Abstract
Objective:
To study the efficacy of growth hormone given to severely burned children from discharge to 12 months after burn and for 12 months after the drug was discontinued.
Summary Background Data:
We have previously shown that low-dose recombinant human growth hormone (rhGH), given to children after a severe thermal injury, successfully improved lean muscle mass, bone mineral content, and growth. The aim of the present study was to investigate long-term functional improvements after treatment.
Methods:
Forty-four pediatric patients with over 40% total body surface area burns were studied for 24 months after burn. Patients were randomized to receive either rhGH (0.05 mg/kg body weight) or placebo. Height, weight, body composition, serum hormones, resting energy expenditure, cardiac function, muscle strength, and number of reconstructive procedures performed were measured during rhGH treatment and for 12 months after treatment was discontinued. Statistical analysis used Tukey's multiple comparison test. Significance was accepted at P < 0.05.
Results:
Height, weight, lean body mass, bone mineral content, cardiac function, and muscle strength significantly improved during rhGH treatment compared with placebo (P < 0.05). This treatment significantly increased GH, IGF-I, and IGFBP-3, whereas serum cortisol decreased (P < 0.05). The number of operative reconstructive procedures was significantly lower with rhGH (P < 0.05). Improvements in height, bone mineral content, and IGF-1 concentrations persisted after rhGH treatment (P < 0.05). No side effects with rhGH were observed.
Conclusions:
Administration of rhGH for 1 year after burn was safe and improved recovery. These salutary effects continued after rhGH treatment was discontinued.
Pediatric burn patients who received low-dose rhGH treatment from hospital discharge to 12 months after a severe burn showed improved growth, body composition, and function. These positive effects persisted after discontinuing rhGH treatment without any negative side effects.
Recovery from a massive burn is characterized by persisting catabolic and hypermetabolic responses.1 The clinical response is characterized by an increase in resting energy expenditure, tachycardia, a negative muscle protein balance, bone wasting, and growth retardation.2–4 These negative clinical responses result in a significant delay in rehabilitation and reintegration of these children back into society. Anabolic agents, such as recombinant human growth hormone (rhGH) have been used successfully to attenuate the hypermetabolic and catabolic response during the acute phase after burn.5,6 Growth hormone, given to severely burned children, has been shown to decrease whole body catabolism, increase protein synthesis, accelerate wound healing, and reverse growth arrest.7–12 The side effects of rhGH have been well described in the literature and indicate that it is safe for children.13 Based on this background, a study at our institution using rhGH for 1 year after burn showed beneficial effects on lean mass and bone mineral content, as well as on height, in children with ≥40% TBSA burns.14 However, it is unknown what effect this drug would have on function or whether endogenous hormone production would recover after drug cessation. To address these questions, we have investigated severely burned children for 24 months after burn, 12 months after discontinuing rhGH, looking additionally at reconstructive procedures, strength, cardiac function, scarring as well as body composition, and endogenous hormone production.
METHODS
Subjects
Forty-four massively burned children were enrolled between 1999 and 2004 in a double-blinded randomized study to test the efficacy of rhGH administered after hospital discharge to 12 months after burn with the patients studied for an additional 12 months after treatment was stopped. Inclusion criteria were: age ≤19 years, TBSA burns of ≥40%, and availability for studies at discharge, 6, 12, 18, and 24 months after injury. This study was approved by the Institutional Review Board at the University of Texas Medical Branch. Informed written consent was obtained from each patient's guardian with the assent of the child prior to enrollment.
Patients were randomized to receive daily 0.05 mg/kg rhGH (Lilly, Indianapolis, IN) or placebo subcutaneously from hospital discharge for up to 12 months after burn. The dose of rhGH was based on the previously demonstrated beneficial effects seen in children with Turner's syndrome.15 Guardians and patients were instructed and supervised in the proper use of the drug, and compliance was checked by questionnaires and by serum levels of insulin-like growth factor-I (IGF-I). Patients were studied at discharge (4–8 weeks after trauma), and 6, 12, 18, and 24 months after injury (Fig. 1). At the time of hospital admission and follow-up, patients were examined by physicians, including a pediatric endocrinologist, and reviewed by a safety committee to screen for compliance and adverse side effects such as hyperglycemia and glucose intolerance. Pubertal development was assessed using the Tanner score,16 and hand and knee x-rays were taken of each subject at each follow-up period to evaluate possible premature closure of epiphyseal plates induced by anabolic agents.
