Abstract
Mutations in the VPS (vacuolar protein sorting) genes of Saccharomyces cerevisiae have been used to define the trafficking steps that soluble vacuolar hydrolases take en route from the late Golgi to the vacuole. The class D VPS genes include VPS21, PEP12, and VPS45, which appear to encode components of a membrane fusion complex involved in Golgi-to-endosome transport. Vps21p is a member of the Rab family of small Ras-like GTPases and shows strong homology to the mammalian Rab5 protein, which is involved in endocytosis and the homotypic fusion of early endosomes. Although Rab5 and Vps21p appear homologous at the sequence level, it has not been clear if the functions of these two Rabs are similar. We find that Vps21p is an endosomal protein that is involved in the delivery of vacuolar and endocytosed proteins to the vacuole. Vacuolar and endocytosed proteins accumulate in distinct transport intermediates in cells that lack Vps21p function. Therefore, it appears that Vps21p is involved in two trafficking steps into the prevacuolar/late endosomal compartment.
INTRODUCTION
The lysosome of animal cells, and similarly the vacuole of the yeast Saccharomyces cerevisiae, is the major protein degradative site within the cell (Jones et al., 1997). As such, the vacuole must receive proteins necessary to carry out its normal functions from the secretory pathway as well as receive proteins that are delivered to the vacuole for degradation. In particular, the vacuole is the target organelle for the vacuolar H+-ATPase, soluble proteases, and membrane-bound phosphatases that get sorted away from bulk secretory traffic at the late Golgi. Additionally, the vacuole is involved in the proteolytic degradation, and hence down-regulation, of the mating pheromone receptors Ste2p and Ste3p. Genetic screens, such as the vacuolar protein-sorting (VPS), peptidase-deficient (PEP), and vacuolar morphology screens, have identified genes involved in vacuolar biogenesis (Bryant and Stevens, 1998). Mutations in many of these genes affect more than one pathway to the vacuole, which seems to correlate with the interconnected nature of the biosynthetic and endocytic pathways.
The soluble vacuolar hydrolase carboxypeptidase Y (CPY) and its receptor Vps10p/Pep1p, as well as the vacuolar H+-ATPase, traffic from the trans-Golgi network (TGN) through the endocytic system en route to the vacuole (Cooper and Stevens, 1996; Bryant et al., 1998a). Vps10p recognizes CPY in the Golgi, and both are packaged into vesicles and delivered to an endocytic compartment designated the prevacuolar compartment (PVC) (Marcusson et al., 1994; Cooper and Stevens, 1996). CPY dissociates from Vps10p in the PVC and proceeds to the vacuole, whereas Vps10p returns to the Golgi to initiate another round of CPY delivery (Cereghino et al., 1995; Cooper and Stevens, 1996). Mutations that disrupt this process cause CPY to be missorted into secretory vesicles and subsequently delivered to the cell surface (Bankaitis et al., 1986; Rothman and Stevens, 1986). This CPY secretion phenotype allowed for the identification of a large number of genes involved in the sorting of soluble vacuolar hydrolases to the vacuole (VPS genes) (Bankaitis et al., 1986; Rothman and Stevens, 1986). The vps mutants can be subdivided into six classes based on morphology (Raymond et al., 1992). These morphological class designations have proven useful in the analysis of the vps mutants, because each class seems to cause a blockage at a specific transport step. In particular, class E genes have been found to be involved in the delivery of CPY and endocytosed proteins to the vacuole from the PVC (Raymond et al., 1992; Davis et al., 1993; Piper et al., 1995; Rieder et al., 1996).
VPS27 is one of the class E VPS genes and controls exit from the PVC in both the forward direction to the vacuole and the return to the Golgi (Piper et al., 1995). Strains that are mutant for VPS27 accumulate late Golgi, vacuolar, and endocytosed proteins in an exaggerated form of the PVC known as the class E compartment (Piper et al., 1995). Because both endocytic and biosynthetic proteins accumulate in this compartment, the PVC has been assumed to be the convergence point for these two pathways to the vacuole. The PVC appears to correspond to a late endosome, as defined kinetically by internalization of the mating pheromone α-factor or internalization of the α-factor receptor Ste2p (Singer-Kruger et al., 1993; Mulholland et al., 1999). Immunoelectron microscopic analysis of this compartment in wild-type cells confirms the data obtained from the characterization of class E VPS mutants (Rieder et al., 1996) in that Ste2p chased into a late endosome labels the same structures as a portion of the CPY pool (Mulholland et al., 1999). Although no overlap between Ste2p and CPY in early endosomes was found, this may be simply a reflection of the relative amounts of time these proteins spend in the early and late endosomes.
The mating pheromone receptors Ste2p and Ste3p are expressed on the plasma membrane of MATa and MATα cells, respectively (Herskowitz, 1989). These receptors are subject to constitutive and ligand-dependent internalization and traverse the endocytic pathway to the vacuole, where they are degraded in a Pep4p protease–dependent manner (Davis et al., 1993; Schandel and Jenness, 1994). The mating pheromone α-factor, which is internalized after being bound by Ste2p, has been used to kinetically divide the endocytic system into early and late endosomes (Singer-Kruger et al., 1993). Exit from these endocytic compartments appears to be dependent on two rab proteins, Vps21p/Ypt51p and Ypt7p (Wichmann et al., 1992; Singer-Kruger et al., 1993; Singer-Kruger et al., 1994). Although these endocytic compartments are separable by gradient fractionation, the identities of resident proteins of the early and late endosomes are largely unknown.
In animal cells, much attention has focused on Rab5, a marker of early endosomes. Rab5 has been shown to be involved in homotypic fusion of early endosomes and internalization of plasma membrane proteins (Gorvel et al., 1991; Li et al., 1994). In addition, many regulators of Rab5 function have been isolated, including the early endosomal autoantigen EEA1 (Simonsen et al., 1998) and the guanine nucleotide exchange factor Rabex-5 (Horiuchi et al., 1997). VPS21, a member of the class D VPS genes, is the structural homologue of Rab5 (Horazdovsky et al., 1994; Singer-Kruger et al., 1994). Also within the class D VPS genes are VAC1/PEP7, which appears to function similarly to EEA1 (Tall et al., 1999); VPS9, which encodes the exchange factor for VPS21 (Hama et al., 1999); PEP12, a member of the syntaxin family (Becherer et al., 1996); and VPS45, a member of the SEC1 family (Cowles et al., 1994; Piper et al., 1994). These genes have all been implicated in the fusion of Golgi-derived vesicles with the PVC. Deletion of VPS21 also results in a defect in the degradation but not the internalization of α-factor (Horazdovsky et al., 1994; Singer-Kruger et al., 1994). The interpretation of these data, however, is made difficult by the fact that these mutant cells also fail to properly sort vacuolar proteases, including Pep4p, the protease required for Ste3p and α-factor degradation (Horazdovsky et al., 1994). Although Vps21p appears to be regulated similarly to Rab5, it is difficult to reconcile the data regarding Vps21p function in the vacuolar transport pathway with the proposed role of Rab5. However, the role of Rab5 in trafficking of lysosomal proteins has not been addressed, nor has the role of Vps21p in endocytosis been firmly established.
The genetic relationships and phenotypes associated with the loss of Vps21p, Pep12p, and Vps45p have led to the postulate that they act together at the PVC to promote fusion of TGN-derived vesicles. Because endocytic traffic also enters the PVC, we sought to investigate the role these proteins might play in delivery of internalized Ste3p to the PVC. Previously, it was found that defects in VPS45 led to blocks that are specific for the biosynthetic route into the PVC, because Ste3p is properly delivered by endocytosis to the vacuole in vps45 cells (Bryant et al., 1998a). We have approached the question of Vps21p involvement in biosynthetic and endocytic trafficking to the vacuole through a characterization of the transport intermediates that accumulate in the absence of Vps21p function. We find that loss of Vps21p function results in a blockage in vacuolar and endocytic traffic into the PVC and that proteins from these two pathways accumulate in different transport intermediates. Thus, the PVC seems to represent the organelle on which vacuolar and endocytic traffic initially converges, and Vps21p controls both trafficking steps into the PVC.
