Abstract
In response to different cellular stresses, a family of protein kinases phosphorylates eIF2α (α subunit of eukaryotic initiation factor-2), contributing to regulation of both general and genespecific translation proposed to alleviate cellular injury or alternatively induce apoptosis. Recently, we reported eIF2α(P) (phosphorylated eIF2α) in the brain during SE (status epilepticus) induced by pilocarpine in mice, an animal model of TLE (temporal lobe epilepsy) [Carnevalli, Pereira, Longo, Jaqueta, Avedissian, Mello and Castilho (2004) Neurosci. Lett. 357, 191–194]. We show in the present study that one eIF2α kinase family member, PKR (double-stranded-RNA-dependent protein kinase), is activated in the cortex and hippocampus at 30 min of SE, reflecting the levels of eIF2α(P) in these areas. In PKR-deficient animals subjected to SE, eIF2α phosphorylation was clearly evident coincident with activation of a secondary eIF2α kinase, PEK/PERK (pancreatic eIF2α kinase/RNA-dependent-protein-kinase-like endoplasmic reticulum kinase), denoting a compensatory mechanism between the two kinases. The extent of eIF2α phosphorylation correlated with the inhibition of protein synthesis in the brain, as determined from polysome profiles. We also found that C57BL/6 mice, which enter SE upon pilocarpine administration but are more resistant to seizure-induced neuronal degeneration, showed very low levels of eIF2α(P) and no inhibition of protein synthesis during SE. These results taken together suggest that PKR-mediated phosphorylation of eIF2α contributes to inhibition of protein synthesis in the brain during SE and that sustained high levels of eIF2α phosphorylation may facilitate ensuing cell death in the most affected areas of the brain in TLE.
Keywords: double-stranded-RNA-dependent protein kinase (PKR), pilocarpine, status epilepticus, α subunit of eukaryotic initiation factor-2 (eIF2α), temporal lobe epilepsy model, translation initiation
Abbreviations: ATF4, activating transcription factor 4; dsRNA, double-stranded RNA; eIF2α, α subunit of eukaryotic initiation factor-2; eIF2α(P), phosphorylated eIF2α; 4E-BP1, eIF4E-binding protein 1; ER, endoplasmic reticulum; GADD34, growth-arrest and DNA-damage-inducible protein 34; HRI, haem-regulated eIF2α kinase; HRP, horseradish peroxidase; IRES, internal ribosomal entry site; ISR, integrated stress response; PKR, dsRNA-dependent protein kinase; PEK/PERK, pancreatic eIF2α kinase/RNA-dependent-protein-kinase-like ER kinase; PACT, PKR-activating protein; PP1, protein phosphatase 1; RPL32, ribosomal protein L32; SE, status epilepticus; TLE, temporal lobe epilepsy
INTRODUCTION
Phosphorylation of the α subunit of the translation initiation factor-2 (eIF2α) has been shown to occur following diverse stress conditions, both in in vitro cell cultures as well as in animal models [1–6]. In addition, it has become increasingly evident that the impaired control of eIF2α phosphorylation is the basis of relevant pathological conditions in humans, such as the Vanishing White Matter disease and the Wolcott–Rallison syndrome, and has been associated with several neuropathologies, such as Alzheimer's and Huntington's diseases and epilepsy [7–12]. Phosphorylation of eIF2α plays a central role in ISR (integrated stress response) that co-ordinates both global and gene-specific translation.
eIF2 participates in translation initiation as a ternary complex, eIF2–GTP–Met-tRNAiMet (initiator methionyl-tRNA), which delivers the initiator tRNA to the 40 S ribosomal subunit [13]. Prior to the joining of the large and small ribosomal subunit, GTP associated with eIF2 is hydrolysed and eIF2-GDP is released. Phosphorylation of eIF2α impedes the exchange of GDP to GTP on eIF2, which is required for subsequent rounds of initiation, leading to a significant reduction in global translation. Coincident with this global down-regulation, phosphorylation of eIF2α induces the translation of key regulatory proteins, such as the transcription activator ATF4 (activating transcription factor 4) [CREB-2 (cAMP-response-element-binding protein-2)] [14,15]. ATF4 directs expression of a number of genes that alleviate cellular damage to stress, or alternatively induce apoptosis [16].