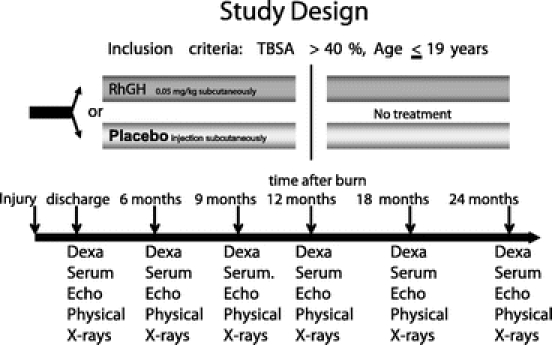
FIGURE 1. Study protocol for body composition including Dexa, height and weight, serum (hormone analysis), and clinical assessment (CA) measures. Clinical assessments include physical examinations and screening for adverse side effects, which are evaluated by a committee of 5 clinical experts, including a pediatric endocrinologist to decide whether the treatment should be discontinued.
Body Composition
Body heights and weights were measured and the percent change calculated for the treatment and placebo group. Total lean body mass (LBM), fat, and bone mineral content (BMC) were measured by dual energy x-ray absorptiometry (Hologic model QDR-4500W, Hologic Inc, Waltham, MA). To minimize systematic deviations, the Hologic system was calibrated daily against a spinal phantom in the anteroposterior, lateral, and single-beam modes. Individual pixels were calibrated against a tissue bar phantom to determine whether the pixel was reading bone, fat, lean tissue, or air.17
Cardiac Function
Echocardiograms were taken prior to discharge and 12 and 24 months after burn. No test subject presented with or previously suffered other concomitant diseases affecting cardiac function, such as diabetes mellitus, coronary artery disease, long-standing hypertension, or hyperthyroidism. Study variables included: resting cardiac output, cardiac index, stroke volume, resting heart rate, and left ventricular ejection fraction. Stroke volume and cardiac output were adjusted for body surface area and expressed as indexes. All ultrasound measurements were made with the HP SONOS 100 CF echocardiogram (Hewlett Packard Imaging System, Andover, MA) with a 3.5-MHz transducer. Recordings were performed with the subjects in a supine position and breathing freely. M-mode tracings were obtained at the level of the tips of the mitral leaflets in the parasternal long axis position and measurements were performed according to the American Society of Echocardiography recommendation. Left ventricular volumes determined at end diastole and end systole were used to calculate ejection fraction, stroke volume, cardiac output, and cardiac index. Three measurements were averaged for data analysis.18
Strength Measurements
Strength testing was conducted on children age ≥7 years using a Biodex System-3 dynamometer (Shirley, NY). The isokinetic test was performed on the dominant leg extensors and tested at an angular velocity of 150°/s. This speed was chosen as it was well tolerated (compared with lower or higher angular speeds) by children of all ages. The patients were seated and their position stabilized with a restraining strap over the midthigh, pelvis, and trunk in accordance to the Biodex System-3 Operator's Manual. All patients were familiarized with the Biodex test. The administrator of the test demonstrated the procedure; then the test procedure was explained to patients who were allowed to practice the actual movement during 3 submaximal repetitions without load as a warmup. More repetitions were not allowed to prevent fatigue. The anatomic axis of the knee joint was aligned with the mechanical axis of the dynamometer before the test. After the 3 submaximal warmup repetitions, 10 maximal voluntary muscle contractions (full extension and flexion) were performed. The maximal repetitions were performed consecutively without rest in between. Three minutes of rest were given to minimize the effects of fatigue before the test sequence was repeated.
Peak torque was calculated by the Biodex software system. The highest peak torque measurement between the 2 trials was selected and corrected for gravitational moments of the lower leg and the lever arm.19
Reconstructive Procedures
A plastic surgeon, not involved in the study and blinded as to drug use, evaluated the patients of both groups at discharge, 6, 9, 12, 18, and 24 months after burn for the need of reconstructive operations to improve functional outcome. The decision was based on adequacy of the function of eyelids, mouth, neck, and joints.
