Abstract
Objectives To determine whether data on proteinuria are useful for refining estimates of risk based on kidney function alone, and whether the results of kidney function tests can be a useful adjunct to data on proteinuria.
Design Analysis of data from a randomised trial. Impaired kidney function was defined as low glomerular filtration rate (< 60 ml/min/1.73 m2) and proteinuria (≥ 1+ protein) on dipstick urinalysis.
Setting Study of cholesterol and recurrent events: a randomised trial of pravastatin 40 mg daily versus placebo.
Participants 4098 men and women with previous myocardial infarction.
Main outcome measures All cause mortality and cardiovascular events.
Results 371 participants died in nearly 60 months of follow-up. Compared with participants without proteinuria or impaired kidney function, patients with both characteristics were at high risk (hazard ratio 2.39, 95% confidence interval 1.72 to 3.30), and those with only proteinuria or only impaired kidney function were at intermediate risk (1.69, 1.32 to 2.16; 1.41, 1.12 to 1.79, respectively) of dying from any cause. The results were similar for cardiovascular outcomes, including new cases of heart failure, stroke, and coronary death or non-fatal myocardial infarction. A graded increase in the risk of all cause mortality was seen for severity of renal impairment and degree of proteinuria by dipstick.
Conclusions The presence or absence of proteinuria on dipstick urinalysis may be used to refine estimates of risk based on kidney function alone.
Introduction
Chronic kidney disease is an independent risk factor for premature death and cardiovascular morbidity. For example, cardiovascular events are 10-20 times more frequent in patients with end stage renal disease than in age and sex matched controls in the general population.1 Recent evidence shows that even mild impairment of kidney function is associated with increased mortality and higher risk of first and recurrent cardiovascular events.2-6
In people with a normal glomerular filtration rate (such as the general population or people being treated for hypertension), proteinuria is associated with an increase in adverse clinical outcomes, even when excretion of protein in the urine is as low as 7 mg/day.7-12 When kidney function is impaired, proteinuria is associated with an increased risk of cardiovascular events, which persists after adjustment for estimated glomerular filtration rate (GFR) and is independent of diabetic status.13,14
Although proteinuria is a fundamental manifestation of kidney disease, in the United States only 25% of people with proteinuria have a low GFR (< 60 ml/min/1.73 m2) and only 25% of people with a low GFR have proteinuria.15 Therefore, few studies examine the relation between adverse outcomes and these two risk factors in combination. We used data from a randomised trial of people with previous myocardial infarction to test the hypothesis that patients with proteinuria and low GFR have higher mortality than those with one or neither characteristic. We wanted to determine whether information on proteinuria was useful for refining estimates of risk based on kidney function alone, and whether the results of kidney function tests can be a useful adjunct to data on proteinuria.
Methods
Study design and patients
Our study of data from a randomised trial was approved by the institutional review board at the University of Alberta. The cholesterol and recurrent events (CARE) study was a randomised trial of pravastatin versus placebo in 4159 people with hyperlipidaemia and previous myocardial infarction.16,17 Men and postmenopausal women were eligible if they had had an acute myocardial infarction 3-20 months before the study, low density lipoprotein cholesterol concentrations of 3.0-4.5 mmol/l, fasting glucose concentrations of no more than 12.2 mmol/l, left ventricular ejection fractions of no less than 25%, no symptoms of congestive heart failure, and were 21-75 years old. Participants were stratified according to clinical centre and randomly assigned in a double blinded fashion to receive either 40 mg of pravastatin (Pravachol, Bristol Myers Squibb) or placebo once daily. The allocation of treatment was concealed by using a centrally maintained code.
