Abstract
Tumour necrosis factor receptor (TNFR)1 is the main receptor responsible for TNF-induced diverse cellular events. In this study, we report that TNFR1 has a crucial role in endoplasmic reticulum (ER) stress-induced Jun amino-terminal kinase (JNK) activation. Although ER stress leads to JNK activation in wild-type mouse embryo fibroblasts, we failed to detect any JNK activation in TNFR1−/− cells. ER stress-induced JNK activation is restored in TNFR1−/− cells when TNFR1 expression is reconstituted. We also found that TNFR1 functions downstream of IRE1 and that IRE1 is present in the same complex with TNFR1 under ER stress condition. Therefore, our study shows a novel role of TNFR1 in mediating ER stress-induced JNK activation.
Keywords: ER stress, JNK, RIP, TNFR1
Introduction
Tumour necrosis factor receptor (TNFR)1 is the main receptor that mediates many TNF-induced cellular events such as septic shock, induction of cytokines, cell proliferation, differentiation and apoptosis (Tracey & Cerami, 1993; Baud & Karin, 2001). In response to TNF treatment, TNFR1 trimerizes and forms a signalling complex with several adaptor proteins, including TNF receptor-associating factor 2 (TRAF2) and TNF receptor-interacting protein (RIP), and elicits diverse cellular responses. It has been shown that TRAF2 and RIP are essential signalling components of TNF-induced activation of the transcription factor nuclear factor-κB (NF-κB) and of mitogen-activated protein (MAP) kinases (Liu et al, 1996; Reinhard et al, 1997; Chen & Goeddel, 2002; Devin et al, 2003). Although TRAF2 was first identified as a protein that interacts with TNFR2, it was subsequently found in the receptor complex of several other TNFR family members, such as CD40, CD30 and TNFR1 (Rothe et al, 1994; Arch et al, 1998; Wajant & Scheurich, 2001). TRAF2 is one of the seven known TRAF family members. All the members of this family have a conserved carboxy-terminal domain, known as the TRAF domain, which mediates receptor/adaptor binding as well as homo- and hetero-oligomerization with other TRAF proteins (Bradley & Pober, 2001; Chung et al, 2002). RIP is a death domain kinase first identified as a Fas (CD95)-interacting protein (Stanger et al, 1995). Later studies showed its crucial role in TNF and TRAIL (TNF-related apoptosis-inducing ligand) signalling (Hsu et al, 1996; Lin et al, 2000). Although they are known as the key effector proteins of TNF signalling, TRAF2 and RIP are also involved in other cellular responses under diverse stress conditions, including oxidative stress and DNA damage (Hur et al, 2003; Shen et al, 2004; Noguchi et al, 2005).
The endoplasmic reticulum (ER) is the organelle in which protein folding occurs and the accumulation of unfolded proteins in the lumen of the ER results in induction of the unfolded protein response (UPR) and in activation of the MAP kinase Jun amino-terminal kinase (JNK; Urano et al, 2000). In response to ER stress, caused by glucose starvation, disturbance of intracellular stores of Ca2+ stores or inhibition of protein glycosylation, protein folding reactions are compromised and malfolded proteins accumulate in the ER lumen. To maintain cellular homeostasis, the stressed cells induce an adaptive response, the so-called UPR (Mori, 2000; Ma & Hendershot, 2001; Patil & Walter, 2001). The UPR is of fundamental importance for the survival of all eukaryotic cells under the condition of ER stress and normal growth conditions. Alteration of the ER processing of unfolded proteins by pathogens or genetic lesions results in disease (Aridor & Balch, 1999). In mammals, the UPR is mediated by three types of ER transmembrane protein: the protein kinase and site-specific endoribonuclease IRE1 (Tirasophon et al, 1998; Wang et al, 1998; Iwawaki et al, 2001); the eukaryotic translation initiation factor 2 kinase PERK/PEK (protein kinase R (PKR)-like endoplasmic reticulum (ER) kinase; Shi et al, 1998; Harding et al, 1999, 2000); and the transcriptional activator ATF6 (Yoshida et al, 1998, 2001; Li et al, 2000). It has also been reported that MAP kinase JNK is activated under ER stress condition and that IRE1 has a crucial role in this process by recruiting TRAF2 (Urano et al, 2000).
