Abstract
The T-cell antigen receptor (TCR•CD3) is a multi-subunit complex that is responsible for triggering an adaptive immune response. It shows high specificity and sensitivity, while having a low affinity for the ligand. Furthermore, T cells respond to antigen over a wide concentration range. The stoichiometry and architecture of TCR•CD3 in the membrane have been under intense scrutiny because they might be the key to explaining its paradoxical properties. This review highlights new evidence that TCR•CD3 is found on intact unstimulated T cells in a monovalent form (one ligand-binding site per receptor) as well as in several distinct multivalent forms. This is in contrast to the TCR•CD3 stoichiometries determined by several biochemical means; however, these data can be explained by the effects of different detergents on the integrity of the receptor. Here, we discuss a model in which the multivalent receptors are important for the detection of low concentrations of ligand and therefore confer sensitivity, whereas the co-expressed monovalent TCR•CD3s allow a wide dynamic range.
Keywords: conformational change, signal transduction, stoichiometry, T-cell antigen receptor, transmembrane interactions
Introduction
How transmembrane (TM) receptors respond to ligands with high sensitivity and plasticity has been intensely studied. Particularly intriguing is the T-cell antigen-receptor complex (TCR•CD3). The complex is composed of the TCRαβ, CD3γε, CD3δε and TCRζζ (CD247) dimers (Fig 1), which are non-covalently bound to each other. The invariant CD3 and TCRζ subunits each contain an extracellular part, a TM region with one potential negative charge and a cytoplasmic tail with several signal-transduction motifs (Alarcón et al, 2003; Malissen, 2003; Reth, 1989). TCRα and TCRβ each contain a variable immunoglobulin (Ig) domain, which together form the binding surface for the ligand, a constant Ig domain and a TM region (Fig 1) that is potentially positively charged and couples TCRαβ to CD3 (Blumberg et al, 1990a; Call et al, 2002; Cosson et al, 1991).
Figure 1.

The T-cell antigen receptor (TCR•CD3) complex consists of the αβ, γε, δε and ζζ dimers. Schematic pictures of the individual components of the TCR•CD3 complex are shown. The variable immunoglobulin domains of TCRαβ (V) bind to the ligand, whereas the cytoplasmic tails of the CD3 subunits (γε and δε) and TCRζζ interact with cytosolic-signalling proteins. Disulphide bridges are shown by thick black lines. Potential charges in the transmembrane regions are also depicted.
The ligand of TCR•CD3 is an antigenic peptide presented by major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). Paradoxically, T cells can be activated by a few antigenic peptides loaded onto the MHC (pMHC; Irvine et al, 2002; Purbhoo et al, 2004), even though the affinity of the pMHC–TCRαβ interaction is low (1–50 μM; Davis et al, 1998). Furthermore, APCs express a 103-fold to 104-fold higher concentration of MHC/self-peptides than antigenic pMHC. MHC/self-peptides by themselves do not elicit a response, although their affinity for TCRαβ could be only tenfold lower than the affinity of the antigenic pMHC. Therefore, it is difficult to understand how this small difference in affinity results in high specificity when there is a huge excess of self-peptides (Boniface et al, 1998). In addition to the extreme sensitivity, T cells still show concentration-dependent responses at 103-fold to 106-fold higher amounts of ligand (Bachmann et al, 1998; Cochran et al, 2000; Irvine et al, 2002). How T cells display an unsaturated response to such a range of pMHC concentrations is also puzzling considering the properties of the receptor.
Crucial to solving these problems are the stoichiometry (the number of copies of each subunit per complex) and the arrangement of the receptor on the cell surface. This review focuses on the advances made concerning the architecture of the TCR•CD3 complex and tries to answer some of the questions that have been raised above.
The monovalent TCR•CD3 model
The present consensus on the composition of the TCR•CD3 complex is that it comprises the TCRαβ, CD3γε, CD3δε and TCRζζ dimers (Fig 1). The minimal stoichiometry possible is therefore αβγεδεζζ. Of particular importance is the receptor's valency—that is, the number of ligand-binding TCRαβ heterodimers per TCR•CD3 complex.