Measuring proteinuria and kidney function
Patients with proteinuria ≥ 2+ on routine dipstick testing or serum creatinine concentrations more than 1.5 times the upper limit of normal before randomisation were excluded from the trial. However, some patients with proteinuria ≥ 2+ and patients in whom repeat urinalysis gave results of < 2+ were enrolled at the discretion of the site investigator. We used the results of the first urinalysis before randomisation to classify patients with respect to proteinuria. Typical dipstick measures of proteinuria were none, trace, 1+,2+, and 3+, which corresponds to urinary protein concentrations of < 0.1, 0.1-0.3, 0.31-1.0, 1.01-3.0, and more than 3.0 g/l. We defined proteinuria as 1+ or greater protein on baseline dipstick urinalysis (Multistix; Ames Miles Bayer) read automatically. We measured baseline serum creatinine in fasting participants with an alkaline picrate method. We estimated GFR using the equation
186×SCr-1.154×age in years-0.203×1.210 (if black)×0.742 (if female) where SCr is serum creatinine in g/dl. This formula agrees with iothalamate measurements of GFR.18 In agreement with recent guidelines, we defined overtly impaired kidney function as GFR < 60 ml/min/1.73 m2 body surface area.18
Study outcomes
The primary outcome was all cause mortality. Secondary outcomes were developing symptomatic congestive heart failure, ischaemic or non-ischaemic stroke, and the composite of fatal coronary disease (fatal myocardial infarction, either definite or probable; sudden death; death during a coronary intervention; and death from other coronary causes) or non-fatal myocardial infarction confirmed by measuring serum creatine kinase. The outcomes committee reviewed deaths without knowing the participant's treatment assignment or laboratory values.
Statistical analysis
Descriptive statistics are reported as medians and interquartile ranges or percentages where appropriate. We used χ2 and Kruskal-Wallis tests to test for differences between four groups defined by the presence and absence of proteinuria and impaired kidney function. We used Cox proportional hazard models to examine the association between proteinuria, kidney dysfunction, and clinical outcomes. On the basis of a priori decisions about potential confounders, we adjusted for the following baseline covariates in all multivariate models: age; ethnic origin (black v other); sex; smoking status; diabetic status; waist to hip circumference ratio; fasting glucose; haemoglobin; serum albumin; low density lipoprotein cholesterol; high density lipoprotein cholesterol; triglycerides; systolic and diastolic blood pressure; country of treatment (USA v Canada); left ventricular ejection fraction; and use of β adrenergic blockers, angiotensin converting enzyme inhibitors, aspirin, and pravastatin. We used the mean of covariates method to produce adjusted survival curves for these final models.19 We determined that the proportional hazard assumption was satisfied by examining plots of the log negative log of the within-group survivorship functions versus log-time, the Schoenfeld residuals, and Kaplan-Meier (observed) versus Cox (expected) survival curves. In a sensitivity analysis, we compared the full model results to the parsimonious model fit with a backwards elimination selection method. Results were similar by using this last approach to those obtained using the fully adjusted model, and we report the results of the backwards elimination selection method. We also evaluated whether risk increased with increasing severity of proteinuria (none, trace, 1+,2+, or > 2) and kidney dysfunction (GFR ≥ 60, 45-59.9, or < 45 ml/min/1.73 m2). All P values are two sided and 95% confidence intervals are provided where appropriate. Analyses were performed with Stata 8 SE software.
Results
Baseline characteristics
Of 4159 participants, 4098 (98.5%) had serum creatinine and proteinuria measured at baseline and were eligible for analysis. Table 1 lists the demographic characteristics of the participants. A total of 2839 (69.3%) participants had neither proteinuria nor impaired kidney function, 707 (17.3%) had only impaired kidney function, 379 (9.3%) had only proteinuria, and 173 (4.2%) had both. Overall, 19.7% (173) of participants with impaired kidney function had proteinuria, and 31.3% (173) of participants with proteinuria had impaired kidney function. The median follow-up was 58.9 months.
Table 1.