Scar Assessment
Scars were evaluated clinically by observers blinded to treatment using the Vancouver Scar Scale.20
Indirect Calorimetry
Resting energy expenditure (REE) was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA). Composition of inspired and expired gases were sampled and analyzed at 60-second intervals. Values obtained during a 5-minute steady state were accepted. The average REE was calculated from steady-state measurements.18
Hormone Panel
Whole blood was withdrawn to determine serum GH, IGF-I, IGF binding protein-3 (IGFBP-3), osteocalcin, parathyroid hormone, insulin, cortisol, total thyroxine (total T4), tri-iodothyronine uptake (T3 uptake), and free thyroxine index. All were measured using enzyme linked immunosorbent assays from Diagnostic Systems Laboratory (Webster, TX).17
Statistical Analysis
Data are presented as means ± SEM. Statistical analysis used Tukey's multiple comparison test, with significance accepted at P < 0.05. Student t test was used for comparing reconstructive procedures with significance accepted at P < 0.05. Statistical software (SigmaStat and SigmaPlot, SPSS, Chicago, IL) was used for analyses.
RESULTS
Demographics
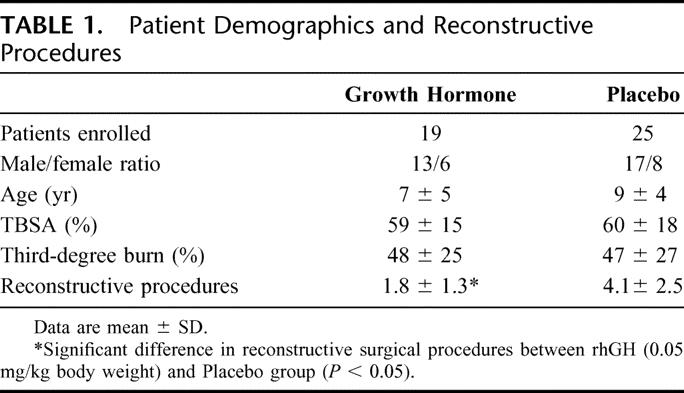
Forty-four patients were studied and randomized to receive rhGH (n = 19) or placebo (n = 25). The groups did not significantly differ in age, gender, ethnicity, and burn size (Table 1). There was no significant difference between groups for caloric intake, which was measured by a 24-hour dietary recall. Fourteen patients in the treatment group and 18 patients in the placebo group successfully completed the study.
TABLE 1. Patient Demographics and Reconstructive Procedures
Body Composition
Percent change in height increased with rhGH compared with placebo for up to 24 months after burn (P < 0.05) (Fig. 2). Bone mineral content was improved during rhGH treatment and continued to improve for 24 months after burn compared with placebo (P < 0.05) (Fig. 3). Percent changes in LBM and weight were higher at 12 months after burn in the rhGH group compared with placebo (P < 0.05) (Fig. 4).
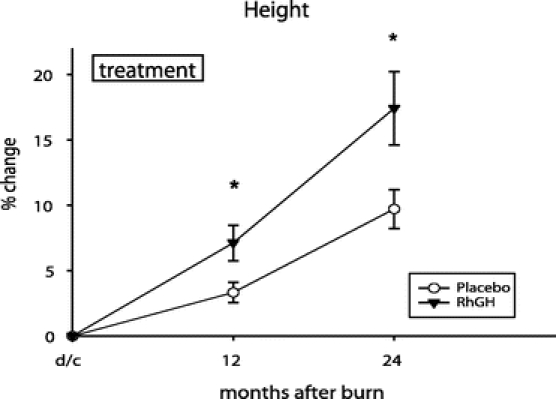
FIGURE 2. Percent change in height from baseline (dc) to 2 years after injury. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
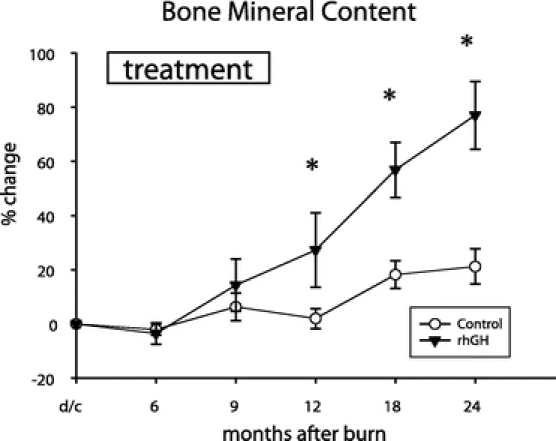
FIGURE 3. Percent change in bone mineral content (BMC) from discharge to 24 months after burn. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
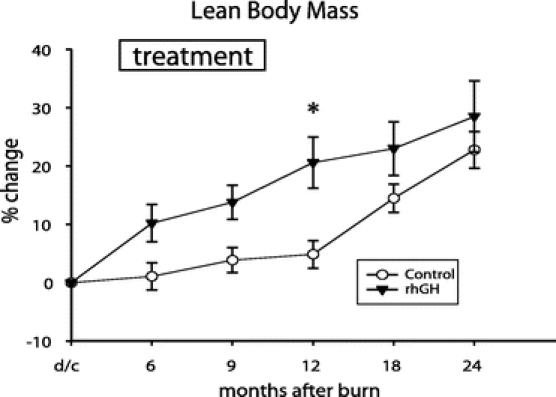
FIGURE 4. Percent change in lean body mass (LBM) when measured by dual-energy x-ray analysis. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
Cardiac Function
Children treated with rhGH showed an improvement in left ventricular function during the treatment period with an increase of 12% ± 24% (SD) in the ejection fraction compared with1% ± 20% for placebo (P < 0.05). Other cardiac measures were not significantly changed with rhGH treatment.