MATERIALS AND METHODS
Materials
Enzymes used in DNA manipulations were from New England Biolabs (Beverly, MA) or Boehringer Mannheim Biochemicals (Indianapolis, IN). Texas Red–conjugated goat anti-rabbit antibodies, biotin-conjugated goat anti-rabbit antibodies, biotin-conjugated goat anti-mouse antibodies, and streptavidin-conjugated FITC were obtained from Jackson Immunoresearch (West Grove, PA). Alexa 594–conjugated goat anti-rabbit and Alexa 594–conjugated goat anti-mouse antibodies were obtained from Molecular Probes (Eugene, OR). Polyclonal antibodies that recognize Vps10p, Vph1p, CPY, alkaline phosphatase (ALP), and Pep12p have been described previously (Roberts et al., 1992; Cooper and Stevens, 1996; Bryant et al., 1998b). mAbs that recognize ALP (1D3), Vph1p (10D7-A7-B2), Vps10p (18C8), and Pep12p (2C3G4) are available commercially from Molecular Probes. mAbs that recognize the c-myc epitope were purified from culture supernatants of hybridoma 9E10 obtained from the American Type Culture Collection (Rockville, MD). Monoclonal and polyclonal antibodies that recognize Ste3p were a generous gift of G. Sprague (Eugene, OR). Fixed Staphylococcus aureus cells (IgG Sorb) were obtained from The Enzyme Center (Malden, MA). [35S]Express label was obtained from New England Nuclear (Boston, MA). Oxalyticase was from Enzogenetics (Corvallis, OR), and zymolyase was obtained from Seikagaku (Tokyo, Japan).
Plasmid Construction
Plasmids used in this study are listed in Table 1. A single c-myc epitope was introduced into VPS21 immediately after the initiating ATG by a PCR-based approach; the PCR product was then subcloned into pRS306 (Sikorski and Hieter, 1989), thus generating pRCP78. pSRG4 was generated by amplifying yeast genomic DNA with the following oligonucleotides: 5KB21 (CGGGATCCCCTGATGAAGATGTCTATGTTTCTCC) and 3KB21 (GGAATTCTCGAG-CGTTATAAGAAACGGAAGAATAT); the resulting PCR product was digested with BamHI/XhoI and ligated into the BamHI/XhoI sites of pRS316 (Sikorski and Hieter, 1989). pSRG22 is derived from pSRG4 and contains a single c-myc epitope in the same position as in pRCP78. pSRG92 was generated by subcloning the BamHI/XhoI fragment from pSRG22 into the BamHI/SalI sites of YEp352 (Hill et al., 1986). pSRG55 was generated by creating a SmaI site immediately downstream of the initiating ATG in pSRG4 by Kunkel mutagenesis with the use of the oligonucleotide VPS21SMA (TGACTGATGTGTTCCCGGGCATTTTTTTGTGTGAT). The XhoI/BamHI fragment from pSRG55 was then subcloned into the XhoI/BamHI sites of Bluescript KS+, thus generating pSRG96. pSRG97 was generated by ligating the SalI(filled-in)/EcoRV fragment from KanMX4 (Wach et al., 1994) into the SmaI/HpaI site of pSRG96. The T39K mutation was made by a two-step PCR procedure with the oligonucleotides 5KB21, 3KB21, 21T39K1 (AATAAGGAGCCCAAGATTGGTGCAGC), and 21T39K2 (GCTGCACCAATCTTGGGCTCCTTATT). The final PCR product was digested with XhoI/BamHI and subcloned into the XhoI/BamHI sites of pRS306.
Table 1.
Plasmids used in this study
| Plasmid | Description | Source |
|---|---|---|
| pKJH2 | vps27Δ∷LEU2 disruption plasmid | Raymond et al., 1992 |
| pLG39 | Integrating GAL1-VPH1 promoter fusion construct | Piper et al., 1994 |
| pRCP78 | Integrating c-myc VPS21 construct | This study |
| pRCP95 | vps10-Δ10*∷LEU2 integrating plasmid | Piper et al., 1997 |
| pSRG4 | VPS21 in pRS316 | This study |
| pSRG92 | 2μ-URA3 c-myc VPS21 | This study |
| pSRG97 | vps21∷KANr disruption plasmid | This study |
| pSRG99 | Integrating vps21-T39K construct | This study |
| pTS18 | CEN URA3 PEP4 plasmid | Ammerer et al., 1986 |
Strains
Yeast strains (Table 2) were constructed with the use of standard genetic techniques and grown in rich media (1% yeast extract, 1% peptone, 2% dextrose [YPD]; 1% yeast extract, 1% peptone, 2% raffinose [YPRaff]) or standard minimal medium lacking appropriate amino acids. All strains were derived from SF838-9D or the congenic PEP4 strain RPY10, which have been described previously (Rothman and Stevens, 1986; Piper et al., 1995).
Table 2.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SF838-9D | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 | Rothman and Stevens, 1986 |
| RPY10 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 PEP4 | Piper et al., 1995 |
| NBY79 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vph1-Δ369∷URA3∷GAL1-VPH1 | Bryant et al., 1998a |
| RPY105 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 PEP4 VPS10∷LEU2∷vps10-Δ10* | Piper et al., 1997 |
| RPY57 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 c-myc VPS21 | This study |
| SGY36 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 PEP4 vps21-T39K | This study |
| SGY37 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps21-T39K | This study |
| SGY46 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps21-T39K vph1-Δ369∷URA3∷GAL1-VPH1 | This study |
| SGY47 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 PEP4 vps21-T39K VPS10∷LEU2∷vps10-Δ10* | This study |
| SGY73 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps27Δ∷LEU2 | This study |
| SGY77 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps27Δ∷LEU2 vph1-Δ369∷URA3∷GAL1-VPH1 | This study |
| SGY78 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps21-T39K vps27Δ∷LEU2 vph1-Δ369∷URA3∷GAL1-VPH1 | This study |
| SGY79 | MATα leu2-3, 112 ura3-52 his4-519 ade6 gal2 pep4-3 vps21∷Kanr | This study |
RPY57 was generated by transforming SF838-9D with pRCP78 linearized with HpaI and selecting for Ura+ transformants. Colonies that expressed the c-myc VPS21 allele were identified by Western analysis and then plated onto medium containing 5-FOA. Colonies were again checked for expression of the c-myc VPS21 allele by Western analysis. SGY36 was derived from RPY10 by transforming with pSRG99 linearized with HpaI. Ura+ transformants were then plated onto medium containing 5-FOA, and vps21-T39K colonies were identified by CPY overlay assay (Rothman and Stevens, 1986). SGY37 was derived from SGY36 by transforming with pLO2010 linearized with EcoRI. Ura+ transformants were then plated onto medium containing 5-FOA, and pep4 mutant colonies were identified by CPY plate assay (Wolf and Fink, 1975). SGY46 was derived from SGY37 by transforming with pLG39 linearized with BstEII and integrated at the VPH1 locus. Ura+ colonies were screened for galactose-inducible expression of VPH1 by Western analysis. SGY47 was derived from SGY36 by transforming with pRCP95 linearized with XbaI and screening the Leu+ transformants by immunoblot analysis for the expression of full-length and truncated Vps10p. SGY79 was derived from SF838-9D by transforming with XbaI/XhoI-digested pSRG97 and screening the Kanr colonies for CPY secretion by overlay assay. SGY73, SGY77, and SGY78 were derived from SF838-9D, NBY79, and SGY46, respectively, by transforming with PstI/BamHI-digested pKJH2 and screening the Leu+ colonies for CPY secretion at 25°C by overlay assay.