Early studies that tried to determine the valency of TCR•CD3 used the detergent digitonin to extract TCR•CD3 from the T-cell membrane. Transgenic mice (Punt et al, 1994) or human T cells (Hou et al, 1994) that simultaneously expressed two distinguishable TCRαβ dimers were used. Specific immunoprecipitation of one dimer did not co-purify the second and vice versa. These data experimentally strengthened the hypothesis that the TCR•CD3 complex is monovalent. It took until 2002 before a complete stoichiometry of all subunits was presented (Call et al, 2002). Using an approach involving the co-purification of TCR•CD3 complexes expressed in an in vitro translation system into endoplasmic reticulum (ER)-derived microsomes (Call et al, 2002, 2004), the αβγεδεζζ stoichiometry was experimentally confirmed for digitonin-extracted TCR•CD3s (Fig 2A). However, as the ζ subunit might only assemble with the other subunits in the Golgi apparatus in vivo (Dietrich et al, 1999), and as TCR•CD3 is post-translationally modified in the secretory pathway, it was unclear whether data obtained in the in vitro translation system reflected the stoichiometry at the plasma membrane of T cells or just the possible stoichiometry in the ER.
Figure 2.

Arrangement of the T-cell antigen receptor (TCR•CD3) complexes on the cell surface before stimulation. The old model shows monovalent receptors with a stoichiometry of αβγεδεζζ distributed evenly on the plasma membrane. The new model has monovalent receptors and a variety of multivalent receptors that co-exist in the intact membrane of unstimulated T cells, and that have been detected by fluorescence resonance-energy transfer and electron microscopy. Due to the lack of certainty, the stoichiometries of the monovalent and multivalent TCR•CD3s are not shown. The monovalent complexes, however, might have the αβγεδεζζ stoichiometry. The multivalent TCR•CD3s might be present in a different lipid context (dark grey). The cell surface also contains large areas that are devoid of any TCR•CD3. TCRαβ is shown in green and CD3 is shown in various shades of blue/turquoise.
The multivalent TCR•CD3 model
Early on, it was noticed that the αβγεδεζζ complex would have an unfavourable imbalance of three potential negative charges in the TM region (Fig 1). This problem could theoretically be solved by adding another TCRαβ heterodimer to the complex, resulting in a bivalent γεαβζζαβδε receptor with a neutral TM region (Fernandez-Miguel et al, 1999; Green, 1991; Rojo & Portoles, 1991). Alternatively, the αβγεδεζζ stoichiometry of the TCR•CD3 complex led to the idea that two acidic amino acids contact one basic amino acid (Call et al, 2002).
The first experimental evidence for a multivalent stoichiometry (that is, more than one TCRαβ dimer) came from hydrodynamic measurements of the TCR•CD3 size after octylglucoside extraction, which revealed that the complex was larger than αβγεδεζζ (Exley et al, 1995). Furthermore, studies of the TCR•CD3 stoichiometry, performed without disrupting the plasma membrane, suggested multivalency (Fernandez-Miguel et al, 1999). Fluorescence resonance-energy transfer (FRET) between two TCRαβs on intact primary double-TCRαβ-transgenic murine T cells proved that at least two TCRαβ dimers are present in one complex. Furthermore, when solubilized with the detergents nonidet P-40 (NP40) and Brij96, two TCRαβ heterodimers could be co-purified. Endoglycosidase H treatment of the receptors showed that it was the surface form that contained at least two TCRαβs (Fernandez-Miguel et al, 1999). These data clearly showed that multiple TCRαβs constitute the receptor, although they did not prove a γεαβζζαβδε stoichiometry. So, how could the discrepancy between the monovalent and multivalent models be resolved?