Baseline characteristics of participants; values are median (interquartile range)
|
No proteinuria
|
Proteinuria
|
||||
|---|---|---|---|---|---|
| Variable | GFR ≥60 (n=2839) | GFR <60 (n=707) | GFR ≥60 (n=379) | GFR <60 (n=173) | P value |
| Demographic variables | |||||
| Age (years) | 58 (50-64) | 65 (59-70) | 60 (52-66) | 65 (60-70) | <0.001 |
| % female | 10.7 | 25.2 | 12.9 | 19.1 | <0.001 |
| % black | 3.0 | 1.7 | 7.9 | 3.5 | <0.001 |
| Body mass index (kg/m2) | 27 (25-30) | 27 (24-30) | 28 (25-31) | 27 (24-29) | <0.001 |
| % with history of hypertension | 37.8 | 49.7 | 51.7 | 70.5 | <0.001 |
| % current smokers | 17.4 | 8.9 | 20.1 | 15.6 | <0.001 |
| % with known diabetes mellitus | 11.5 | 15.3 | 28.0 | 22.5 | <0.001 |
| % treated in the US (v Canada) | 65.5 | 65.5 | 71.0 | 66.5 | 0.20 |
| Drugs | |||||
| Pravastatin | 50.3 | 48.4 | 48.3 | 52.6 | 0.62 |
| β blocker | 38.7 | 41.9 | 41.2 | 42.2 | 0.35 |
| Angiotensin converting enzyme inhibitor | 12.5 | 17.7 | 15.0 | 26.0 | <0.001 |
| Aspirin | 84.6 | 81.6 | 79.4 | 79.2 | 0.01 |
| Lipid status | |||||
| Total cholesterol (mmol/l) | 5.4 (5.1-5.8) | 5.5 (5.1-5.8) | 5.4 (5.1-5.7) | 5.4 (5.0-5.8) | 0.009 |
| Low density lipoprotein cholesterol (mmol/l) | 3.6 (3.3-3.9) | 3.6 (3.3-3.9) | 3.5 (3.3-3.8) | 3.6 (3.3-3.8) | 0.05 |
| High density lipoprotein cholesterol (mmol/l) | 0.98 (0.85-1.1) | 1.0 (0.85-1.2) | 0.96 (0.83-1.1) | 0.93 (0.83-1.1) | 0.003 |
| Triglycerides (mmol/l) | 1.6 (1.2-2.1) | 1.6 (1.2-2.2) | 1.6 (1.3-2.3) | 1.7 (1.3-2.3) | 0.40 |
| Renal function, blood pressure, and ejection fraction | |||||
| GFR (ml/min/1.73m2) | 75 (68-84) | 55 (51-58) | 74 (67-84) | 53 (47-57) | <0.001 |
| Serum creatinine (μmol/l) | 97 (88-106) | 123 (115-133) | 97 (88-106) | 123 (123-141) | <0.001 |
| Systolic blood pressure (mm Hg) | 126 (116-140) | 130 (120-142) | 132 (120-145) | 136 (120-150) | <0.001 |
| Diastolic blood pressure (mm Hg) | 80 (70-85) | 80 (70-85) | 80 (72-88) | 80 (74-90) | <0.001 |
| Ejection fraction (%) | 53 (45-61) | 53 (44-62) | 54 (45-61) | 50 (44-59) | 0.04 |
| Laboratory variables | |||||
| Haemoglobin (g/l) | 150 (140-160) | 150 (140-160) | 150 (140-160) | 150 (140-150) | <0.001 |
| Serum albumin (g/l) | 42 (41-44) | 42 (40-43) | 42 (40-43) | 41 (40-43) | <0.001 |
Proteinuria was ≥1+ on routine dipstick urinalysis; GFR ≥60/<60 is estimated glomerular filtration rate ≥60/<60 ml/min/1.73 m2.
Association with all cause mortality
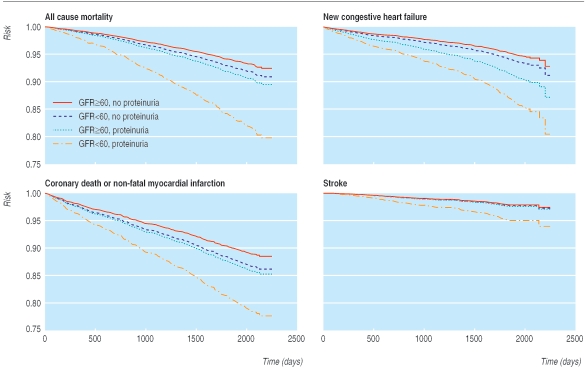
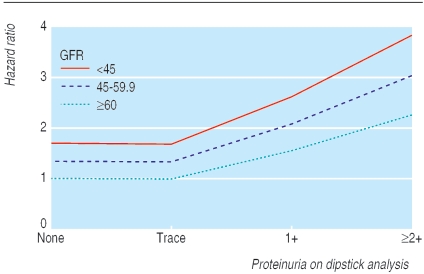
The unadjusted risk of all cause mortality was significantly higher in patients with both proteinuria and impaired kidney function (27.2%) than in those with neither condition (7.1%; P < 0.001 by using χ2; table 2). A Cox model that adjusted for age, ethnic origin, and sex, showed that proteinuria (hazard ratio 2.00, 95% confidence interval 1.58 to 2.53) and impaired kidney function (1.41, 1.12 to 1.77) were significantly associated with the risk of all cause mortality. Impaired kidney function and proteinuria were independently associated with all cause mortality when entered separately into the fully adjusted model (1.41, 1.12 to1.79 and 1.69, 1.32 to 2.16). Participants with both characteristics were at highest risk (2.39, 1.72 to 3.30), and participants with neither characteristic were at lowest risk (1.0) (table 2; fig 1). The risk of all cause mortality increased with the severity of renal impairment (P for trend 0.003) and degree of proteinuria by dipstick (P for trend < 0.001) (table 3; fig 2).