In this report, we show that TNFR1 mediates ER stress-induced JNK activation independently of TNF. By using RIP−/− and TNFR1−/− fibroblasts, we found that RIP and TNFR1 are required for JNK activation in response to ER stress. ER stress induces JNK activation in wild-type (WT) but not RIP−/− or TNFR1−/− fibroblasts. Ectopic expression of RIP or TNFR1 in RIP−/− or TNFR1−/− fibroblasts restored ER stress-induced JNK activation. Overexpression of IRE1α failed to activate JNK in TNFR1−/− cells. Our co-immunoprecipitation experiments indicated that TNFR1 might interact with IRE1α and that IRE1α might be present in the same complex with TNFR1 and RIP following ER stress. Therefore, our study shows that TNFR1 is the crucial mediator of ER stress-induced JNK activation.
Results and Discussion
JNK activation by ER stress requires RIP and TNFR1
It has been suggested that IRE1α mediates ER stress-induced JNK activation by recruiting TRAF2 (Urano et al, 2000). As we found that RIP is also required for TNF-induced JNK activation, we tested whether RIP is involved in ER stress-induced JNK activation. As shown in Fig 1A, when WT fibroblasts were treated with thapsigargin (Tg) or tunicamycin (Tu), which induce ER stress by the depletion of ER Ca2+ stores or inhibition of protein glycosylation, respectively, JNK activity, measured by in vitro kinase assay (Liu et al, 1996), is elevated. However, we failed to detect any JNK activation after Tg or Tu treatment in RIP−/− fibroblasts (Fig 1A). The protein levels of JNK1 and RIP in WT and RIP−/− cells were detected with an anti-JNK1 or an anti-RIP antibody, as shown in Fig 1A. This observation was further confirmed with anti-phospho-JNK antibody (Fig 2C). To prove that the lack of JNK activation in RIP−/− cells is due to the absence of RIP protein, we established RIP−/− cells that were stably transfected with RIP (Fig 1B). As shown in Fig 1C, ER stress-induced JNK activation was recovered in RIP-reconstituted RIP−/− cells, even though the protein level of RIP was much lower in RIP-reconstituted cells than in WT cells (Fig 1B). These results indicate that RIP, like TRAF2, is essential for JNK activation in response to ER stress.
Figure 1.
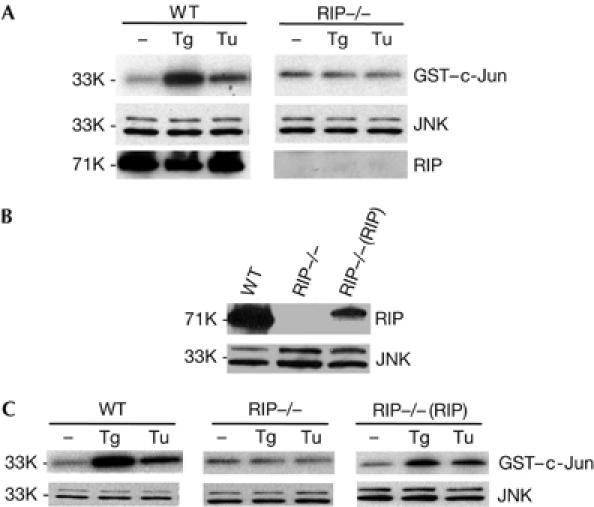
RIP is required for the activation of JNK induced by the ER stress agents. (A) WT and RIP−/− cells were treated with 2 μM Tg and 15 μg/ml Tu. Cell extracts were used for JNK kinase assay and western blot. (B) Protein expression levels in WT, RIP−/− and RIP−/−(RIP) cells. (C) Reconstitution of ER stress agent-induced JNK activation in RIP−/−(RIP) cells. WT, RIP−/− and RIP−/−(RIP) cells were treated with Tg or Tu. Cell extracts were used for JNK kinase assay and western blot. ER, endoplasmic reticulum; JNK, Jun amino-terminal kinase; RIP, tumour necrosis factor receptor-interacting protein; Tg, thapsigargin; Tu, tunicamycin; WT, wild type. Numbers on the left are relative molecular masses.
Figure 2.