Pulse-Chase Immunoprecipitations
The fates of newly synthesized Vps10p-Δ10*, CPY, and ALP were followed by immunoprecipitation of the protein, as described previously (Nothwehr et al., 1995; Piper et al., 1995; Cooper and Stevens, 1996). The fate of newly synthesized Ste3p was followed by immunoprecipitation of the protein in a manner essentially as described for ALP. Briefly, yeast cultures were grown overnight at 25°C in synthetic minimal medium lacking methionine to OD600 = 1. A total of 0.5 OD600 of cells per time point to be analyzed were transferred to fresh medium and incubated at either 25 or 36°C for 5 min before labeling. Labeling was initiated by the addition of 100 μCi of [35S]Express label per 0.5 OD600 of cells and then chased for specified times by the addition of excess unlabeled methionine and cysteine (final concentration of 100 μg/ml). The chase was terminated by the addition of 20 mM sodium azide and chilling the cells on ice. Cells were converted to spheroplasts and lysed with the use of 2% SDS for CPY and 1% SDS/8 M urea for all other proteins. Lysates were then adjusted to 0.1% SDS, 0.1% Triton X-100, 0.8 M urea, and 20 mM Tris-HCl, pH 8.0, before the addition of the appropriate antibody (1 μL of CPY or Vps10p; 2 μL of ALP or Ste3p). After a 2-h incubation at 4°C, S. aureus cells (IgG Sorb) were added for an additional hour.
Fluorescence Microscopy
Indirect immunofluorescence microscopy for the localization of Vps10p, Vph1p, Pep12p, and ALP was performed as described previously (Roberts et al., 1991; Nothwehr et al., 1995; Bryant et al., 1998b). Indirect immunofluorescence microscopy for the localization of c-myc Vps21p was essentially as described previously, with the c-myc mAb used at a final concentration of 2 μg/ml. For experiments involving the induction from the GAL1 promoter in temperature-sensitive mutants, cells were grown to OD600 = 1 in YPRaff medium at 25°C. The culture was then split, galactose was added to 2%, and the cells were incubated at either 25 or 37°C. After 30 min, cycloheximide was added to a final concentration of 100 μg/ml, and cells were fixed after 15 min if incubated at 37°C and after 45 min if incubated at 25°C. For experiments not involving galactose induction, cells were grown overnight under the appropriate selection in synthetic medium. Cells were then diluted to ∼0.25 OD600 in YPD medium and allowed to go through two cell divisions before fixation. Cells were fixed by the addition of formaldehyde to a final concentration of 3% for 10 min, collected by centrifugation, and followed with an incubation in 2% paraformaldehyde/50 mM potassium phosphate, pH 7.0, for 15 h. Cells were converted to spheroplasts and permeabilized by treatment with 1% SDS for 2 min. Cells were washed in 1.2 M sorbitol and allowed to adhere to poly-l-lysine–coated slides. Incubation of cells with the primary antibody was performed at 22°C for 2 h. Secondary and tertiary antibodies underwent 1-h incubations at 22°C. Images were obtained from either a Bio-Rad (Richmond, CA) confocal microscope (Figures 1 and 2) or a Zeiss (Thornwood, NY) microscope fitted with Nomarski optics (Figures 6 and 8).
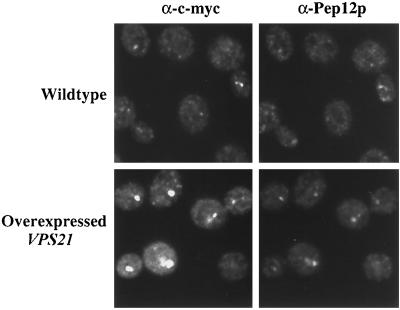
Figure 1.
Vps21p and Pep12p localize to endosomal membranes. c-myc VPS21 pep4-3 (RPY57) and pep4-3 (SF838-9D) overexpressing VPS21 (pSRG92) were grown to midlog phase before fixation. Vps21p was labeled with mouse anti-c-myc antibodies and Alexa 594–conjugated secondary antibody. Pep12p was labeled with rabbit anti-Pep12p antibodies, followed by biotinylated secondary antibody and FITC-conjugated streptavidin. Staining was visualized by confocal microscopy.
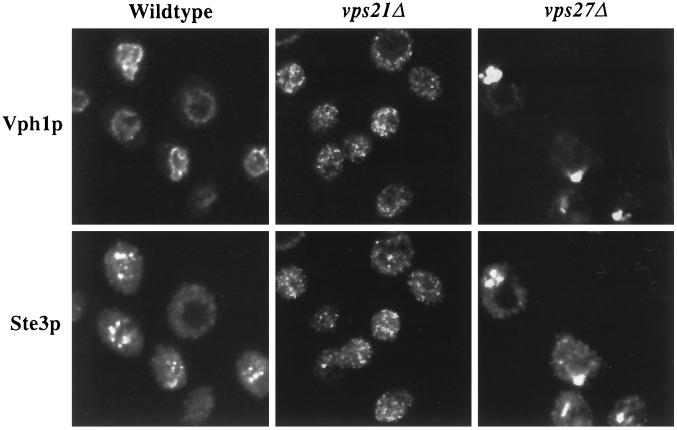
Figure 2.
vps21Δ and vps27Δ cells accumulate Ste3p and Vph1p in trafficking intermediates. pep4-3 (SF838-9D), vps27Δ pep4-3 (SGY73), and vps21Δ pep4-3 (SGY79) cells were grown to midlog phase at 25°C before fixation. Vph1p was labeled with rabbit anti-Vph1p antibodies and Alexa 594–conjugated secondary antibody. Ste3p was labeled with mouse anti-Ste3p antibodies, followed by biotinylated secondary antibody and FITC-conjugated streptavidin.
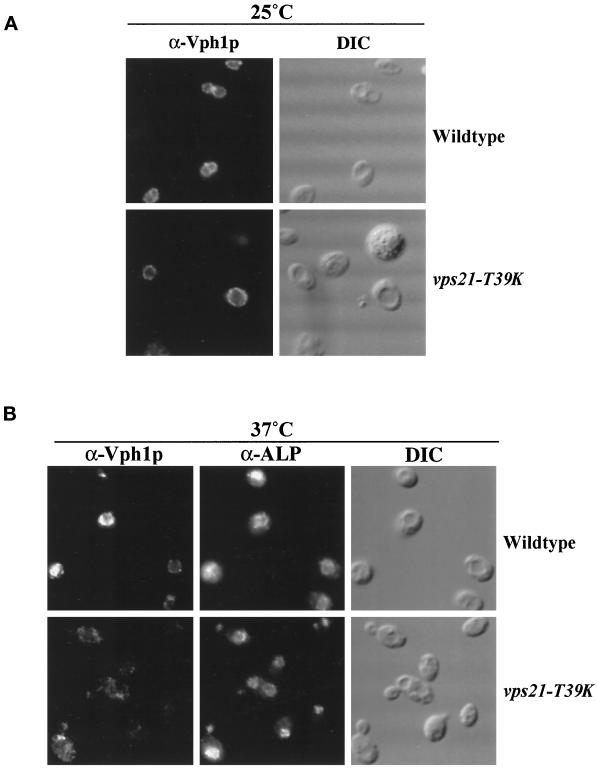
Figure 6.
VPS21 is required for the delivery of Vph1p but not for the delivery of ALP to the vacuole. The production of Vph1p was under the control of the galactose-inducible GAL1 promoter in both pep4-3 (NBY79) and vps21-T39K pep4-3 (SGY46) strains. Cells were grown to midlog phase at 25°C, galactose was added, and the cells were incubated for 30 min at either 25°C (A) or 37°C (B). Protein synthesis was terminated by the addition of cycloheximide, followed by incubation for an additional 45 min at 25°C (A) or for 15 min at 37°C (B) before fixation. Vph1p was labeled with rabbit anti-Vph1p antibodies and Texas Red–conjugated secondary antibody. ALP was labeled with mouse anti-ALP antibodies, followed by biotinylated secondary antibody and FITC-conjugated streptavidin.