A unifying model of TCR•CD3 valency
The TM domains have a fundamental role in the interactions between TCRαβ and the CD3 subunits (Blumberg et al, 1990a; Call et al, 2002; Cosson et al, 1991). Therefore, the choice of detergent is crucial for maintaining TM interactions. Conversely, the detergent should be effective enough to dissolve the membrane around the protein complex. Also, the assumption that only one defined complex exists might not be true. Therefore, the experimental approach used to study a multiprotein complex should be able to discriminate between complexes of different stoichiometries.
Blue-native polyacrylamide-gel electrophoresis (BN-PAGE; Schägger & von Jagow, 1991; Schamel & Reth, 2000) with different detergents was used to study the size and stoichiometry of TCR•CD3 (Hellwig et al, 2005; Schamel et al, 2005). In contrast to co-purification and FRET, BN-PAGE determines the relative abundance and number of different complexes that are formed by a given protein. Furthermore, the complexes are formed in their native environment, which allows post-translational modifications and interactions with other proteins, such as chaperones, to occur.
When the TCR•CD3 complex was extracted in digitonin from murine and human primary T cells, as well as T-cell lines, a single monovalent complex with the αβγεδεζζ stoichiometry was detected by BN-PAGE (Hellwig et al, 2005; Schamel et al, 2005; Zapata et al, 2004). By contrast, the detergents Brij96 and Brij98 allowed the separation of a variety of complexes of different sizes (Hellwig et al, 2005; Schamel et al, 2005). Control experiments excluded the possibility that Brij96 caused an artefactual aggregation of TCR•CD3 (Schamel et al, 2005). The large TCR•CD3 complexes were located at the plasma membrane as they became tyrosine-phosphorylated on pMHC stimulation. For confirmation, one TCRβ was co-purified with another from Brij96 lysates, indicating that the Brij96-solubilized TCR•CD3 complexes have at least two TCRβ chains (Schamel et al, 2005). Co-purification, however, was not possible using digitonin extraction.
The co-existence of TCR•CD3 complexes with different valencies identified by BN-PAGE in Brij96 and other detergents is in accordance with the previously mentioned FRET data on intact T cells. Furthermore, electron microscopy (EM) of immunogold-labelled primary human T cells, as well as human and murine T-cell lines, showed that before stimulation, monovalent TCR•CD3 complexes are co-expressed with a variety of multivalent TCR•CD3s (Schamel et al, 2005; Fig 2B). The same conclusion has been reached by using a distinct EM technique (B. Lillemeier & M.M. Davis, unpublished data). At their largest, these multivalent TCR•CD3 complexes consist of around 20 CD3 and TCRαβ dimers (Schamel et al, 2005). The pre-formed clusters are too small to be seen by confocal microscopy, and are therefore different from the so-called TCR•CD3 patches or microclusters that can be detected by light microscopy after TCR•CD3 stimulation and subsequent receptor aggregation (Mossman et al, 2005; Yokosuka et al, 2005).
In summary, multivalent TCR•CD3s are co-expressed with monovalent receptors on the resting T-cell surface (Fig 2). As expected, different detergents have different effects on the integrity of the TCR•CD3 complexes (Fig 3). Brij96 is the mildest detergent, extracting all TCR•CD3s while leaving the multivalent and monovalent receptors intact. By contrast, digitonin disrupts the multivalent receptors, yielding only the monovalent stoichiometry. Taking into account the different effects of the detergents, the co-existence of monovalent and multivalent TCR•CD3s is in agreement with all published data (Blumberg et al, 1990b; Call et al, 2002, 2004; de la Hera et al, 1991; Exley et al, 1995; Fernandez-Miguel et al, 1999; Hou et al, 1994; Punt et al, 1994; Schamel et al, 2005; Zapata et al, 2004).
Figure 3.