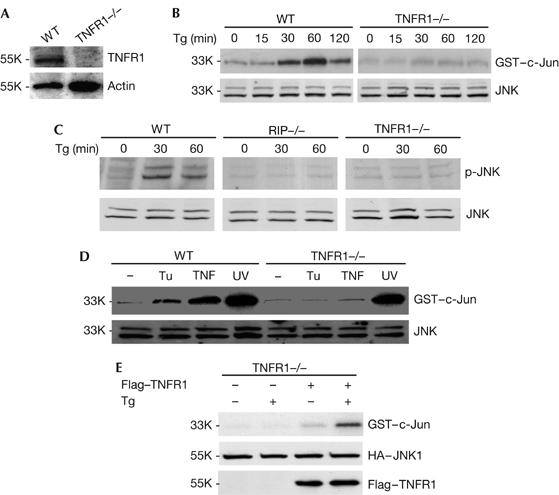
TNFR1 is required for the activation of JNK induced by the ER stress agents. (A) Western blot shows the TNFR1 level in WT and TNFR1−/− cells. (B) WT and TNFR1−/− cells were treated with 2 μM Tg for 0, 15, 30, 60 and 120 min. Cell extracts were used for JNK kinase assay and western blot. (C) WT, RIP−/− and TNFR1−/− cells were treated with 2 μM Tg for 0, 30 and 60 min. Cell extracts were used for western blotting with anti-phospho-JNK1 and anti-JNK antibodies. (D) WT and TNFR1−/− cells were treated with 15 μg/ml Tu for 2 h, 30 ng/ml TNF for 15 min and UV for 1 h. Cell extracts were used for JNK kinase assay and western blot. (E) TNFR1−/− cells were transiently transfected with plasmids HA–JNK1 and Flag–TNFR1, and then subjected to Tg treatment for 1 h. Cell extracts were used for JNK kinase assay and western blot. ER, endoplasmic reticulum; HA, haemagglutinin; JNK, Jun amino-terminal kinase; RIP, tumour necrosis factor receptor-interacting protein; TNFR1, tumour necrosis factor receptor 1; Tg, thapsigargin; Tu, tunicamycin; WT, wild type. Numbers on the left are relative molecular masses.
As both TRAF2 and RIP are key effector molecules for TNF signalling, we examined whether TNFR1 is involved in ER stress-induced JNK activation. To do so, WT and TNFR1−/− fibroblast cells (Fig 2A) were treated with Tg or Tu. As shown in Fig 2B, JNK activation was detected at 30 min after Tg treatment, it peaked at 1 h and was still detectable at 2 h in WT fibroblast cells. In contrast, no obvious JNK activation was detected in response to Tg in TNFR1−/− fibroblast cells. This observation is further confirmed by anti-phospho-JNK antibody (Fig 2C). Similarly, Tu-induced JNK activation was found only in WT cells but not in TNFR1−/− cells (Fig 2D). As controls, TNF- and UV-induced JNK activation was also examined in these cells. Whereas UV treatment led to robust JNK activation in both WT and TNFR1−/− cells, TNF activates JNK only in WT and not in TNFR1−/− cells. These results indicated that TNFR1 is required for ER stress-induced JNK activation. To test whether ectopic expression of TNFR1 in TNFR1−/− cells could restore ER stress-induced JNK activation, we transiently transfected a plasmid encoding Flag-tagged TNFR1 into TNFR1−/− cells. As shown in Fig 2E, JNK activation by Tg was reconstituted when Flag–TNFR1 was presented in TNFR1−/− cells. Haemagglutinin (HA)–JNK and TNFR1 protein levels in these experiments are shown in Fig 2E as controls. Therefore, these findings indicate that, in addition to TRAF2 and RIP, TNFR1 might be essential for ER stress-induced JNK activation.