Strength Measurements
Strength measures significantly improved with rhGH at 12 months after burn compared with those receiving placebo (Fig. 5). There was no significant effect on leg strength once the drug was discontinued.
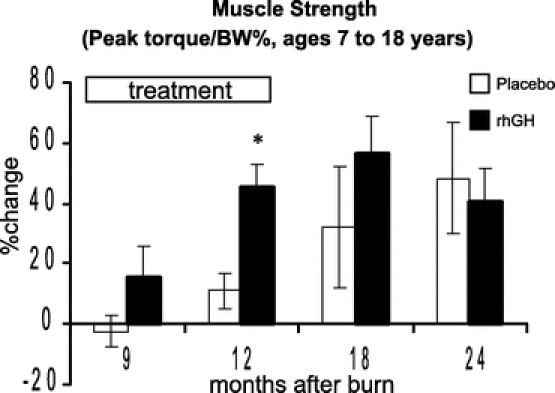
FIGURE 5. Percent change in muscle strength (Nm/kg BW). Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
Reconstructive Procedures
The number of reconstructive procedures from hospital discharge to the end of the study period was significantly lower in the group receiving rhGH compared with placebo (Table 1).
Scar Assessment
The scar evaluation did not reveal any significant differences between groups.
Indirect Calorimetry
There was no significant difference between rhGH and placebo in the percent of predicted REE, with the decrease in predicted REE similar over time in each group.
Hormone Panel
Recombinant human growth hormone administration increased serum GH, IGF-1, and IGFBP-3 and decreased cortisol concentrations when compared with placebo (P < 0.05) (Figs. 6–9). Effects on IGF-1 and cortisol serum levels persisted for 1 year after rhGH was discontinued (P < 0.05). Osteocalcin was elevated 18 months after burn in the rhGH group compared with placebo (P < 0.05). Insulin, parathyroid hormone, total thyroxine (total T4), tri-iodothyronine uptake (T3 uptake), and free thyroxine index were not significantly different between groups.
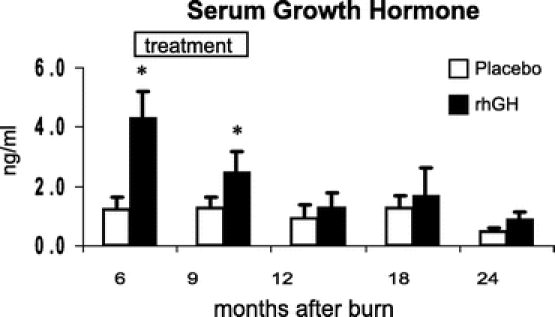
FIGURE 6. Serum concentrations of human growth hormone with time after burn. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).