Influence of different detergents on the integrity of the multivalent T-cell antigen receptor (TCR•CD3) complex. The detergents Brij96, Brij98, lubrol and octylglucoside keep the multivalent TCR•CD3 intact. Digitonin disrupts the multivalent complexes, resulting in the αβγεδεζζ stoichiometry. In contrast to murine TCR•CD3, the human receptor is disassembled into its dimeric constituents by nonidet P-40 (NP40) and TritonX-100 (TX-100; San Jose et al, 1998). Sodium dodecyl sulphate (SDS) treatment leads to unfolding of the subunits and subsequent destruction of all non-covalent interactions.
Architecture of the multivalent TCR•CD3 complexes
In the 2B4 T-cell line, approximately one-half of the TCRαβ chains are present in monovalent TCR•CD3 complexes, whereas the others are present in multivalent complexes (Schamel et al, 2005). This ratio varies between different cell types for a given TCRαβ dimer (Hellwig et al, 2005), indicating that factors other than TCR•CD3 determine the degree of multimerization before stimulation.
As visualized by EM, the small and large TCR•CD3 complexes are located in close proximity to each other on the cell surface, leaving large areas devoid of any TCR•CD3 (Fig 2; I. Arechaga, H.M.v.S., J. M. Valpuesta, W.W.A.S. & B.A., unpublished data). In these experiments, the multivalent complexes were predominantly found as linear assemblies, in which the gap between two adjacent gold particles was not always resolved. As the resolution of the EM was higher than 1 nm and the predicted diameter of one TCR•CD3 unit is larger than the 10 nm gold particles used (Sun et al, 2004), it was concluded that the TCR•CD3 units are close enough to establish protein–protein contacts. The organization of the multivalent complexes in linear arrays rather than in random clusters further supports the existence of protein–protein interactions as the driving force for the formation of multivalent complexes. Nevertheless, the disassembly of multivalent TCR•CD3 by cholesterol extraction (Schamel et al, 2005) suggests that lipid–protein interactions also have a structural role within the multimers. It is likely that cholesterol confers stability to the multivalent, but not monovalent, complexes. This conclusion is supported by a study indicating that two different TCR•CD3 populations exist on the surface of living cells: one that is sensitive and one that is insensitive to cholesterol extraction (Uhlin et al, 2003). Owing to its cholesterol dependence, the multivalent TCR•CD3 could be present in lipid rafts, whereas the monovalent receptor could be excluded. As cholesterol levels are low in the ER membrane (Holthuis & Levine, 2005), it is questionable whether the multivalent TCR•CD3 was present in the in vitro translation experiments (Call et al, 2002, 2004).
The stoichiometry of the TCR•CD3 is still unresolved
Although the stoichiometry of the TCR•CD3 is most relevant in the intact membrane, at present this information is only available for the complex in detergent micelles. The stoichiometries in detergents milder than digitonin remain unknown. When the mild detergent lubrol, which keeps the multivalent receptors intact (Fig 2; M.S. & W.W.A.S., unpublished data), was used in the in vitro translation approach to extract the receptor, a 1:1:1:1 ratio of αβ:γε:δε:ζζ was found (Call et al, 2004). As it is not known whether the multivalent complexes form in the ER, it is difficult to interpret the lubrol-derived data. However, they could indicate that the monovalent TCR•CD3 has the αβγεδεζζ stoichiometry. Another important question is whether the multivalent receptor clusters are multimers of the monovalent form or contain a different ratio between the individual subunits. An attractive hypothesis is that there is a dynamic equilibrium between monovalent and multivalent receptors on the cell surface.
Until the stoichiometry issue is clarified, it will not be possible to conclude whether each potential positive charge in the TCRαβ TM region is counterbalanced by one (Fernandez-Miguel et al, 1999; Green, 1991) or two (Call et al, 2002) potential negative charges. In this context, it is worth mentioning that the extensive mutagenesis study of the charged amino acids in the TM regions of the individual TCR•CD3 subunits that determined the interaction of the different charges (Call et al, 2002) was only conducted in digitonin micelles and therefore might not necessarily reflect the interactions in the intact membrane.