IRE1α interacts with TNFR1 following ER stress
As both TNFR1 and TNFR2 are able to mediate TNF signalling, we tested whether TNFR2 is required for ER stress-induced JNK activation. As shown in Fig 3A, whereas Tg treatment failed to elevate JNK activity in TNFR1−/− cells, JNK activation by Tg was relatively normal in TNFR2 fibroblast cells. These results indicate that ER stress-induced JNK activation is specifically mediated by TNFR1. Because TRAF2 and RIP, two key effector molecules of TNFR1 signalling, are also involved in ER stress-induced JNK activation, these data indicate that ER stress engages the TNFR1 pathway to activate JNK. One possibility is that ER stress may result in the production or release of TNF, which then activates TNFR1 signalling, leading to JNK activation. To explore this possibility, an antagonistic TNFR1 antibody was used to block TNFR1 signalling. For this, WT fibroblast cells were incubated with this antibody 30 min before Tg or TNF treatment. As shown in Fig 3B, the administration of this TNFR1 antibody completely blocked TNF-induced JNK activation but had little effect on Tg-induced JNK activation. Therefore, it is unlikely that ER stress-induced JNK activation is due to the production or release of TNF in cells under ER stress.
Figure 3.
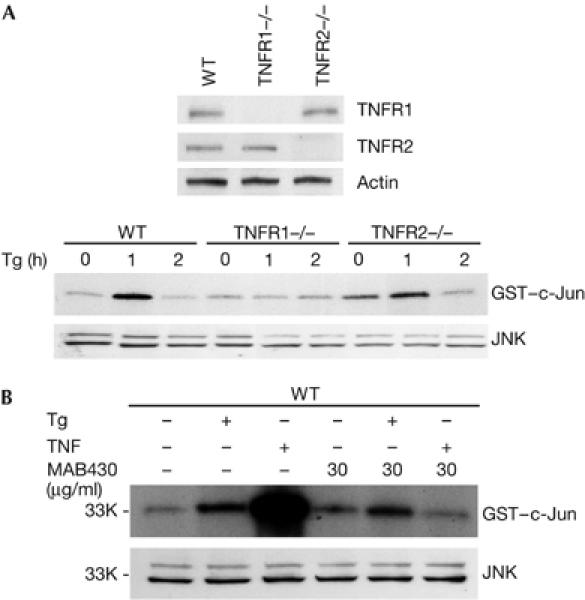
ER stress-induced JNK activation is mediated by TNFR1. (A) Western blot shows protein expression levels in wild-type, TNFR1−/− and TNFR2−/− cells (top panel) and cells were treated with Tg for 0, 1 and 2 h. Cell extracts were used for JNK kinase assay and western blot. (B) WT cells blocked with 30 μg/ml TNFR1 antibody (R&D Systems, MAB430) for 30 min, and then treated with 30 ng/ml TNF and 2 μM Tg for 15 min and 1 h, respectively. Cell extracts were used for JNK, kinase and western blot. ER, endoplasmic reticulum; JNK, Jun amino-terminal kinase; TNFR, tumour necrosis factor receptor; Tg, thapsigargin; WT, wild type. Numbers at the left are relative molecular masses.
It has been reported that overexpression of IRE1α leads to the activation of JNK. To help understand the mechanism by which TNFR1 mediates ER stress-induced JNK activation, we tested whether JNK activation by IRE1α overexpression requires TNFR1. For this, we co-transfected HA–JNK1 and Flag–IRE1α plasmids into WT and TNFR1−/− fibroblast cells, and JNK activity was measured by an in vitro kinase assay after immunoprecipitation of HA–JNK1. As shown in Fig 4A, overexpression of IRE1α activates JNK in WT fibroblast cells, but not in TNFR1−/− cells. These results indicate that TNFR1 may function downstream of IRE1α in the process of JNK activation in response to ER stress. The early study by Urano et al (2000) showed that IRE1α mediates ER stress-induced JNK activation by interacting with TRAF2. As our data indicate that TNFR1 is downstream of IRE1α, we tested whether IRE1α interacts with TNFR1. We co-transfected 293 cells with Flag–IRE1α and green fluorescent protein (GFP)–TNFR1 plasmids and carried out immunoprecipitation experiments with anti-GFP antibody. As shown in Fig 4B, Flag-tagged IRE1α was co-immunoprecipitated with GFP–TNFR1 and the treatment of Tg increased the interaction between IRE1α and TNFR1. As it is known that IRE1α interacts with TRAF2 and that TRAF2 is a key effector of TNFR1 signalling, we performed similar co-immunoprecipitation experiments in TRAF2−/− fibroblast cells to rule out the possibility that the interaction between IRE1α and TNFR1 is mediated by TRAF2, and similar results to those in Fig 4B were obtained with TRAF2−/− cells (data not shown). These results indicate that IRE1α interacts with TNFR1 independently of TRAF2. As TNFR1 normally localizes within cell plasma membrane, whereas IRE1α is at ER, it is important to know whether TNFR1 localizes at ER after ER stress. To investigate this possibility, we stained WT MEF cells with anti-TNFR1 antibody and anti-calnexin (CNX), which specifically labels ER. As shown in Fig 4C, there is a small amount of TNFR1 localized at ER before Tg treatment. But the level of TNFR1 at ER is markedly increased after Tg treatment. Therefore, these results imply that TNFR1 accumulates in ER under ER stress condition. As a control, we used the same anti-TNFR1 antibody to stain TNFR1−/− cells and did not observe any specific staining (data not shown). Finally, we examined whether endogenous IRE1α and TNFR1 form a complex in response to ER stress. As shown in Fig 4D, after Tg treatment, WT fibroblast cells were collected at different periods of time and immunoprecipitation experiments were carried out with an anti-TNFR1 antibody. Both RIP and IRE1α were co-precipitated with TNFR1 in response to Tg treatment, indicating that TNFR1, IRE1α and RIP might form a complex in cells under ER stress.