FIGURE 7. Effects of rhGH on Insulin-like growth factor-1 with time. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
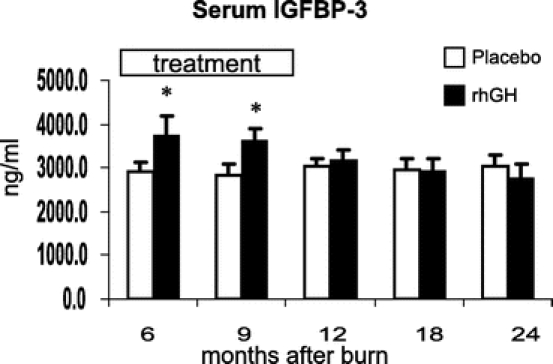
FIGURE 8. Effects of rhGH on insulin-like growth factor binding-protein-3 with time. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
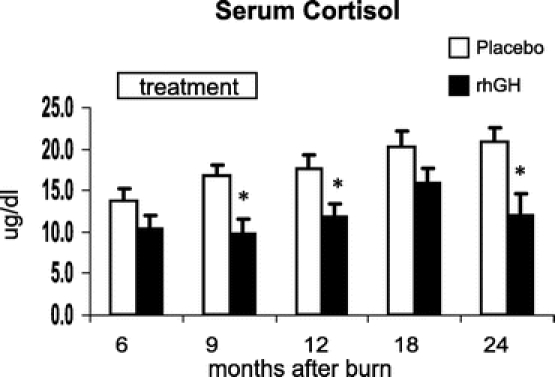
FIGURE 9. Serum concentrations of cortisol with time after burn. Values are mean ± SEM. *Significant difference between rhGH and placebo (P < 0.05).
Side Effects
No adverse side effects such as hyperglycemia, changes in the predicted Tanner scores, or premature closure of growth plates were observed during the study period.
DISCUSSION
Fluid resuscitation, early burn wound excision and closure, early enteral nutrition, as well as infection control have significantly improved survival of severely burned children.1 With these successful developments, burn care has focused on rehabilitation. Previous studies indicate that the hypermetabolic and catabolic response after a massive burn persists for at least 1 year following wound closure.21,22 A net loss in lean body mass for up to 9 months after trauma has been demonstrated.23 Recombinant human growth hormone has been shown to improve the catabolic response during the acute phase after burn in both children and adults.5,9 The effects of rhGH administration given from hospital discharge to 12 months after burn have been previously studied at this institution, with height, weight, bone mineral content, and lean body mass significantly improved when compared with placebo.14 In that study, rhGH was given to pediatric burn patients for a period of 8 to 11 months, but there were concerns regarding a rebound phenomenon from possible suppression of endogenous growth hormone production once rhGH was discontinued. It was also unclear whether benefits would continue after drug cessation or whether there would be functional benefits from the treatment.
Our current study showed a significant increase in height and bone mineral content during the treatment period, which continued to improve in the year after rhGH was discontinued. A possible rebound phenomenon, regarding effects on body composition after treatment was discontinued, was not observed for any of the analyzed variables.
Functional improvements with rhGH treatment have not been previously described. We report the effects of rhGH on body function for up to 2 years after burn. Based on our previous studies, we chose muscle strength, reflected as peak torque, as a relevant measurement as it requires a combination of several complex body systems, including muscles, nerves, and overall cognitive function.19 We observed that the increase in lean body mass was concomitant with a significant improvement in muscle strength during the first year of the study period in children treated with rhGH when compared with placebo. We also analyzed left ventricular function by echocardiography, which had improved significantly after 1 year of rhGH administration. In addition to improved function, the number of reconstructive procedures performed by a blinded and independent plastic surgeon whose indication for surgery followed an algorithm for functional improvement was reduced over 50% in patients treated with rhGH compared with placebo in the first 2 years after injury. A previous study investigating the effects of long-term treatment with rhGH did not show any adverse effect of rhGH on scar maturation or immunohistochemical characteristics in severely burned children when compared with placebo.20 We speculate that the improvements seen with rhGH in body function have led to a higher daily activity level, thus preventing scar contractures during the critical first 12 months of scar maturation leading to reduced requirements for reconstructive procedures for functional needs.