Receptor pre-clustering for higher sensitivity
We have recently shown that multivalent TCR•CD3s are activated at low agonistic pMHC concentrations, whereas monovalent TCR•CD3s remain unaffected (Schamel et al, 2005). This finding could be explained by three mechanisms. First, multivalent TCR•CD3 complexes probably enhance the avidity towards the ligand, which is expressed in clusters on the surface of APCs (Anderson et al, 2000; Krishna et al, 1992; Kropshofer et al, 2002). One consequence might be that at low ligand doses, only the multivalent complexes are bound, whereas monovalent TCR•CD3s require higher ligand concentrations (Schamel et al, 2005). This is especially evident when MHC/self-peptides with intermediate affinity to TCRαβ are considered; they cannot activate the TCR•CD3 on their own (Fig 4C), but they can enhance the sensitivity of T-cell activation at low agonist concentrations (Krogsgaard et al, 2005; Wülfing et al, 2002; Yachi et al, 2005). Under these conditions, only multimeric forms of TCR•CD3 might become activated due to their high avidity for the pMHC heterodimers compared with the monovalent form (Fig 4C). Monovalent TCR•CD3 would be activated only at high agonist doses (Fig 5). Second, the multivalent complexes might allow the propagation of the activation signal from ligand-bound TCRαβ to the neighbouring receptors in the same multimer, thereby amplifying the effect of binding and explaining the high sensitivity of T cells. In this model, the endogenous MHC/self-peptides also have a role in occupying the non-agonist-bound TCRαβ within a TCR•CD3 multimer and thereby activating additional receptors (Fig 5). Third, the linear arrays of multivalent complexes could help a single pMHC to serially trigger several receptors (Valitutti et al, 1995).
Figure 4.
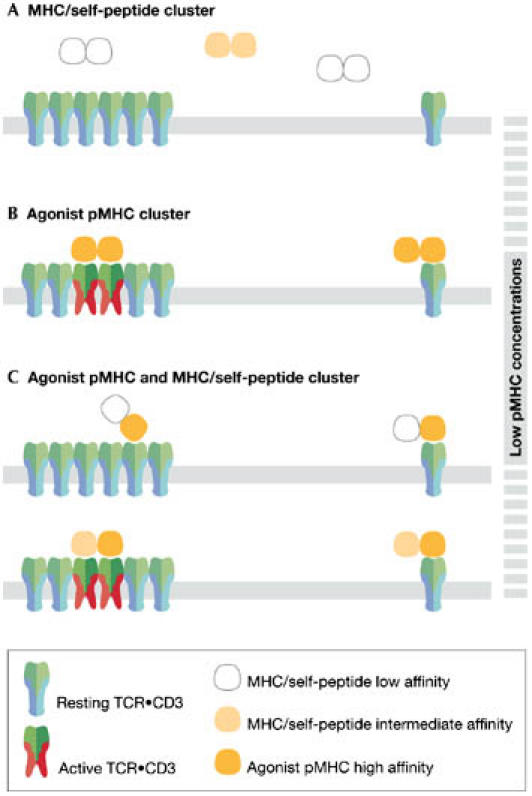
Multivalent T-cell antigen receptors (TCR•CD3s) have higher avidity towards the ligand than monovalent TCR•CD3s. (A) Major histocompatibility complex (MHC)/self-peptide clusters (white or yellow) do not bind with sufficient avidity to either TCR•CD3 form and thus do not activate T cells. (B) Clusters of MHC containing two agonistic peptides bind with sufficient avidity to the multivalent, but not the monovalent, TCR•CD3. Consequently, at low pMHC concentrations, only multivalent TCR•CD3s are activated. (C) In clusters with one antigenic pMHC (orange) and MHC/self-peptides of intermediate affinity (yellow), the average affinity of both interactions will determine whether the multivalent TCR•CD3s can be bound with a sufficiently high avidity for T-cell activation. If the affinity of the MHC/self-peptide is low (upper panel), then TCR•CD3 triggering does not occur. If the affinity of the MHC/self-peptide is above a certain threshold, then, in combination with the agonist pMHC, the avidity might be high enough to trigger multivalent TCR•CD3 (lower panel). This avidity is not sufficient to cluster and activate two monovalent TCR•CD3s. Thus, small amounts of agonistic pMHC presented by an antigen-presenting cell might preferentially trigger multivalent TCR•CD3.