Table 2.
Association between proteinuria, kidney dysfunction, and clinical outcomes
|
Hazard ratio
|
|||||
|---|---|---|---|---|---|
|
Adjusted for age, ethnic origin, and sex
|
Fully adjusted*
|
||||
| Outcome | Unadjusted event rate (%) | Ratio (95% CI) | P value | Ratio (95% CI) | P value |
| All cause mortality | |||||
| No proteinuria: | |||||
| GFR ≥60 | 201/2839 (7.1) | 1.0 | 1.0 | ||
| GFR <60 | 74/707 (10.5) | 1.20 (0.91 to 1.58) | 0.203 | 1.41 (1.12 to 1.79) | 0.004 |
| Proteinuria: | |||||
| GFR ≥60 | 49/379 (12.9) | 1.61 (1.17 to 2.20) | 0.003 | 1.69 (1.32 to 2.16) | <0.001 |
| GFR <60 | 47/173 (27.2) | 3.31 (2.39 to 4.59) | <0.001 | 2.39 (1.72 to 3.30) | <0.001 |
| Coronary death or non-fatal myocardial infarction | |||||
| No proteinuria: | |||||
| GFR ≥60 | 293/2839 (10.3) | 1.0 | 1.0 | ||
| GFR <60 | 87/707 (12.3) | 1.19 (0.93 to 1.53) | 0.173 | 1.28 (1.03 to 1.60) | 0.03 |
| Proteinuria: | |||||
| GFR ≥60 | 60/379 (15.8) | 1.47 (1.11 to 1.95) | 0.007 | 1.43 (1.14 to 1.81) | 0.003 |
| GFR <60 | 38/173 (22.0) | 2.31 (1.63 to 3.26) | <0.001 | 1.84 (1.35 to 2.50) | <0.001 |
| New symptomatic heart failure | |||||
| No proteinuria: | |||||
| GFR ≥60 | 150/2839 (5.3) | 1.0 | 1.0 | ||
| GFR <60 | 65/707 (9.2) | 1.28 (0.94 to 1.73) | 0.115 | 1.31 (1.01 to 1.71) | 0.04 |
| Proteinuria: | |||||
| GFR ≥60 | 50/379 (13.2) | 2.13 (1.54 to 2.95) | <0.001 | 1.83 (1.40 to 2.40) | <0.001 |
| GFR <60 | 36/173 (20.8) | 3.30 (2.28 to 4.79) | <0.001 | 2.41 (1.68 to 3.45) | <0.001 |
| Stroke | |||||
| No proteinuria: | |||||
| GFR ≥60 | 71/2839 (2.5) | 1.0 | 1.0 | ||
| GFR <60 | 28/707 (4.0) | 1.07 (0.68 to 1.69) | 0.761 | 1.25 (0.84 to 1.84) | 0.27 |
| Proteinuria: | |||||
| GFR ≥60 | 16/379 (4.2) | 1.41 (0.82 to 2.45) | 0.216 | 1.33 (0.87 to 2.03) | 0.19 |
| GFR <60 | 15/173 (8.7) | 2.61 (1.48 to 4.61) | 0.001 | 1.66 (0.95 to 2.90) | 0.08 |
Proteinuria was defined by ≥1+ proteinuria on routine dipstick urinalysis; GFR ≥60/<60 means estimated glomerular filtration rate ≥60/<60 ml/min/1.73m2. The number of participants in each category was 2839 (no proteinuria, GFR ≥60), 707 (no proteinuria, GFR <60), 379 (proteinuria, GFR ≥60), and 173 (proteinuria, GFR <60).