The serum hormone panel provides indications as how the effects of rhGH might be mediated. One proposed mechanism of action of rhGH is through stimulation of hepatic production of IGF-1 and its binding protein IGFBP-3.13 This is supported by our results in which serum growth hormone, IGF-1, and IGFBP-3 were significantly elevated compared with placebo. After discontinuing treatment 12 months after burn, serum concentrations of growth hormone and IGFBP-3 were no longer significantly different compared with placebo. Interestingly, IGF-1 was still significantly elevated at 18 months after burn, at a time when rhGH had been discontinued for 6 months. Elevated levels of IGF-1 may reflect an additional production induced by the increased LBM in those children who received rhGH.24 The compliance of patients receiving rhGH injections at home has been correlated to IGF-1 serum levels.25 Besides the elevation of endogenous anabolic hormones, rhGH significantly reduced serum cortisol concentrations, which is a known mediator of catabolic reactions.26,27 This decrease in cortisol was observed during rhGH treatment as well as in the following year after the drug was discontinued. The significant increase of osteocalcin, a marker for bone turnover, at 18 months after burn in those receiving rhGH may be associated with the increase in height and BMC in the second year of the study relative to placebo. The nutritional intake was not different between groups; thus, we attribute these findings to the effect of rhGH. Adverse side effects associated with anabolic therapy such as glucose intolerance, precocious sexual development, or early epiphysial closure were not noted at follow-up visits.
The high cost for approximately 10 months of rhGH treatment has been of some concern. With the number of reconstructive operations required during the first 2 years after discharge reduced by 50%, the cost of rhGH is offset by reduced surgical expenses. For example, 10 months of drug treatment of the average patient in this study (7-year-old, 34.5 kg body weight) receiving 0.05 mg/kg rhGH per day would cost approximately $18,000. Surgical expenses for each operative intervention including the day of hospital stay would be approximately $8000. The average patient in the placebo group required a total of 4 operations, resulting in $32,000 in expenses, whereas the average patient in the rhGH group required only 1.8 operations, resulting in $14,400 plus $18,000 for rhGH, thus resulting in total expenses of $32,400 or nearly the same cost as those receiving placebo. The reduced time in pain and recovery is considered well worth any small additional cost.
CONCLUSION
Severely burned children who received rhGH after hospital discharge showed improved body composition and function as well as hormone metabolism requiring fewer reconstructive procedures when compared with placebo. RhGH given subcutaneously at a daily dose of 0.05 mg/kg body weight after hospital discharge is safe for severely burned children and successfully attenuates burn induced catabolism and improves body function.
ACKNOWLEDGMENTS
The authors thank D. Benjamin, W. Benjamin, S. McEntire, M. Buffalo, M. Celis, J. Huddleston, L. Jecker, M. Kelly, G. Kulp, J. Martinez, S. Ojeda, and J. Wilkins for their technical assistance.
Discussions
Dr. William G. Cioffi, Jr. (Providence, Rhode Island): Dr. Herndon and his colleagues are to be congratulated for extending our knowledge of the treatment of what we now know to be a chronic disease with significant long-term metabolic and physiologic consequences. This follow-up report responds to our previous criticisms in which long-term functional data were lacking and are now presented. They have clearly demonstrated that the administration of low-dose growth hormone for a year following injury has long-term beneficial effects which persist for a year following the cessation of treatment.
The strengths of the study are its long-term nature, the randomization process, and the large number of variables which were measured. There is no doubt that the provision of an anabolic agent improves lean body mass, bone density, and other measures. However, before adopting this expensive treatment, $18,000 a year, as Dr. Herndon pointed out for a child, we need some more data.
Specifically, how do the results relate to normal children? In previous studies, you have always had a normal control group. If I take an anabolic agent, my performance will be improved, and one might argue that we should all then take anabolic agents. So what about normal control data? Do the growth hormone kids catch up and surpass normal controls?
Why a 30% fallout rate? A third of your randomized patients did not complete the trial. What were the results in those groups? When should treatment be stopped? The most important data was the reduction in reconstructive surgery requirements. However, what kinds of procedures were reduced?
Given that you state that scarring was not different between the two groups, I presume using the Vancouver scar assessment or something similar, how do you really explain this? So it is important to know what procedures were decreased and whether these are really a decrease in contractual releases.
Finally, you have shown a lack of short-term untoward effects. You now have kids out for multiple years following growth hormone treatment, and like many anabolic agents, there might be significant long-term consequences. What data do you have on that?
Again, Dr. Herndon is to be congratulated for following up previous criticisms providing long-term functional data and showing us that this expensive treatment should have prolonged beneficial effects in these children.
Dr. David N. Herndon (Galveston, Texas): I did not report the relationship of long-term data to normals in this presentation. These patients, in fact, do not catch up to normal growth rates. They are still grossly small being grossly under normal for lean body mass at 2 years post-injury, which makes doubly important your question of how long should therapy persist if you are going to bring these patients back to normal and should not that be your index of treatment? I think a very legitimate question is when should you stop?