Figure 5.

Multivalent T-cell antigen receptors (TCR•CD3s) allow spreading of the signal within the multimer. (A) At low agonist–major histocompatibility complex peptide (pMHC) concentrations, the multimeric TCR•CD3 complexes become preferentially activated. This might be due to their higher avidity for multimeric antigenic pMHC (orange). TCRαβs of multivalent TCR•CD3s that are not engaged by antigenic pMHC can bind to MHC/self-peptide (white) and thereby become activated. By amplifying the effect of a few pMHC-binding events to neighbouring receptors within a TCR•CD3 multimer, the T cell might be able to respond to low pMHC doses. (B) At medium agonist–pMHC concentrations, the multimers are saturated. (C) At high agonist–pMHC concentrations, monovalent TCR•CD3s begin to be activated. This allows the T cell to sense high doses of antigen when the multivalent receptors are saturated. (D) Only at high ligand concentrations, when all multivalent and monovalent receptors are engaged, is the T-cell response saturated. APC, antigen-presenting cell.
In conclusion, the role of the multimers might be to enable T cells to respond to low concentrations of antigen, for example, at the onset of an immune response (Fig 4). Mathematical modelling has shown that cooperative effects within the receptor multimers amplify small differences in ligand affinity by several orders of magnitude (Schamel et al, 2006). Thus, multivalent TCR•CD3s might be more effective in discriminating between distinct pMHC than monovalent TCR•CD3s. This resolves the paradox of the high specificity–low affinity TCR•CD3–pMHC interaction.
Monovalent TCR•CD3s raise the saturation threshold
T cells respond to a wide range of pMHC concentrations (Bachmann et al, 1998; Cochran et al, 2000; Irvine et al, 2002). When the multivalent TCR•CD3 complexes are already saturated, the monovalent receptors can still be activated at increasing ligand doses (Schamel et al, 2005). Indeed, theoretical calculations suggest that cells co-expressing monovalent and multivalent forms of a given receptor can show high sensitivity to low concentrations of ligand and concentration-dependent responses to high ligand concentrations (Bray et al, 1998). Thus, the presence of monovalent TCR•CD3 might endow the T cell with the capacity to produce a concentration-dependent response even at high pMHC doses when multivalent TCR•CD3s are already saturated (Fig 5).
Conclusions
The controversy over the TCR•CD3 stoichiometry has been resolved by combining advanced biochemical methods using different detergents in combination with non-invasive techniques, such as FRET and immuno-gold EM. The demonstration of the co-existence of monovalent and multivalent TCR•CD3 complexes on the surface of a T cell is a foundation on which new hypotheses can be generated and tested experimentally. Hopefully, we can now take a big step forwards in understanding the TCR•CD3 complex, as well as T-cell biology as a whole.
Acknowledgments
We thank M. Reth for discussions, and M.M. Davis and B. Lillemeier for sharing unpublished data. This work was supported by grant SAF2005-00937 from the Comisión Interministerial de Ciencia y Tecnología (to B.A.) and by German–Spanish joint grants HA20020094 and D/0232957 from the Deutscher Akademischer Austauschdienst. W.W.A.S. was supported by a Marie Curie Individual Fellowship from the European Union, and by the Deutsche Forschungsgemeinschaft through the Emmy Noether programme and the SFB620. The institutional support of the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa is acknowledged.