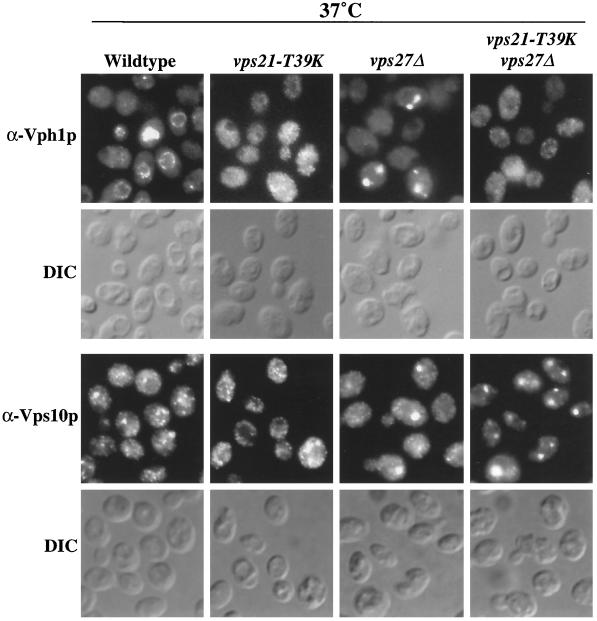
Figure 8.
VPS21 is epistatic to VPS27 for Vph1p trafficking. The production of Vph1p was under the control of the galactose-inducible GAL1 promoter in all strains. pep4-3 (NBY79), vps21-T39K pep4-3 (SGY46), vps27Δ pep4-3 (SGY77), and vps21-T39K vps27Δ pep4-3 (SGY78) strains were grown to midlog phase at 25°C, galactose was added, and the cells were incubated for 30 min at 37°C. Protein synthesis was terminated by the addition of cycloheximide, followed by incubation for an additional 15 min at 37°C before fixation. Vph1p and Vps10p were labeled with rabbit anti-Vph1p or rabbit anti-Vps10p antibodies, respectively, and Alexa 594–conjugated secondary antibody. DIC, differential interference contrast.
Subcellular Fractionation
Cells were fractionated with the use of differential centrifugation after osmotic lysis, as described previously (Horazdovsky and Emr, 1993). Cells were grown in rich medium (10 ml) to OD600 = 1 and then incubated in 1 ml of 50 mM Tris-HCl, pH 8, 1% 2-mercaptoethanol for 10 min at 30°C. Cells were then converted to spheroplasts by treatment with zymolyase (150 μg/ml) in 1 ml of 1.2 M sorbitol, 50 mM potassium phosphate, pH 7.5, 1 mM magnesium chloride at 30°C for 40 min. Spheroplasts were washed once in 1.2 M sorbitol before osmotic lysis in 1.3 ml of cold 0.2 M sorbitol, 50 mM Tris-HCl, pH 7.5, 1 mM EDTA. Unbroken cells were removed by centrifugation for 5 min at 500 × g (this and all subsequent centrifugation steps were performed at 4°C), yielding 1.2 ml of whole cell extract. One milliliter of whole cell extract was subjected to centrifugation at 13,000 × g for 10 min to yield a P13 (pellet) fraction. The resulting supernatant fraction was subjected to centrifugation at 100,000 × g for 30 min, yielding P100 (pellet) and S100 (supernatant) fractions. The P13 and P100 pellets were resuspended in 200 μl of 8 M urea, 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, 10% 2-mercaptoethanol. Proteins were precipitated from the S100 and 0.2 ml of the whole cell extract with the use of 10% trichloroacetic acid (TCA) and resuspended in 200 and 40 μl, respectively, of 8 M urea, 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, 10% 2-mercaptoethanol.
Sucrose Gradients
P13 membranes were prepared from 20 OD of cells essentially as described for differential centrifugation, except that the membranes were pelleted onto 100 μl of 60% sucrose, 10 mM HEPES, pH 7.5. The supernatant was removed, 300 μL of 10 mM HEPES, pH 7.5, was added, and the membranes were resuspended by pipetting up and down. The P13 membranes were then loaded at the top of an 11.4-ml 40–60% linear sucrose gradient that contained 10 mM HEPES, pH 7.5, throughout. The samples were centrifuged in a SW41-Ti rotor at 38,000 rpm for 18 h at 4°C. Sixteen fractions were collected from the top of the gradient, 5 μg of BSA was added as carrier, and the proteins were precipitated with 10% TCA. The TCA pellet was washed in acetone and then resuspended in 50 μl of 8 M urea, 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, 10% 2-mercaptoethanol.
RESULTS
Vps21p Localizes to Endosomal Membranes
To assess the localization of Vps21p, an N-terminal c-myc–tagged allele was generated and integrated into the genome as the sole copy of VPS21. This strain was indistinguishable from the wild-type strain with respect to CPY sorting and processing of vacuolar proteins (our unpublished observations), and thus the tagged VPS21 allele is fully functional. Figure 1 shows double labeling of Vps21p and Pep12p. Pep12p has been shown previously to fractionate away from Golgi and vacuolar marker proteins (Becherer et al., 1996), consistent with Pep12p residing in an endosomal/prevacuolar compartment. Consistent with this subcellular fractionation data, Pep12p and Vps21p exhibited highly punctate staining patterns characteristic of endosomal proteins by indirect immunofluorescence microscopy (Figure 1).
The highly punctate nature of Vps21p and Pep12p staining made determination of the extent of colocalization difficult; therefore, we repeated this experiment in a strain that overexpressed the c-myc VPS21 allele. Overexpression of VPS21 resulted in a collapse of the Vps21p punctate staining pattern into one to three larger staining structures per cell (Figure 1) (Singer-Kruger et al., 1995) in a manner analogous to that seen with overexpression of Rab5 in mammalian cells (Bucci et al., 1990). Rab5 controls early endosome fusion, and this altered endosomal morphology caused by Rab5 overexpression has been interpreted as being a result of increased homotypic fusion of endosomes (Gorvel et al., 1991). Although overexpression of VPS21 in yeast appears to alter endosomal morphology, the sorting of CPY and recycling of Vps10p were unaffected in these cells (our unpublished observations), making overexpression of VPS21 a useful tool for the analysis of endosomal markers by immunofluorescence. Double labeling of Pep12p and Vps21p is shown in Figure 1 in a strain that overexpressed VPS21. Overexpression of VPS21 altered the distribution of Pep12p such that Pep12p now clearly colocalized with Vps21p in fewer, but larger, structures.
Ste3p and Vph1p Are Mislocalized in vps21Δ Cells
Ste3p is the mating pheromone receptor present on the plasma membrane of MATα cells. After internalization, the receptor follows the endocytic pathway to the vacuole, where it is degraded. In wild-type cells, Ste3p is delivered to the lumen of the vacuole in multivesicular bodies (Odorizzi et al., 1998) and degraded in a Pep4p protease–dependent manner with a half-life of ∼20 min at 30°C (Davis et al., 1993). As a consequence of rapid internalization and delivery to the vacuole, Ste3p localizes to the vacuole in cells that lack Pep4p (Davis et al., 1993). Many vacuolar proteins traverse the secretory pathway as far as the TGN, where they are sorted away from bulk secretory traffic into vesicles destined for the PVC (Bryant and Stevens, 1998). The 100-kDa subunit of the vacuolar H+-ATPase, Vph1p, is an integral membrane protein that reaches the vacuole via the PVC, and its delivery to the PVC is blocked in many class D vps mutants, such as vps45 (Piper et al., 1997; Bryant et al., 1998a). Consistent with Ste3p being stable in the lumen of the vacuole in pep4 cells (Odorizzi et al., 1998), we observed Ste3p inside the limiting vacuolar membrane, as defined by Vph1p, an integral membrane subunit of the vacuolar H+-ATPase (Figure 2). VPS27 functions after the convergence of the biosynthetic and endocytic pathways, as demonstrated by the accumulation of both Ste3p and Vph1p in the exaggerated PVC class E compartment (Figure 2) (Piper et al., 1995; Bryant et al., 1998a). Indirect immunofluorescence staining for both Ste3p and Vph1p was punctate and nonvacuolar in vps21Δ cells (Figure 2). Therefore, Vps21p is involved in the vacuolar delivery of Ste3p and Vph1p.