Factors adjusted for: age, sex, ethnic origin, smoking status, diabetic status, waist to hip circumference ratio, fasting glucose, haemoglobin, serum albumin, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, systolic and diastolic blood pressure, country of treatment (US v Canada), left ventricular ejection fraction, and use of drugs (β adrenergic blockers, angiotensin converting enzyme inhibitors, aspirin, or pravastatin).
Fig 1.
Time to clinical outcomes by proteinuria and kidney dysfunction
Table 3.
Association between proteinuria, kidney dysfunction, and all cause mortality
| Grade | No | Fully adjusted hazard ratio (95% CI) | P value |
|---|---|---|---|
| ≥2+ proteinuria | |||
| GFR <45 | 10 | 3.84 (2.12 to 6.96) | <0.001 |
| GFR 45-59.9 | 30 | 3.04 (1.87 to 4.94) | <0.001 |
| GFR ≥60 | 55 | 2.26 (1.46 to 3.52) | <0.001 |
| 1+ proteinuria | |||
| GFR <45 | 28 | 2.62 (1.56 to 4.40) | <0.001 |
| GFR 45-59.9 | 105 | 2.08 (1.44 to 3.00) | <0.001 |
| GFR ≥60 | 324 | 1.55 (1.17 to 2.05) | 0.002 |
| Trace proteinuria | |||
| GFR<45 | 21 | 1.68 (0.99 to 2.86) | 0.06 |
| GFR 45-59.9 | 119 | 1.33 (0.91 to 1.96) | 0.15 |
| GFR ≥60 | 479 | 0.99 (0.73 to 1.34) | 0.95 |
| No proteinuria | |||
| GFR<45 | 48 | 1.7 (1.08 to 2.67) | 0.02 |
| GFR 45-59.9 | 519 | 1.34 (1.05 to 1.72) | 0.02 |
| GFR ≥60 | 2360 | 1.0 |
Adjusted for age, sex, ethnic origin, smoking status, diabetic status, waist to hip circumference ratio, fasting glucose, haemoglobin, serum albumin, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, systolic and diastolic blood pressure, country of treatment (US v Canada), left ventricular ejection fraction, and use of drugs (β adrenergic blockers, angiotensin converting enzyme inhibitors, aspirin, or pravastatin) (all at baseline).
Fig 2.
Adjusted risk of all cause mortality according to proteinuria and kidney dysfunction
After full adjustment, interaction between impaired kidney function and proteinuria on mortality was of borderline significance (P = 0.046). The risk associated with concomitant proteinuria and kidney dysfunction (compared with the risk in participants with neither characteristic) was qualitatively similar in models with and without the interaction term (2.78, 1.98 to3.92; 2.39, 1.72 to 3.30, respectively), indicating that the interaction was of modest clinical importance. Results were similar when participants with diabetes mellitus at baseline were excluded (data not shown).
Association with other adverse clinical outcomes
Results were similar for other adverse outcomes, including cardiovascular death or non-fatal myocardial infarction, the development of new congestive heart failure, and stroke. For all three outcomes, risk was qualitatively higher for participants with both proteinuria and impaired kidney function than for participants with one or neither characteristic (all P < 0.001 by χ2; table 2; fig 1), and tests for interaction were non-significant (all P > 0.15).
Discussion
We found that impaired kidney function and proteinuria often exist independently, so that the presence of both these conditions could be used to identify people at high risk of death.15 Among survivors of myocardial infarction who were clinically stable, patients with proteinuria and impaired kidney function were more than twice as likely to die as patients with one or neither abnormality. These results were consistent for a range of adverse clinical outcomes, including all cause mortality, stroke, new congestive heart failure, and cardiovascular death or non-fatal myocardial infarction. We also found a dose effect for proteinuria and kidney dysfunction: increased risk was associated with greater proteinuria and lower GFR. Thus the presence or absence of proteinuria on routine urinalysis could help refine estimates of risk that are based on kidney function alone.
Comparison with other studies
Many studies with a wide range of participants have found an association between adverse outcomes and kidney dysfunction.3,4,20-23 Several studies have shown an association between urinary protein excretion (overt proteinuria and microalbuminuria) and the risk of death or cardiovascular events.7-10 Despite this, data on how proteinuria and kidney function together affect prognosis are lacking. Studies have either not reported data on both characteristics, or they have reported that one characteristic is independently associated with risk after controlling for the other. Our findings are consistent with the findings of the heart outcomes prevention evaluation study.3 However, that analysis excluded participants with proteinuria on dipstick urinalysis and did not report findings for all cause mortality. Given the low cost and ready availability of estimates of kidney function based on urinalyses and serum creatinine, our finding is likely to be clinically useful.