References
- Alarcón B, Gil D, Delgado P, Schamel WWA (2003) Initiation of TCR signaling: regulation within CD3 dimers. Immunol Rev 191: 38–46 [DOI] [PubMed] [Google Scholar]
- Anderson HA, Hiltbold EM, Roche PA (2000) Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol 1: 156–162 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Speiser DE, Zakarian A, Ohashi PS (1998) Inhibition of TCR triggering by a spectrum of altered peptide ligands suggests the mechanism for TCR antagonism. Eur J Immunol 28: 3110–3119 [DOI] [PubMed] [Google Scholar]
- Blumberg RS, Alarcón B, Sancho J, McDermott FV, Lopez P, Breitmeyer J, Terhorst C (1990) Assembly and function of the T cell antigen receptor. Requirement of either the lysine or arginine residues in the transmembrane region of the α chain. J Biol Chem 265: 14036–14043 [PubMed] [Google Scholar]
- Blumberg RS, Ley S, Sancho J, Lonberg N, Lacy E, McDermott F, Schad V, Greenstein JL, Terhorst C (1990) Structure of the T-cell antigen receptor: evidence for two CD3ε subunits in the T-cell receptor–CD3 complex. Proc Natl Acad Sci USA 87: 7220–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface JJ, Rabinowitz JD, Wülfing C, Hampl J, Reich Z, Altman JD, Kantor RM, Beeson C, McConnell HM, Davis MM (1998) Initiation of signal transduction through the T cell receptor requires the peptide multivalent engagement of MHC ligands. Immunity 9: 459–466 [DOI] [PubMed] [Google Scholar]
- Bray D, Levin MD, Morton-Firth CJ (1998) Receptor clustering as a cellular mechanism to control sensitivity. Nature 393: 85–88 [DOI] [PubMed] [Google Scholar]
- Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW (2002) The organizing principle in the formation of the T cell receptor–CD3 complex. Cell 111: 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call ME, Pyrdol J, Wucherpfennig KW (2004) Stoichiometry of the T-cell receptor–CD3 complex and key intermediates assembled in the endoplasmic reticulum. EMBO J 23: 2348–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran JR, Cameron TO, Stern LJ (2000) The relationship of MHC–peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity 12: 241–250 [DOI] [PubMed] [Google Scholar]
- Cosson P, Lankford SP, Bonifacino JS, Klausner RD (1991) Membrane protein association by potential intramembrane charge pairs. Nature 351: 414–416 [DOI] [PubMed] [Google Scholar]
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y (1998) Ligand recognition by α/β T cell receptors. Annu Rev Immunol 16: 523–544 [DOI] [PubMed] [Google Scholar]
- de la Hera A, Muller U, Olsson C, Isaaz S, Tunnacliffe A (1991) Structure of the T cell antigen receptor (TCR): two CD3ε subunits in a functional TCR/CD3 complex. J Exp Med 173: 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Kastrup J, Lauritsen JP, Menne C, von Bulow F, Geisler C (1999) TCRζ is transported to and retained in the Golgi apparatus independently of other TCR chains: implications for TCR assembly. Eur J Immunol 29: 1719–1728 [DOI] [PubMed] [Google Scholar]
- Exley M, Wileman T, Mueller B, Terhorst C (1995) Evidence for multivalent structure of T-cell antigen receptor complex. Mol Immunol 32: 829–839 [DOI] [PubMed] [Google Scholar]
- Fernandez-Miguel G, Alarcón B, Iglesias A, Bluethmann H, Alvarez-Mon M, Sanz E, de la Hera A (1999) Multivalent structure of an αβ T cell receptor. Proc Natl Acad Sci USA 96: 1547–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green NM (1991) Biological membranes. The semiotics of charge. Nature 351: 349–350 [DOI] [PubMed] [Google Scholar]
- Hellwig S, Schamel WW, Pflugfelder U, Gerlich B, Weltzien HU (2005) Differences in pairing and cluster formation of T cell receptor α- and β-chains in T cell clones and fusion hybridomas. Immunobiology 210: 685–694 [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Levine TP (2005) Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6: 209–220 [DOI] [PubMed] [Google Scholar]