Ste3p and Vph1p Accumulated in Different Transport Intermediates in vps21Δ Cells
Our results indicate that VPS21 controls the delivery of both endocytic and biosynthetic proteins into the PVC. In animal cells, lysosomal traffic appears to pass from the Golgi to the early endosome (Ludwig et al., 1991; Press et al., 1998). Therefore, a block in early-to-late endosome traffic would be expected to cause endocytic and biosynthetic proteins to accumulate in the same early endosome or early-to-late endosome trafficking intermediates. To address the issue of pathway convergence in yeast, we sought to determine the nature of the transport intermediate in which Vph1p and Ste3p are trapped in vps21 mutant cells. It is not possible to resolve the Vph1p- and Ste3p-containing trafficking intermediates by immunofluorescence microscopy; therefore, we attempted to determine whether the trafficking intermediates could be separated by subcellular fractionation.
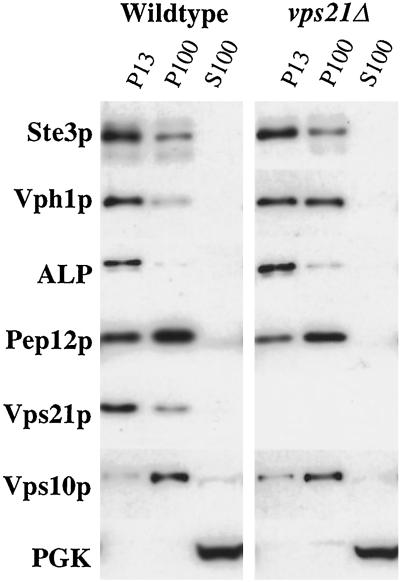
Lysates from wild-type and vps21Δ cells were subjected to fractionation, and the fractions were probed by immunoblotting for marker proteins of various intracellular compartments. Vacuolar membrane proteins such as ALP and Vph1p were recovered in the P13 fraction from wild-type cells (Figure 3). In pep4 mutant cells, Ste3p was stable and accumulated in the lumen of the vacuole (Figure 2). Consequently, Ste3p fractionated as a vacuolar protein in pep4 cells. The lighter membranes (P100 fraction) include Golgi membranes and transport vesicles. Vps10p is a Golgi membrane protein and localized predominantly to the P100 fraction in both wild-type and vps21Δ cells (Figure 3). Although Ste3p no longer reached the vacuole (Figure 2), it still fractionated predominantly with P13 membranes in vps21Δ cells (Figure 3). Vph1p fractionated in approximately equal amounts between the P13 and P100 membrane fractions from vps21Δ cells (Figure 3). The fraction of Vph1p that was recovered with P13 membranes from vps21Δ cells did not appear to be vacuolar, as indicated by immunofluorescence microscopy (Figure 2). Electron microscopy of vps45 and vps21 strains has revealed the accumulation of 40- to 60-nm transport in these cells (Horazdovsky et al., 1994; Piper et al., 1994; Webb et al., 1997). The Vph1p that fractionated in the P100 membranes of vps21Δ cells is presumably trapped in these vesicles.
Figure 3.
The Ste3p and Vph1p trafficking intermediates that accumulate in vps21Δ cells are partially separable by differential centrifugation. pep4-3 (SF838-9D) and vps21Δ pep4-3 (SGY79) strains were subjected to differential centrifugation to yield low-speed pellet (P13), high-speed pellet (P100), and S100 fractions, as described in MATERIALS AND METHODS. The amount of Ste3p, Vph1p, ALP, Pep12p, Vps21p, Vps10p, and phosphoglycerate kinase (PGK) in each of the fractions was assessed by immunoblot analysis.
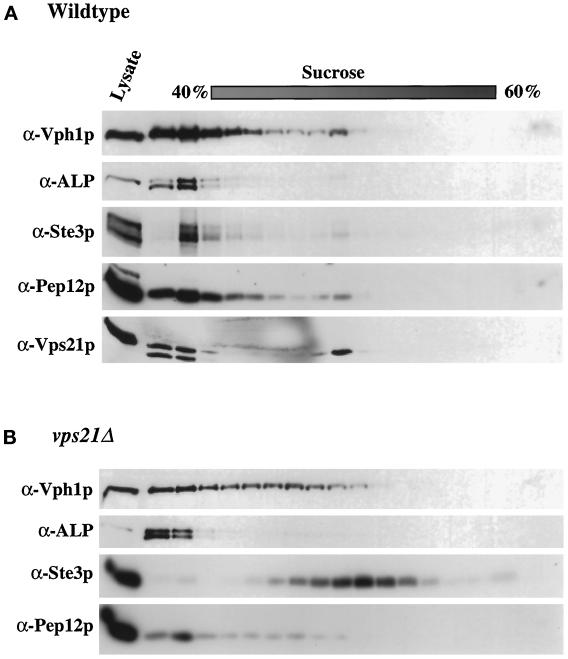
Although Ste3p- and Vph1p-containing membranes from vps21Δ cells largely fractionated away from each other, it was still possible that a portion of Vph1p was trapped in the same transport intermediate as Ste3p. To further investigate this possibility, P13 membranes were subjected to sucrose density gradient fractionation. In wild-type cells, vacuolar and endosomal proteins fractionated near the top of the gradient, predominantly in the first three fractions (Figure 4). In membranes from the vps21Δ strain, ALP, Vph1p, and Pep12p continued to fractionate near the top of the gradient, although Vph1p extended deeper into the gradient than was observed for wild-type membranes. However, Ste3p shifted into the middle of the gradient and peaked in fractions away from Vph1p, Pep12p, or ALP (Figure 4). These data suggest that Ste3p and Vph1p do not accumulate in the same trafficking intermediate in vps21Δ mutant cells. Furthermore, Ste3p fractionated away from Pep12p (Figure 4), a result consistent with a model in which Vps21p controls membrane traffic into the Pep12p-containing compartment.
Figure 4.
Sucrose density gradient fractionation of P13 membranes from pep4-3 (SF838–9D) (A) and vps21Δ pep4-3 (SGY79) (B) cells. P13 membranes were prepared and applied to the top of a 40–60% linear sucrose density gradient. Samples were centrifuged for 18 h, and then 16 fractions were removed from the top of the gradient, as described in MATERIALS AND METHODS. The amount of Vph1p, ALP, Ste3p, Pep12p, and Vps21p in each of the fractions was assessed by immunoblot analysis.
VPS21 Controls Biosynthetic Traffic into the PVC
Class D VPS genes, such as VPS21, are thought to be responsible for the delivery of CPY-containing vesicles to the PVC (Horazdovsky et al., 1994). Null mutations in these genes result in phenotypes consistent with their proposed role, such as the accumulation of 40- to 60-nm vesicles, failure to mature intracellular CPY, and decreased protease activity in the vacuole (Cowles et al., 1994; Horazdovsky et al., 1994; Piper et al., 1994; Becherer et al., 1996; Webb et al., 1997).