Four eIF2α kinases are known in mammals, which are each activated by different stress conditions [17]. GCN2 is activated by nutrient deprivation, UV irradiation and proteasome inhibition [5,13,18,19]. The activity of PEK/PERK [pancreatic eIF2α kinase/RNA-dependent-protein-kinase-like ER (endoplasmic reticulum) kinase], an ER transmembrane protein, is stimulated by an increase in unfolded proteins in the ER. This so-called ER stress can be experimentally induced by drugs that impair N-linked glycosylation of proteins, block disulphide bond formation, or prevent ER calcium influx mediated by SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) [2,3]. PKR [dsRNA (double-stranded RNA)-dependent protein kinase] is activated by dsRNA produced by viral infection and by the stress-activated protein PACT (PKR-activating protein) [20]. Finally, the eIF2α kinase HRI (haem-regulated eIF2α kinase), found predominantly in erythroid cells, is activated by haem deprivation and arsenite stress [21,22]. Activation of these eIF2 kinases is proposed to involve the relief of their distinct inhibitory domains allowing for their dimerization. This dimerization then triggers an autophosphorylation event at the kinase activation loop that facilitates the recognition and phosphorylation of eIF2α [23,24]. The model presented suggests that each environmental stress is uniquely recognized by a single eIF2α kinase. However, recent studies indicate that this is a simplified view. For example, analysis of PEK/PERK+/+ and PEK/PERK−/− mouse embryo fibroblast showed that eIF2α phosphorylation requires PEK/PERK early in response to ER stress, but with extended stress conditions there was significant eIF2α kinase activity even in the PEK/PERK-depleted cells [25]. Prolonged amino acid depletion also results in the phosphorylation of eIF2α in GCN2−/− cells [26]. Furthermore, there are certain stress conditions, such as oxidative stress, where there can be activation of multiple eIF2α kinases, depending on the cell type, dosage of the environmental agent and duration of exposure [22,27,28]. In addition, both PKR and PEK/PERK have been implicated in the resistance of cells to vesicular stomatitis virus infection [29]. These observations suggest that more than one eIF2α kinase can function during a given stress condition, mediating either additive or compensatory mechanisms.
We have recently described the extensive eIF2α phosphorylation in the mice brain during SE (status epilepticus) induced by pilocarpine, a cholinergic agonist [11]. This is a well-characterized animal model of TLE (temporal lobe epilepsy), where a period of continuous convulsions (SE) is followed by a silent period that can vary from 14 to 20 days. After this silent period, animals develop spontaneous recurrent epileptic seizures, with neuronal degeneration primarily in the hippocampus, cortex and amigdala [30]. Immunohistochemistry analysis indicated that at 30 min following SE onset, the cortex (layer V) and hippocampus displayed high levels of eIF2α(P) (phosphorylated eIF2α) [11].
In the present study, we show that PKR is activated in the hippocampus and cortex early in SE. In PKR−/− animals, we detected the activation of PEK/PERK, suggesting a compensatory mechanism of eIF2α kinase activation. We also provide a quantitative temporal analysis of the rates of dephosphorylation of eIF2α following SE onset. The levels of eIF2α(P) parallel the extent of inhibition of protein synthesis. Given the known downstream targets of this signalling pathway, our results suggest that phosphorylation of eIF2α by PKR during SE may have implications in the ensuing cell death observed in the most affected brain areas.
MATERIALS AND METHODS
This study was conducted using methods approved by the Animal Care and Use Ethic Committee of the Universidade Federal de São Paulo, in accordance with the Guide for Care and Use of Laboratory Animals adopted by the NIH (National Institutes of Health; Bethesda, MD, U.S.A.).
Animals and drug treatment
All experiments were conducted in Swiss albino mice, except where otherwise indicated. Seizures were induced by intraperitoneal injections of pilocarpine hydrochloride (Merck, Quimitra, Brazil). The pilocarpine dose was adjusted to each mouse strain as the smallest possible for induction of SE in an efficient manner: 200 mg/kg for the Swiss mice, 250 mg/kg for the 129Sv mice and 300 mg/kg for C57BL/6. SE was characterized as previously defined [30]. For most animals, seizures progressed to SE within approx. 10 min after pilocarpine injection. Thionembutal was used, where indicated, at a dose of 30 mg/kg. Animals were killed at the indicated times after SE onset. Experiments were conducted with at least three groups of four animals each, except where otherwise indicated.
Extract preparation
For polysome analysis, whole brains were homogenized with 400 μl of buffer A: 50 mM Hepes/KOH (pH 7.5), 140 mM potassium acetate, 4 mM magnesium acetate, 2.5 mM dithiothreitol, 0.32 M sucrose, 2 mM benzamidine, 2 mM EGTA, 10 μg/ml pepstatin A, 1 μg/ml leupeptin and 1 μg/ml antipain [31]. The supernatant was obtained by centrifugation at 14000 g for 10 min at 4 °C. Extracts of whole brain and brain parts employed in immunoblot assays were prepared in a buffer containing 1% Triton, 150 mM NaCl, 20 mM Hepes (pH 7.5), 10% (v/v) glycerol, 1 mM EDTA, 100 mM NaF, 10 mM tetrasodium pyrophosphate, 1 mM PMSF, 4 μg/ml aprotinin and 2 μg/ml pepstatin.