Figure 4.
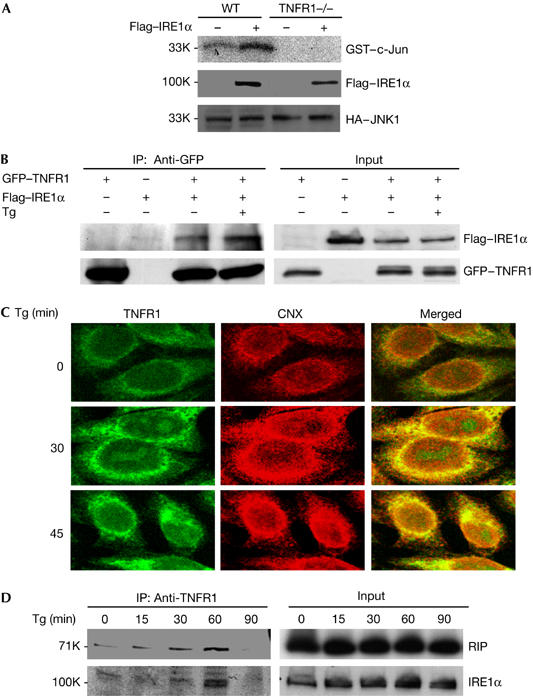
IRE1α interacts with TNFR1 and forms a complex with TNFR1 and RIP in response to ER stress. (A) WT and TNFR1−/− cells were co-transfected with plasmids HA–JNK1 and Flag–IRE1α overnight. Cell extracts were immunoprecipitated by anti-HA antibody, and then used for JNK kinase assay. (B) 293 cells were transiently transfected with plasmids GFP–TNFR1 and Flag–IRE1α, and then left without treatment or treated with Tg for 1 h. Cell extracts were used for immunoprecipitation with anti-GFP antibody. (C) The colocalization of TNFR1 with ER marker protein calnexin (CNX). WT cells were treated with 2 μM Tg for 30 or 45 min. The cells were immunostained with anti-TNFR1 antibody (R&D Systems) and anti-calnexin (CNX, BD). Confocal images indicate the colocalization of TNFR1 and CNX in ER in response to Tg treatment. (D) WT cells were treated with 2 μM Tg and collected for immunoprecipitation with anti-TNFR1 antibody. ER, endoplasmic reticulum; GFP, green fluorescent protein; HA, haemagglutinin; JNK, Jun amino-terminal kinase; RIP, tumour necrosis factor receptor-interacting protein; TNFR1, tumour necrosis factor receptor 1; Tg, thapsigargin; WT, wild type. Numbers at the left are relative molecular masses.
Previous studies have suggested that JNK activation is crucial for ER-induced apoptosis (Nishitoh et al, 2002; Nawrocki et al, 2005). As we found that TNFR1 and RIP are required for ER stress-induced JNK activation, we next tested whether TNFR1 and RIP are involved in ER stress-induced apoptosis. As shown in supplementary Fig 1 online, both TNFR1- and RIP-null MEFs are more resistant than WT MEF cells to ER stress-induced apoptosis. We also examined Bip/GRP78 expression and XBP1 protein in TNFR1−/− cells after Tg treatment. Bip/GRP78 messenger RNA level and the level of the spliced form of XBP1 protein are decreased in TNFR1−/− cells (supplementary Fig 2 online). We examined ASK1 activation as well and did not detect any ASK1 activation in WT or TNFR1−/− cells after Tg treatment (data not shown).