Most endocytic assays in yeast rely on proteolytic degradation of internalized plasma membrane proteins. Interpretation of degradation assays in vps21Δ strains is made difficult by the fact that these strains have decreased proteolytic activity in the vacuole because the trafficking of soluble vacuolar hydrolases is blocked. We sought to isolate a conditional temperature-sensitive allele of VPS21 that could be rapidly inactivated upon exposure to the nonpermissive temperature. This type of allele would allow the vacuole to maintain proteolytic activity and thus would make the correlation between a degradation defect and a trafficking defect more direct. We screened extensively for such an allele, but we were unable to isolate a temperature-sensitive VPS21 that could be rapidly inactivated upon exposure to the nonpermissive temperature. We then made two effector domain mutations, T39K and G138D, that are analogous to mutations that result in temperature-sensitive alleles of another rab, YPT1 (Jedd et al., 1995). vps21-G138D makes a protein that is functional at all temperatures (our unpublished data); however, vps21-T39K appeared to encode a temperature-sensitive version of Vps21p.
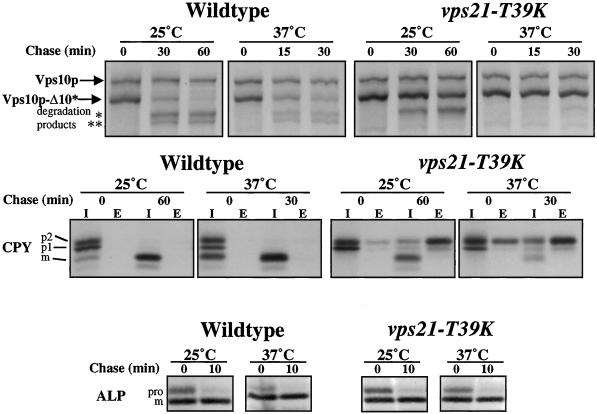
CPY and a truncated form of the CPY receptor, Vps10p-Δ10*, are resident vacuolar proteins that are subjected to Pep4p protease–dependent processing in the vacuole (Piper et al., 1997). The processing kinetics of CPY and Vps10p-Δ10* were measured in wild-type and vps21-T39K cells at 25 and 37°C (Figure 5). In wild-type cells, all of the CPY was found in the intracellular fraction in the mature form at both 25 and 37°C, consistent with its delivery to the vacuole. By contrast, cells carrying the T39K mutation in VPS21 secreted a portion of CPY at both temperatures (∼60%) (Figure 5); however, processing of the intracellular pool to the mature form was temperature dependent. At the permissive temperature of 25°C, 75% of the internal CPY was proteolytically processed to the mature form (Figure 5). However, upon shift to 37°C, only 30% of the intracellular pool was found in the mature form, consistent with newly synthesized CPY being trapped in transport vesicles (Figure 5). Similarly, Vps10p-Δ10* was delivered to the vacuole and processed with a half-time of ∼15 min in wild-type cells, but this processing was blocked in vps21-T39K cells at 37°C (Figure 5). Consistent with placing Vps21p function at the Golgi-to-PVC transport step, ALP, which bypasses the PVC and uses an alternative pathway to the vacuole (Cowles et al., 1997; Piper et al., 1997; Stepp et al., 1997), was processed with wild-type kinetics in vps21-T39K cells (Figure 5). Furthermore, the block in processing observed for CPY and Vps10p-Δ10* was not a result of decreased protease activity in the vacuole, because ALP was rapidly processed even after vps21-T39K cells were incubated at the nonpermissive temperature for 120 min before metabolic labeling (our unpublished results).
Figure 5.
VPS21 is required for proteolytic processing of Vps10p-Δ10* and CPY but not for proteolytic processing of ALP. Wild-type (RPY10) and vps21-T39K (SGY36) cells were incubated at 25°C or for 5 min at 37°C before the addition of [35S]Express. Cells were labeled for 10 min and then chased for the indicated times. Vps10p, Vps10p-Δ10*, CPY, and ALP were immunoprecipitated from cell lysates, as described in MATERIALS AND METHODS. The experimental procedure was repeated three times, and results of a representative experiment are shown.
We then characterized the effect of the vps21-T39K mutation on the trafficking of vacuolar membrane proteins by immunofluorescence. Newly synthesized Vph1p (whose synthesis was driven by the galactose-inducible GAL1 promoter) was found on the vacuolar membrane of both wild-type and vps21-T39K cells at 25°C, as defined by the depression visualized with Nomarski optics (Figure 6A). Newly synthesized Vph1p was also found on the vacuolar membrane of wild-type cells at 37°C (Figure 6B). By contrast, vps21-T39K cells, incubated at 37°C for 5 min before induction of Vph1p synthesis by galactose addition, failed to deliver newly synthesized Vph1p to the vacuolar membrane, as defined by localization of ALP, which follows a VPS21-independent path to the vacuole (Bryant et al., 1998a). Instead of vacuolar membrane staining, these cells exhibited a dispersed, punctate staining pattern for Vph1p (Figure 6B) similar to that seen for Vph1p in vps21Δ cells (Figure 2). These results indicate that rapid loss of Vps21p function leads to an immediate block in transport along the CPY pathway to the vacuole.
Ste3p Degradation Is Delayed in vps21-T39K Cells
We have demonstrated that the vps21-T39K allele is partially functional at the permissive temperature of 25°C. Although sorting of vacuolar proteins is not efficient, the vacuoles are proteolytically active, as demonstrated by the processing of ALP with wild-type kinetics (Figure 5). Therefore, degradation of Ste3p can be used as an assay for its delivery to the vacuole in vps21-T39K cells.
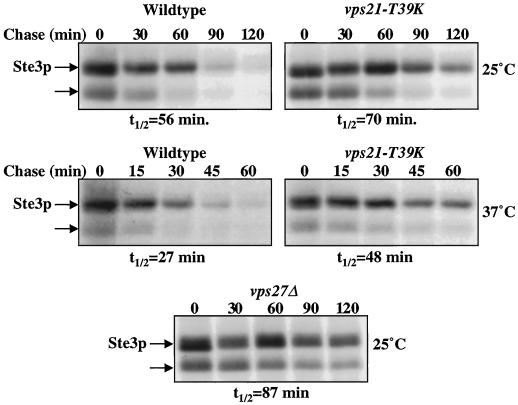
The requirement for VPS21 in the turnover of Ste3p was assessed by measuring the half-life of the receptor in vps21-T39K cells at the permissive and nonpermissive temperatures. Ste3p appears as two distinct bands that are found exclusively in MATα strains and not in the congenic MATa strain (our unpublished results). Ste3p is ubiquitylated and phosphorylated before internalization, accounting for the observation that the lower band chased into the upper band over time (Figure 7) (Roth and Davis, 1996). At the permissive temperature for CPY sorting (25°C), vps21-T39K cells were modestly inhibited in Ste3p degradation compared with wild-type cells, showing a half-life of degradation of 70 min compared with 56 min in wild-type cells (a 25% increase). Class E VPS genes, such as VPS27, are involved in the formation of multivesicular bodies (Odorizzi et al., 1998). Consistent with this, vps27Δ mutant cells were also impaired in the degradation of Ste3p. Ste3p half-life in a vps27Δ strain was 87 min at 25°C, a 55% increase over that of the wild-type cells (Figure 7). However, if cells were metabolically labeled after a 5-min incubation at 37°C, the half-life of Ste3p degradation decreased to 27 min in wild-type cells and 48 min in vps21-T39K cells. Therefore, the turnover of Ste3p was slowed by 78% in vps21-T39K cells at 37°C relative to wild-type cells at 37°C. Although the half-life increase for Ste3p in vps21-T39K and vps27Δ cells is modest, it correlates with the accumulation of Ste3p in nonvacuolar intermediates in vps21Δ and vps27Δ cells (Figure 2).
Figure 7.
VPS21 and VPS27 are required for the Pep4p protease–dependent degradation of Ste3p. Wild-type (RPY10) and vps21-T39K (SGY36) cells were incubated at 25°C or for 5 min at 37°C before the addition of [35S]Express. vps27Δ (SGY73) cells harbored the PEP4 plasmid (pTS18) and were incubated at 25°C before the addition of [35S]Express. Cells were labeled for 10 min and then chased for the indicated times. Ste3p was immunoprecipitated from cell lysates, as described in MATERIALS AND METHODS. The experimental procedure was repeated three times, and results of a representative experiment are shown.