You asked what operative procedures were decreased. I feel a larger number of patients should be treated with this agent for perhaps a longer period of time; 2 years is probably an appropriate time. I can respond that, in our analyses of operative procedures, the ones that appear to be decreased are most prominently releases of axillas and elbows.
The long-term effects, 10 or 15 years after treatment, on whether these patients reach adolescent full growth or whether they do not require an analysis of when these patients reach puberty to make sure that a benefit is being achieved.
Dr. Steven E. Wolf (Fort Sam Houston, Texas): What was most intriguing to me was the long-term effects on cortisol as well as IGF-1 that you had in this patient population. Even 6 months after treatment, they were still different from the control group. My question would be: what about normal controls? For those particular hormones specifically?
The second question would be, why did growth hormone affect the levels of cortisol? Is it the chicken or the egg? Is that the actual mechanism by which several of the things that you see are taking place? Or is it just an associated finding?
Dr. David N. Herndon (Galveston, Texas): It is really intriguing that the administration of growth hormone for a year affects long-term production of IGF-1 and cortisol. It has long been known that there is a reciprocal relationship between IGF-1 and cortisol. Whether there is a direct interaction or an indirect interaction is going to have to be determined through more detailed studies that are more mechanistic in nature that look at the linkages of signal transduction pathways.
The effect on cortisol is profound, and the levels of cortisol in the growth hormone-treated patients are, in fact, returning to normal levels, whereas in the placebo-treated patients they are profoundly elevated for 2 years after injury. One possible explanation, of course, is the linkage of IGF-1 and growth hormones.
Why does IGF-1 remain persistently elevated after cessation of the growth hormone? A possible explanation is the change in body stature and the fact that lean body mass itself can stimulate the production of IGF-1 through indirect mechanisms. This, however, is only speculative and needs further study.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): In children, growth occurs in spurts and I wonder how did you identify when a growth spurt was occurring in the two groups of patients? Could the effect of growth hormone have been that it just stimulated more growth spurts or prolonged the duration or accentuated the magnitude of growth spurts in the treated patients?
Secondly, the need for operation can be a subjective assessment. You said that you thought that the difference in operations between the two groups was due to lesser scar contractures across joints, which would be burn site dependent. Were the burn sites in these two groups sufficiently different so that it was just a difference in location of the burns that accounted for the difference in operations? Otherwise, if the difference in operations was a consequence of cosmetic reasons, was that because of a difference in the character of the scar or did that correlate with differences in histologic or biochemical characteristics of the scars?
Thirdly, it is puzzling that the muscle strength fell between 18 and 24 months while lean body mass continued to increase. Does that mean that the treated patients are making nonfunctional lean body mass? If so, what is that?
Then fourthly, you have been interested in the microarray assessment of genomic change in burn patients. Was there any differential change in the genomic response in those who got growth hormone and those who didn't?
Dr. David N. Herndon (Galveston, Texas): The question about growth spurts is entirely pertinent. In a larger series in which we looked at the effect of growth hormone on growth that was reported in the Lancet, we did show that growth hormone has its primary effects during the non-growth spurt periods. These children were mean age of 7 and the variation was quite small, so most of the patients were in the same growth characteristic. That was arranged by design, but a much larger series of patients is being analyzed in which growth hormone has been given over prolonged periods of time in multiple age groups up to and including puberty. This is to look specifically at the issue of growth spurt effect. It may well be you should only give growth hormone during the non-growth spurt period and to a specific patient population at a specific time. This is a needed refinement to these studies for prescribing information.
The need for operative procedures is truly subjective. The size of burns is quite large, over 40% of the total body surface. And the mean burn size is 60% with 50% being third degree; thus, most of the body surface is involved. There were no differences in the involvement of axillas, elbows, wrists, and feet and knees, which were the primary areas operated upon in the operative interventions that have been specified.
How growth hormone prevents the need for operations is entirely speculative. It is based on increased activity, which will need to be analyzed by putting activity monitors on these patients over time and analyzing exactly what activity occurs at the elbows and the axillas over time.
Indeed, we looked biochemically at the scar, as Dr. Cioffi alludes to, and did Vancouver scar scales in which there were no gross differences in the scar between the two groups. We also did detailed biochemical analyses, to be reported elsewhere, that show no great difference in biochemical characteristics with growth hormone over time.