TNFR1 is known as the key receptor responsible for eliciting diverse cellular events in response to TNF. Our study shows that TNFR1 is a crucial mediator of ER stress-induced JNK activation. As TNFR1 signalling can activate NF-κB, we also examined whether ER stress leads to the activation of NF-κB, as reported recently (Mauro et al, 2006). However, we failed to detect any NF-κB activation after Tg or Tu treatment on examining IκBα degradation (data not shown). One possible explanation is that certain modifications, such as ubiquitination, of crucial signalling molecules, which are necessary for activating NF-κB by TNFR1, did not take place when the IRE1α, TNFR1 and RIP complex formed in response to ER stress. This may be true, because we did not find any ubiquitinated RIP in the IRE1α/TNFR1 complex (Fig 4D).
Earlier reports have suggested that TNF is important in ER stress and that several TRAF proteins including TRAF2 and TRAF7 are crucial mediators of ER stress responses (Urano et al, 2000; Xu et al, 2004; Xue et al, 2005). However, our current study provided evidence that TNFR1 also has a key role in ER stress-induced JNK activation and apoptosis. More importantly, our work shows a novel function of TNFR1 independently of its ligand, TNF. Our finding that TNFR1, RIP and IRE1α form a complex in response to ER stress implies that IRE1α recruits TNFR1 to form a signalling complex, which in turn leads to JNK activation.
Methods
Reagents. Tg and Tu were from Sigma (St Louis, MO, USA). Antibodies of TNFR1 and TNFR2 were from R&D Systems (Minneapolis, MN, USA), anti-JNK1 was from Pharmingen (San Jose, CA, USA) and anti-phospho-JNK1 was from Cell Signaling (Beverly, MA, USA). The antibody against RIP was from Transduction Laboratories (San Jose, CA, USA). Anti-HA and anti-GFP were from Santa Cruz (Santa Cruz, CA, USA), and anti-actin and anti-Flag were purchased from Sigma.
Cell culture. WT, TNFR1−/−, TNFR2−/− and RIP−/− mouse 3T3-like fibroblast cells and 293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 μg/ml). RIP-reconstituted stable cell lines (RIP−/−(RIP)) were also cultured in this medium, including 300 μg/ml hygromycin B.
Western blot analysis. After treatment as described in the figure legends, cells were collected and lysed in M2 buffer (20 mM Tris-HCl pH 7.0, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulphonyl fluoride, 20 mM glycerol phosphate, 1 mM sodium vanadate, 1 μg/ml leupeptin). The cell lysate (20 μg) from each sample was fractionated by SDS–polyacrylamide gel electrophoresis and immunoblotted. The blots were visualized with enhanced chemiluminescence in accordance with the manufacturer's instructions (Amersham, Piscataway, NJ, USA).
Kinase assays. Transfected or non-transfected cells were treated with Tg or Tu as indicated in the figure legends and then collected in M2 lysis buffer. JNK1 was immunoprecipitated with anti-JNK1 or anti-HA antibody and collected with protein A–Sepharose beads (Roche, Penzberg, Germany). JNK kinase activities were determined using GST–c-Jun (1–79) substrates.
Immunoprecipitation assay. 293 cells were co-transfected with plasmids GFP–TNFR1 and Flag–IRE1α and then treated with Tg. Non-transfected WT cells were treated as indicated in the figure legend. Then, the cells were collected in M2 lysis buffer. The immunoprecipitation experiments were carried out with anti-GFP antibody or anti-TNFR1 antibody. Proteins were resolved by SDS–polyacrylamide gel electrophoresis and detection was accomplished by western blot.