VPS21 Is Epistatic to VPS27 for Trafficking of Vph1p
Vps10p-Δ10* and CPY were not proteolytically processed in vps21-T39K cells at the nonpermissive temperature (Figure 5), consistent with their accumulation in a proteolytically inactive trafficking intermediate along the pathway. Previous studies of vps27 mutant strains have shown that newly synthesized Vph1p accumulates in the class E compartment, an exaggerated form of the PVC (Piper et al., 1995; Bryant et al., 1998a). These observations suggest that VPS21 controls a trafficking step before fusion of TGN-derived vesicles with the PVC. To address the epistatic relationship of VPS21 and VPS27, we constructed a vps21-T39K vps27Δ strain and followed the fate of Vph1p synthesized after shift to the nonpermissive temperature for the vps21-T39K mutant. The vps21-T39K vps27Δ and vps21-T39K strains cultured at 37°C exhibited the same diffuse distribution of Vph1p, as opposed to the accumulation of Vph1p in the class E compartment in vps27Δ cells (Figure 8). In the same strains, the steady-state distribution of the recycling CPY receptor, Vps10p, was also visualized. In wild-type cells, Vps10p cycles between the TGN and the PVC and localizes to the TGN at steady state, thus displaying a punctate staining pattern (Figure 8) (Cereghino et al., 1995; Cooper and Stevens, 1996). Mutation of VPS27 altered this distribution because Vps10p could no longer traffic from the PVC back to the TGN; consequently, it accumulated in the class E compartment within vps27Δ cells (Figure 8) (Bryant et al., 1998a). In vps21-T39K cells at 37°C, Vps10p staining was punctate, consistent with its localization being predominantly TGN or vesicular (Figure 8). However, in vps27Δ and vps21-T39K vps27Δ strains, Vps10p was limited to one to three larger staining structures, consistent with it being trapped in the class E compartment (Figure 8). Because the vps21-T39K and vps21-T39K vps27Δ strains had been at the nonpermissive temperature for only 45 min, the steady-state distribution of Vps10p remained qualitatively unchanged (Golgi/vesicular in vps21-T39K cells and class E compartment in vps21-T39K vps27Δ cells). Therefore, the class E compartment is still present in the vps21-T39K vps27Δ cells, yet Vph1p staining is diffuse as a result of vps21-T39K mutation blocking a trafficking step before vesicle fusion with the PVC.
DISCUSSION
Newly synthesized soluble vacuolar hydrolases, such as CPY, are diverted from bulk secretory traffic at the TGN and trafficked through the PVC en route to the vacuole (Figure 9) (Graham and Emr, 1991). The level of the α-factor receptor Ste3p on the plasma membrane is regulated through a process of continual internalization and degradation in the vacuole (Davis et al., 1993). As such, Ste3p also traffics through the PVC, but it takes a route different from CPY (Figure 9). Vps27p controls traffic out of the PVC in both the anterograde direction to the vacuole and the retrograde direction to the Golgi. Loss of VPS27 results in a failure to deliver both vacuolar and endocytosed proteins to the vacuole (Piper et al., 1995). Vacuolar and endocytosed proteins, such as Vph1p and Ste3p, instead accumulate in an exaggerated form of the PVC, termed the class E compartment (Piper et al., 1995). Our data indicate that vps21 mutant cells also fail to deliver both endocytic and vacuolar proteins to the vacuole. Unlike vps27 cells, however, vps21 cells accumulated Vph1p and Ste3p in distinct trafficking intermediates, suggesting that Vps21p functions at two different membrane trafficking steps before convergence of the vacuolar and endocytic trafficking pathways. Finally, our epistasis analysis indicates that Vps21p acts upstream of Vps27p for the trafficking of Vph1p.
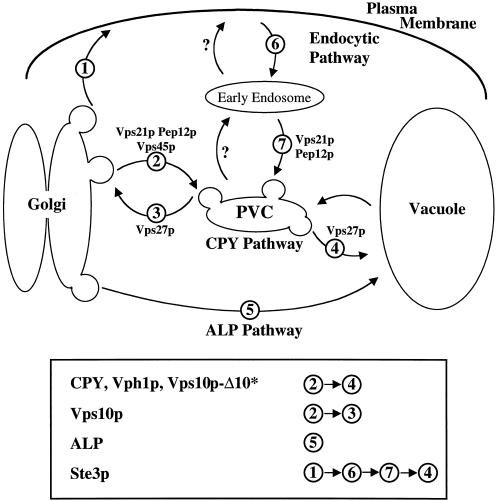
Figure 9.
Transport pathways to the vacuole. The CPY pathway is taken by soluble vacuolar hydrolases and the membrane proteins Vps10p-Δ10* and Vph1p. This pathway is sensitive to mutation in VPS21 or VPS27. Vps21p controls Golgi-derived traffic into the PVC, and Vps27p controls traffic leaving the PVC for either the Golgi or the vacuole. The ALP pathway is not affected by mutation in VPS21 or VPS27. Endocytosed proteins, such as Ste3p, traffic via the plasma membrane and through the PVC en route to the vacuole. Vacuolar delivery of Ste3p is dependent on VPS21 and VPS27. The question marks refer to pathways for which there is little or no evidence in yeast.
Rab proteins are a large and diverse family of ras-like GTPases (Schimmoller et al., 1998). The large number of rabs and their specific localization within eukaryotic cells lead to the hypothesis that each population of transport vesicles possesses its own unique rab protein (Novick and Zerial, 1997). It has been proposed that rab proteins control the fidelity of vesicular fusion by regulating SNARE pairing, possibly through interaction in a GTP-dependent manner with regulatory proteins such as members of the Sec1p-like family (Schimmoller et al., 1998). Our data strongly suggest that Vps21p functions in two transport steps: TGN to PVC and early endosome to PVC. The only other rab protein that has been implicated in two transport steps is Ypt1p, which functions in endoplasmic reticulum to cis-Golgi and cis-Golgi to medial-Golgi transport (Jedd et al., 1995). The multiple roles for Vps21p and Ypt1p are not necessarily inconsistent with the proposal that rabs regulate SNARE pairing. Many SNARE proteins have been shown to act at multiple transport steps (Gotte and Fischer von Mollard, 1998), and these rabs may simply be controlling the formation of SNARE complexes that function in multiple transport steps.
vps21 cells accumulate 40- to 50-nm vesicles, similar to those found in vps45 and pep12 cells (Cowles et al., 1994; Horazdovsky et al., 1994; Piper et al., 1994; Becherer et al., 1996). VPS21 and VPS45 interact genetically, and the phenotypes associated with mutation in these genes suggest that they act together at the Golgi-to-PVC transport step (Tall et al., 1999). Small transport vesicles, such as those that accumulate in vps45 and vps21 cells, are expected to fractionate with the lighter P100 membranes. As such, the Vph1p pool in vps45 cells is found nearly exclusively in the P100 fraction (Bryant et al., 1998a). Interestingly, a significant portion of the Vph1p pool (∼50%) and all of the Ste3p pool were found with the heavier P13 fraction of membranes from vps21Δ cells, suggesting that they are not in the same type of transport intermediate that accumulates in vps45 cells. Furthermore, the Ste3p intermediate was quite dense and peaked further into a 40–60% sucrose gradient than vacuolar and PVC proteins. These data are consistent with Ste3p being trapped in an early endosomal compartment. Internalized α-factor, which follows the same path to the vacuole as Ste3p, associates first with a heavy membrane fraction, followed by a second, lighter-density membrane fraction, in an energy- and time-dependent manner (Singer-Kruger et al., 1993). These intermediates, therefore, have been defined as early and late endosomes. These biochemically defined endocytic intermediates probably correspond to the vesicular/tubular network and perivacuolar compartments identified by electron microscopy of cells that had endocytosed nanogold particles (Prescianotto-Baschong and Riezman, 1998).