Implications of the study
We do not know how concomitant proteinuria and renal insufficiency mediate increased cardiovascular risk, but several possibilities exist. Firstly, proteinuria and impaired kidney function often coexist with other cardiovascular risk factors.24-27 Secondly, patients with renal disease might be less likely to receive beneficial treatments.6,28,29 Although we controlled for these two factors, we cannot exclude the possibility of residual confounding. Thirdly, proteinuria and impaired kidney function may be markers of endothelial dysfunction, inflammation, or severity of vascular disease, including atherosclerosis that is not yet clinically evident.30-35 Finally, patients with proteinuria and impaired kidney function may be more likely to have clinically relevant kidney disease than those with either characteristic alone.
Our findings indicate that studies of the relation between chronic kidney disease and death should consider stratifying patients on the presence or absence of proteinuria (and studies evaluating the risk associated with proteinuria should stratify on kidney function). The proportion of patients with proteinuria may have varied in previous studies examining this issue; this might partly explain the heterogeneity in the reported size of the association between chronic kidney disease and death.31
Strengths and limitations of the study
In the CARE study, outcomes were measured according to pre-specified criteria by people who were unaware of kidney function or the results of urinalysis. We also adjusted for many potential confounders, including comorbidity, use of drugs, left ventricular ejection fraction, and other laboratory results, which reduced the risk of bias. However, our study does have limitations. Firstly, although the analysis was retrospective, the study hypothesis was formulated before starting analyses. Secondly, we analysed a selected population (clinically stable survivors of myocardial infarction) that may not be representative of the general population. Thirdly, we did not determine the cause of renal dysfunction in the participants.
Fourthly, baseline dipstick urinalysis and measurements of serum creatinine were performed only once, but the resulting loss of precision (compared with measuring more than once) would be expected to bias towards the null, so that the true relation between proteinuria, impaired kidney function, and death is probably stronger than our findings indicate. However, the use of dipstick urinalysis probably resulted in a stronger association between proteinuria and adverse outcomes than if a more sensitive marker of urinary protein had been used.
Fifthly, although serum creatinine was measured in a central laboratory, we did not calibrate our GFR assay against the reference laboratory assay used to develop the GFR equation. This may have led to misclassification of some patients with respect to disease status, which again would be expected to bias towards the null. Finally, the number of participants in some groups (especially the group with proteinuria and impaired kidney function) was small.
What is already known on this topic
Many studies have shown that impaired kidney function and proteinuria are risk factors for all cause mortality and cardiovascular events
Data are lacking on how these common laboratory tests can be used together to predict risk
What this study adds
Higher risk of mortality was associated with heavier proteinuria on dipstick urinalysis and lower kidney function, and the risk associated with these conditions was additive
The results of kidney function tests and urinalysis improve the accuracy of estimates of risk
Natasha Wiebe (research associate, University of Alberta) performed statistical analyses and a detailed statistical review. Thanks to Tim Craven for his assistance. The CARE study was initiated by the investigators and was funded by Bristol-Myers-Squibb. This study was not supported by industry.
Contributors: MP had the idea for the analysis, and MT designed the detailed analysis plan, with input from MP and GC. MT wrote the first draft and is guarantor. All authors helped to interpret the data and to revise the article.
Funding: MT was funded by a population health investigator award from the Alberta Heritage Foundation for Medical Research and by the Canadian Institutes of Health Research. PJ was funded by the Stanley J Sarnoff Endowment for Cardiovascular Science.
Competing interests: None declared.
