, Boye E, Steen HB (1986) Timing of initiation of chromosome-replication in individual Escherichia coli cells. EMBO J 5: 1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L, Maaløe O (1973) Autorepressor model for control of DNA replication. Nature 241: 133–135 [DOI] [PubMed] [Google Scholar]
- Von Freiesleben U, Rasmussen KV, Schaechter M (1994) SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol Microbiol 14: 763–772 [DOI] [PubMed] [Google Scholar]