Ste3p is not trapped on the plasma membrane of vps21Δ cells, indicating that Vps21p acts after internalization. Therefore, there are at least two endocytic trafficking events after internalization, and they can be genetically separated based on a requirement for VPS21 or VPS27. Traffic into the PVC requires the function of Vps21p, whereas traffic out of the PVC requires the function of Vps27p. Our genetic data correspond well with recent morphological data that have revealed the existence of three distinct endocytic trafficking intermediates (Prescianotto-Baschong and Riezman, 1998; Mulholland et al., 1999). Consistent with our assigning VPS21 function before VPS27, we found by sucrose density gradient that Ste3p fractionated away from the PVC syntaxin Pep12p. Our data, therefore, support a model in which Vps21p controls traffic from the early endosome into the late endosome or PVC (Figure 9).
The vps21-T39K mutation blocks the transport of Vps10p-Δ10*, CPY, Vph1p, and Ste3p to the vacuole. The effect of the temperature-dependent inactivation of this allele on the trafficking of these proteins was rapid; thus, we infer that Vps21p is likely to be involved directly in the transport of each of these proteins. Inactivation of VPS21 function had no effect on the delivery and proteolytic processing of ALP. This result demonstrates two important points: first, that VPS21 is involved strictly in trafficking through the endosomal system, because ALP bypasses the endosomes and is delivered directly to the vacuole (Cowles et al., 1997; Piper et al., 1997; Stepp et al., 1997); and second, that the vacuole of a vps21-T39K cell is proteolytically active. Therefore, the processing block observed in vps21-T39K cells at the nonpermissive temperature for CPY, Vps10p-Δ10*, and Ste3p is a transport block.
There has been debate regarding whether Vps21p functions like its mammalian homologue, Rab5. The role that Rab5 plays in endocytosis and homotypic fusion of early endosomes is well characterized (Bucci et al., 1990; Gorvel et al., 1991; Li et al., 1994). Although Vps21p is an endocytic protein that appears to be involved in homotypic fusion of endosomes, the pleiotropic phenotypes associated with loss of Vps21p have made its role in endocytosis less clear (Horazdovsky et al., 1994; Singer-Kruger et al., 1994, 1995). The vps21-T39K strain displays a delay in Ste3p processing after brief exposure to the nonpermissive temperature. This processing delay is not a result of general protease deficiency, because ALP, a vacuolar protein that follows a VPS21-independent pathway to the vacuole, is proteolytically processed with wild-type kinetics. Furthermore, by sucrose density gradient, Ste3p-containing membranes separated from ALP membranes in vps21Δ cell lysates. These data argue that, like Rab5, Vps21p is involved in endosomal protein trafficking.
Vps21p controls the transport of biosynthetic vacuolar and endocytosed proteins to the vacuole. These transport events are distinct, and Vps21p controls both trafficking events. Although there are no data that directly address the role of Rab5 in lysosomal protein trafficking, recent data suggest that soluble lysosomal hydrolases do not travel directly from the TGN to the late endosome. Newly synthesized cathepsin D, a soluble lysosomal hydrolase, appears to transit early endosomes before reaching the late endosome (Press et al., 1998). These results predict that a role for Rab5 in lysosomal trafficking will likely be found.
Loss of Vps21p causes Vph1p and Ste3p to accumulate in distinct transport intermediates. Therefore, it appears that Vps21p controls two different transport steps. Genetic and physical data indicate that Vps21p, Vps45p, and Pep12p function together at the TGN-to-PVC transport step (Burd et al., 1997; Tall et al., 1999). Loss of Vps45p does not appear to affect vacuolar delivery of Ste3p (Bryant et al., 1998a); therefore, it seems that although Vps21p is a common component of both transport steps, it does not necessarily interact with the same proteins to mediate membrane fusion. Experiments are under way to identify the other components of early endosome-to-PVC transport.
ACKNOWLEDGMENTS
We thank Laurie Graham for affinity-purified anti-Vph1p antibodies. This work was supported by grant GM32448 from the National Institutes of Health to T.H.S. and a National Institutes of Health predoctoral traineeship to S.R.G.
REFERENCES
- Ammerer G, Hunter CP, Rothman JH, Saari GC, Valls LA, Stevens TH. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986;6:2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Johnson LM, Emr SD. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent endocytic route. Eur J Cell Biol. 1998a;76:43–52. doi: 10.1016/S0171-9335(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Weisman LS, Stevens TH. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol. 1998b;142:651–663. doi: 10.1083/jcb.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Hauri HP, Simons K, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1990;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghino JL, Marcusson EG, Emr SD. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol Biol Cell. 1995;6:1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Emr SD, Horazdovsky BF. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J Cell Sci. 1994;107:3449–3459. doi: 10.1242/jcs.107.12.3449. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NG, Horecka JL, Sprague GF. cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J-P, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gotte M, Fischer von Mollard G. A new beat for the SNARE drum. Trends Cell Biol. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Tall GG, Horazdovsky BF. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J Biol Chem. 1999;274:15284–15291. doi: 10.1074/jbc.274.21.15284. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989;342:749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Hill JE, Myers AM, Koerner TJ, Tzagoloff A. E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Horazdovsky BF, Busch GR, Emr SD. VPS21 encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Emr SD. The VPS16 gene product associates with a sedimentable protein complex and is essential for vacuolar protein sorting in yeast. J Biol Chem. 1993;268:4953–4962. [PubMed] [Google Scholar]
- Horiuchi H, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EW, Webb GC, Hiller MA. Molecular Biology of the Yeast Saccharomyces cerevisiae. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. [Google Scholar]
- Li G, Barbieri MA, Colombo MI, Stahl PD. Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J Biol Chem. 1994;269:14631–14635. [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Konopka J, Singer-Kruger B, Zerial M, Botstein D. Visualization of receptor-mediated endocytosis in yeast. Mol Biol Cell. 1999;10:799–817. doi: 10.1091/mbc.10.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins are transported to the plasma membrane and then to the vacuole in vps1 mutant yeast cells. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, postGolgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Prescianotto-Baschong C, Riezman H. Morphology of the yeast endocytic pathway. Mol Biol Cell. 1998;9:173–189. doi: 10.1091/mbc.9.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press B, Feng Y, Hoflack B, Wandinger-Ness A. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–1089. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Banta LM, Kohrer K, McCaffery JM, Emr SD. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Nothwehr SF, Stevens TH. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992;119:69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Raymond CK, Yamashiro CT, Stevens TH. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- Roth AF, Davis NG. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Stevens TH. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Schandel KA, Jenness DD. Direct evidence for ligand-induced internalization of the yeast alpha-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier J-M, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B, Frank R, Crausaz F, Riezman H. Partial purification and characterization of early and late endosomes from yeast. J Biol Chem. 1993;268:14376–14386. [PubMed] [Google Scholar]
- Singer-Kruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo J-S, Gallwitz D, Zerial M. Role of three Rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Kruger B, Stenmark H, Zerial M. Yeast Ypt51p and mammalian Rab5: counterparts with similar function in the early endocytic pathway. J Cell Sci. 1995;108:3509–3521. doi: 10.1242/jcs.108.11.3509. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall GG, Hama H, DeWald DB, Horazdovsky BF. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Webb G, Zhang J, Garlow S, Wesp A, Riezman H, Jones E. Pep7p provides a novel protein that functions in vesicle-mediated transport between the yeast Golgi and endosome. Mol Biol Cell. 1997;8:871–895. doi: 10.1091/mbc.8.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann H, Hengst L, Gallwitz D. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p) Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Fink GR. Proteinase C (carboxypeptidase Y) mutant of yeast. J Bacteriol. 1975;123:1150–1156. doi: 10.1128/jb.123.3.1150-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]