Ethical approval: The institutional review board at the University of Alberta, Canada.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998;32(suppl 3): S112-9. [DOI] [PubMed] [Google Scholar]
- 2.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The hypertension detection and follow-up program cooperative group. Hypertension 1989;13(suppl): I80-93. [DOI] [PubMed] [Google Scholar]
- 3.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med 2001;134: 629-36. [DOI] [PubMed] [Google Scholar]
- 4.Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol 2001;12: 218-25. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003;108: 2154-69. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 2002;137: 555-62. [DOI] [PubMed] [Google Scholar]
- 7.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004;110: 32-5. [DOI] [PubMed] [Google Scholar]
- 8.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: a prospective study. Am J Med 2000;109: 1-8. [DOI] [PubMed] [Google Scholar]
- 9.Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med 2003;139: 901-6. [DOI] [PubMed] [Google Scholar]
- 10.Hillege HL, Fidler V, Diercks GF, van Gilst WH, De Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002;106: 1777-82. [DOI] [PubMed] [Google Scholar]
- 11.Grimm RH, Svendsen KH, Kasiske B, Keane WF, Wahi MM. Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT research group. Multiple risk factor intervention trial. Kidney Int Suppl 1997;63: S10-4. [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351: 1296-305. [DOI] [PubMed] [Google Scholar]
- 13.Anavekar NS, Gans DJ, Berl T, Rohde RD, Cooper W, Bhaumik A, et al. Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: a case for albuminuria. Kidney Int Suppl 2004;92: S50-5. [DOI] [PubMed] [Google Scholar]
- 14.Damsgaard EM, Froland A, Jorgensen OD, Mogensen CE. Microalbuminuria as predictor of increased mortality in elderly people. BMJ 1990;300: 297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int 2002;61: 2165-75. [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335: 1001-9. [DOI] [PubMed] [Google Scholar]
- 17.Sacks FM, Pfeffer MA, Moye L, Brown LE, Hamm P, Cole TG, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the cholesterol and recurrent events trial (CARE). Am J Cardiol 1991;68: 1436-46. [DOI] [PubMed] [Google Scholar]
- 18.NKF-K/DOQI. Clinical practice guidelines for chronic kidney disease. Am J Kidney Dis 2002;39(suppl 1(2)): S76. [Google Scholar]
- 19.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol 1996;143: 1059-68. [DOI] [PubMed] [Google Scholar]
- 20.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 1999;56(6): 2214-9. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int 2002;62: 997-1004. [DOI] [PubMed] [Google Scholar]
- 22.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004;109: 1004-9. [DOI] [PubMed] [Google Scholar]
- 23.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004;351: 1285-95. [DOI] [PubMed] [Google Scholar]
- 24.Hannedouche T, Albouze G, Chauveau P, Lacour B, Jungers P. Effects of blood pressure and antihypertensive treatment on progression of advanced chronic renal failure. Am J Kidney Dis 1993;21(suppl 2): 131-7. [DOI] [PubMed] [Google Scholar]
- 25.Hakim RM, Lazarus JM. Progression of chronic renal failure. Am J Kidney Dis 1989;14: 396-401. [DOI] [PubMed] [Google Scholar]
- 26.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int 1997;51: 1908-19. [DOI] [PubMed] [Google Scholar]
- 27.Locatelli F, Manzoni C, Marcelli D. Factors affecting progression of renal insufficiency. Miner Electrolyte Metab 1997;23: 301-5. [PubMed] [Google Scholar]
- 28.Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis 2001;37: 484. [PubMed] [Google Scholar]
- 29.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J 2002;144: 226-32. [DOI] [PubMed] [Google Scholar]
- 30.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 2001;103: 1869-74. [DOI] [PubMed] [Google Scholar]
- 31.Kasiske BL. The kidney in cardiovascular disease. Ann Intern Med 2001;134: 707-9. [DOI] [PubMed] [Google Scholar]
- 32.Garg AX, Blake PG, Clark WF, Clase CM, Haynes RB, Moist LM. Association between renal insufficiency and malnutrition in older adults: results from the NHANES III. Kidney Int 2001;60: 1867-74. [DOI] [PubMed] [Google Scholar]
- 33.Stenvinkel P, Wanner C, Metzger T, Heimburger O, Mallamaci F, Tripepi G, et al. Inflammation and outcome in end-stage renal failure: does female gender constitute a survival advantage? Kidney Int 2002;62: 1791-8. [DOI] [PubMed] [Google Scholar]
- 34.Stuveling EM, Hillege HL, Bakker SJ, Asselbergs FW, de Jong PE, Gans RO, et al. C-reactive protein and microalbuminuria differ in their associations with various domains of vascular disease. Atherosclerosis 2004;172: 107-14. [DOI] [PubMed] [Google Scholar]
- 35.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol 2004;15: 1897-903. [DOI] [PubMed] [Google Scholar]