- Hou X, Dietrich J, Kuhlmann J, Wegener AM, Geisler C (1994) Structure of the T cell receptor in a Ti α Vβ2, α Vβ8-positive T cell line. Eur J Immunol 24: 1228–1233 [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM (2002) Direct observation of ligand recognition by T cells. Nature 419: 845–849 [DOI] [PubMed] [Google Scholar]
- Krishna S, Benaroch P, Pillai S (1992) Tetrameric cell-surface MHC class I molecules. Nature 357: 164–167 [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM (2005) Agonist/endogenous peptide–MHC heterodimers drive T cell activation and sensitivity. Nature 434: 238–243 [DOI] [PubMed] [Google Scholar]
- Kropshofer H, Spindeldreher S, Rohn TA, Platania N, Grygar C, Daniel N, Wolpl A, Langen H, Horejsi V, Vogt AB (2002) Tetraspan microdomains distinct from lipid rafts enrich select peptide–MHC class II complexes. Nat Immunol 3: 61–68 [DOI] [PubMed] [Google Scholar]
- Malissen B (2003) An evolutionary and structural perspective on T cell antigen receptor function. Immunol Rev 191: 7–27 [DOI] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML (2005) Altered TCR signaling from geometrically repatterned immunological synapses. Science 310: 1191–1193 [DOI] [PubMed] [Google Scholar]
- Punt JA, Roberts JL, Kearse KP, Singer A (1994) Stoichiometry of the T cell antigen receptor (TCR) complex: each TCR/CD3 complex contains one TCRα, one TCRβ and two CD3ε chains. J Exp Med 180: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM (2004) T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol 5: 524–530 [DOI] [PubMed] [Google Scholar]
- Reth M (1989) Antigen receptor tail clue. Nature 338: 383. [DOI] [PubMed] [Google Scholar]
- Rojo JM, Portoles P (1991) A symmetrical view of the T-cell receptor–CD3 complex. Immunol Today 12: 377–378 [DOI] [PubMed] [Google Scholar]
- San Jose E, Sahuquillo AG, Bragado R, Alarcón B (1998) Assembly of the TCR/CD3 complex: CD3εδ and CD3εγ dimers associate indistinctly with both TCRα and TCRβ chains. Evidence for a double TCR heterodimer model. Eur J Immunol 28: 12–21 [DOI] [PubMed] [Google Scholar]
- Schamel WW, Reth M (2000) Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13: 5–14 [DOI] [PubMed] [Google Scholar]
- Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcón B (2005) Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med 202: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel WW, Risueno RM, Minguet S, Ortiz AR, Alarcón B (2006) A conformation- and avidity-based proofreading mechanism for the TCR–CD3 complex. Trends Immunol 27: 176–182 [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231 [DOI] [PubMed] [Google Scholar]
- Sun ZY, Kim ST, Kim IC, Fahmy A, Reinherz EL, Wagner G (2004) Solution structure of the CD3εδ ectodomain and comparison with CD3εγ as a basis for modeling T cell receptor topology and signaling. Proc Natl Acad Sci USA 101: 16867–16872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin M, Masucci MG, Levitsky V (2003) Pharmacological disintegration of lipid rafts decreases specific tetramer binding and disrupts the CD3 complex and CD8 heterodimer in human cytotoxic T lymphocytes. Scand J Immunol 57: 99–106 [DOI] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A (1995) Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375: 148–151 [DOI] [PubMed] [Google Scholar]
- Wülfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM (2002) Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol 3: 42–47 [DOI] [PubMed] [Google Scholar]
- Yachi PP, Ampudia J, Gascoigne NR, Zal T (2005) Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol 6: 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Dustin ML, Saito T (2005) Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol 6: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Zapata DA, Schamel WW, Torres PS, Alarcón B, Rossi NE, Navarro MN, Toribio ML, Regueiro JR (2004) Biochemical differences in the αβ T cell receptor. CD3 surface complex between CD8+ and CD4+ human mature T lymphocytes. J Biol Chem 279: 24485–24492 [DOI] [PubMed] [Google Scholar]