We did, however, look at recombinant gene changes in scar over time, and at fat, muscle, and blood. We see very characteristic changes in muscle, fat, skin, in relationship to burn, with only a few genes that varied with recombinant human growth hormone. We are currently finalizing these results. They give us insight into where to look for other potential agents that may mitigate the hypermetabolic response in a salutary way.
Dr. Cioffi asked what happened to the third of the patients that were not reported? Why did they fail to continue this treatment? Seventy percent of our patients come from Mexico, and we have lost some patients which may have affected the study. We need a totally captive patient population, which we are working towards.
Footnotes
Supported by National Institutes of Health Grant Nos. P50-GM60338-06 and T32-GM08256-15, National Institute for Disability and Rehabilitation Research Grant No. H133A020102-05, and Shriners Hospital Grant Nos. 8952 and 8480. Recombinant human growth hormone was provided as gift from the Eli Lilly Corp. (Indianapolis, IN).
Reprints: David N. Herndon, MD, FACS, Shriners Hospitals for Children, 815 Market Street, Galveston TX 77550. E-mail: dherndon@utmb.edu.
REFERENCES
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1900. [DOI] [PubMed] [Google Scholar]
- 2.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass following severe burn injury in children. J Pediatr. 1995;126:252–256. [DOI] [PubMed] [Google Scholar]
- 3.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore D, Aulick L. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1280. [DOI] [PubMed] [Google Scholar]
- 5.Byrne TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg. 1993;218:400–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knox J, Demling RH, Wilmore DW, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma. 1995;39:526–532. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor site healing in severely burned children. Ann Surg. 1990;212:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilpin DA, Barrow RE, Rutan RL, et al. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg. 1994;220:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore DC, Honeycutt D, Jahor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126:38–43. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi WG, Gore DC, Rue LW, et al. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann Surg. 1994;220:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone: treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. [DOI] [PubMed] [Google Scholar]
- 12.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study [Research letter]. Lancet. 1999;354:1789. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez RJ, Wolf SE, Barrow RE, et al. Growth hormone therapy in burns: a safe therapeutic approach. Ann Surg. 1998;228:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart DW, Herndon DN, Klein G, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rongen-Westerlaken C, Wit JM, De Muinck Keizer-Schrama SM, et al. Growth hormone treatment in Turner syndrome accelerates growth and skeletal maturation: Dutch Growth Hormone Working Group. Eur J Pediatr. 1992;151:477–481. [DOI] [PubMed] [Google Scholar]
- 16.Tanner JM. Growth at Adolescence, 2nd ed. Oxford: Blackwell, 1962:28–39. [Google Scholar]
- 17.Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mlcak RP, Suman OE, Murphy K, et al. Effects of growth hormone on anthropometric measurements and cardiac function in children with thermal injury. Burns. 2005;31:60–66. [DOI] [PubMed] [Google Scholar]
- 19.Suman OE, Spiess RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;90:1474–1480. [DOI] [PubMed] [Google Scholar]
- 20.De Oliveira GV, Sanford A, Murphy K, et al. Growth hormone effects on hypertrophic scar formation: a randomized controlled trial of 62 burned children. Wound Repair Reg. 2004;12:404–411. [DOI] [PubMed] [Google Scholar]
- 21.Mlcak RP, Suman OE, Cortiella J, et al. Longitudinal assessment of the hypermetabolic response to thermal injury in children. Intensive Care Med. 2003;29:94–97. [Google Scholar]
- 22.Milner EA, Cioffi WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994;37:167–170. [DOI] [PubMed] [Google Scholar]
- 23.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. [DOI] [PubMed] [Google Scholar]
- 24.Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32:181–186. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins JP, Suman OE, Benjamin DA, et al. Comparison of self-reported and monitored compliance of daily injection of human growth hormone in burned children. Burns. 2003;29:697–701. [DOI] [PubMed] [Google Scholar]
- 26.Hahn TJ, Halstead LR, Teitelbaum SI, et al. Altered mineral metabolism in glucocorticoid induced osteopenia: effect of 25-hydroxyvitamin D administration. J Clin Invest. 1979;64:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein GL, Bi LX, Sherrard DJ, et al. Evidence of supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int. 2004;15:468–474. [DOI] [PubMed] [Google Scholar]