Immunostaining assay. WT MEF cells were double-stained with anti-TNFR1 and ER marker protein CNX antibody. Cells were treated with 2 μM Tg for 30 or 45 min, washed with 1 × PBS three times and then fixed with 4% paraformaldehyde. After washing with 1 × PBS, cells were blocked with 20% normal donkey serum for 30 min, and then incubated with the mixture of 1:100 biotinylated anti-TNFR1 antibody (R&D Systems) and 1:100 anti-CNX (McAb, BD) for 60 min. After washing with 1 × PBS, cells were incubated with the secondary antibody mixture of 1:100 avidin–fluorescein isothiocyanate and 1:1000 anti-mouse IgG with Alexa Fluor 594 (Molecular Probes, Eugene, OR, USA) for 30 min. Finally, cells were mounted and observed with confocal microscopy.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig 1
Acknowledgments
We thank Dr S. Nedospasov for TNF-R2−/− MEF cells and Dr M. Kelliher for RIP−/− MEF cells. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- Arch RH, Gedrich RW, Thompson CB (1998) Tumor necrosis factor receptor-associated factors (TRAFs): a family of adapter proteins that regulates life and death. Genes Dev 12: 2821–2830 [DOI] [PubMed] [Google Scholar]
- Aridor M, Balch WE (1999) Integration of endoplasmic reticulum signaling in health and disease. Nat Med 5: 745–751 [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11: 372–377 [DOI] [PubMed] [Google Scholar]
- Bradley JR, Pober JS (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20: 6482–6491 [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV (2002) TNFR1 signaling: a beautiful pathway. Science 296: 1634–1635 [DOI] [PubMed] [Google Scholar]
- Chung JY, Park YC, Ye H, Wu H (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 115: 679–688 [DOI] [PubMed] [Google Scholar]
- Devin A, Lin Y, Liu ZG (2003) The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep 4: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904 [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4: 387–396 [DOI] [PubMed] [Google Scholar]
- Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, Liu ZG (2003) The death domain kinase RIP has an essential role in DNA damage-induced NFκB activation. Genes Dev 17: 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata K, Tsuru A, Kohno K (2001) Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol 3: 158–164 [DOI] [PubMed] [Google Scholar]
- Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS (2000) ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol 20: 2096–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG (2000) The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IκB kinase and c-Jun N-terminal kinase. Mol Cell Biol 20: 6638–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M (1996) Dissection of TNF receptor 1 effecter functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87: 565–576 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM (2001) The unfolding tale of the unfolded protein response. Cell 107: 827–830 [DOI] [PubMed] [Google Scholar]
- Mauro C, Crescenzi E, De Mattia R, Pacifico F, Mellone S, Salzano S, de Luca C, D'Adamio L, Palumbo G, Formisano S, Vito P, Leonardi A (2006) Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281: 2631–2638 [DOI] [PubMed] [Google Scholar]
- Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101: 451–454 [DOI] [PubMed] [Google Scholar]
- Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K Jr, Huang P, Abbruzzese JL, McConkey DJ (2005) Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res 65: 11658–11666 [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue JI, Ichijo H (2005) Recruitment of TRAF family proteins to the ASK1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 280: 37033–37040 [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13: 349–355 [DOI] [PubMed] [Google Scholar]
- Reinhard C, Shamoon B, Shyamala V, Williams LT (1997) Tumor necrosis factor-α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J 16: 1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Wong SC, Enzel WJ, Goeddel DV (1994) A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78: 681–692 [DOI] [PubMed] [Google Scholar]
- Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG (2004) Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol 24: 5914–5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18: 7499–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Leder P, Lee TH, Kim E, Seed B (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81: 513–523 [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease(Ire1p) in mammalian cells. Genes Dev 12: 1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A (1993) Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol 9: 317–343 [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Hardign HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666 [DOI] [PubMed] [Google Scholar]
- Wajant H, Scheurich P (2001) Tumor necrosis factor receptor-associated factor (TRAF)2 and its role in TNF signaling. Int J Biochem Cell Biol 33: 19–32 [DOI] [PubMed] [Google Scholar]
- Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17: 5708–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LG, Li LY, Shu HB (2004) TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J Biol Chem 279: 17278–17282 [DOI] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H (2005) Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J Biol Chem 280: 33917–33925 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273: 33741–33749 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Negishi M, Mori K (2001) Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y(CBF) and activating transcription factors 6 and 6 that activates the mammalian unfolded protein response. Mol Cell Biol 21: 1239–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig 1