Polysome profiles
Extracts [20 units of A260 (absorbance at 260 nm)] were loaded on 7–47% sucrose gradients, prepared with a buffer containing 20 mM Tris/HCl (pH 7.6), 3 mM magnesium acetate and 100 mM KCl. The samples were ultracentrifuged in a Beckman rotor SW41 at 39000 rev./min for 150 min. Gradient profiles were obtained by continuous UV monitoring at A254.
RNA isolation and Northern blots
Gradient fractions (0.9 ml) were collected into tubes containing 2 ml of 8 M guanidinium chloride; 3 ml of 100% ethanol was added and incubated overnight at −20 °C. RNA was precipitated by centrifugation at 2800 g for 30 min. The pellets were resuspended in 400 μl of diethyl pyrocarbonate-treated distilled water and the RNA was precipitated with 0.3 M NaOAc (pH 4.5) and ethanol. Brain total RNA was obtained by TRIzol® extraction as described by the manufacturer (Life Technologies). The RNAs were resuspended in gel-loading buffer and loaded on to 1.4% (w/v) agarose/formaldehyde gels. The RNA was transferred to Hybond-N membranes (Amersham Biosciences) by capillarity and immobilized by UV cross-linking. The membranes were prehybridized [1% BSA, 1 mM EDTA, 0.25 M Na2PO4, pH 7.5, and 7% (w/v) SDS] at 65 °C for 2 h and hybridized with the denatured probes [β-actin and RPL32 (ribosomal protein L32)] in the same solution overnight, followed by two 15 min washes with 1× SSC (0.15 M NaCl and 0.015 M sodium citrate) and 0.1% SDS at 65 °C. The probes used were obtained by reverse transcriptase–PCR from RNA isolated from brain extracts using oligonucleotides derived from the published murine sequences (RPL32: fw5′-ATGGCTGCCCTCCGGCCTCT-3′ and rv5′-CTACTCATTTTCTTCGCTGCGTAGC-3′; β-actin: fw5′-CGAGGCCCAGAGCAAGAGAG-3′ and rv5′-AGGAAGAGGATGCGGCAGTGG-3′) and labelled with [α-32P]dCTP by PCR using the same set of oligonucleotides. Analysis of the data was performed on Typhoon (Amersham Biosciences).
Immunoblots
Immunoblot analysis of the phosphorylated form of eIF2α and total eIF2α was performed as described previously [11]. For the detection of PKR(P), membranes were blocked with 0.1% Tween 20 and 5% (w/v) non-fat milk in PBS, and incubated with antibodies directed to the phosphorylated Thr446 residue [1:2000; a gift from Dr Antonis Koromilas (McGill University, Montreal, ON, Canada)] in PBS-Tween 20 (0.14 M NaCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3, and 0.1% Tween 20) and 5% (w/v) BSA, overnight at 4 °C. Detection was performed with Protein A–HRP (horseradish peroxidase), followed by ECL® (enhanced chemiluminescence). For the detection of total PKR, the previous membrane was stripped of the antibodies by incubation with 100 mM 2-mercaptoethanol, 2% SDS and 62.5 mM Tris/HCl (pH 6.7) at 50 °C for 30 min. The membrane was blocked as before and incubated with antibodies against total PKR (1:2000; Santa Cruz Biotechnology) in TBS (Tris-buffered saline; 150 mM NaCl and 50 mM Tris/HCl, pH 7.4)-Tween 20 and 5% BSA, overnight at 4 °C. Detection was as described above. For GADD34 (growth-arrest and DNA-damage-inducible protein 34), membranes were incubated with anti-GADD34 antibodies (Santa Cruz Biotechnology) in TBS-Tween 20 and 2.5% BSA, followed by detection with Protein A–HRP. Immunoblots for PEK/PERK(P) were performed on samples subjected to SDS/PAGE (7% gel). The membrane was incubated with anti-PEK/PERK antibodies (1:1000; Santa Cruz Biotechnology) in TBS-Tween 20 and 4% non-fat milk overnight, followed by detection with anti-rabbit IgG–HRP antibodies and ECL®. Immunoblots for 4E-BP1 (eIF4E-binding protein 1) employed polyclonal rabbit antibodies against total 4E-BP1 (1:1000; Cell Signaling Technology).
RESULTS
PKR is activated in the brain during SE
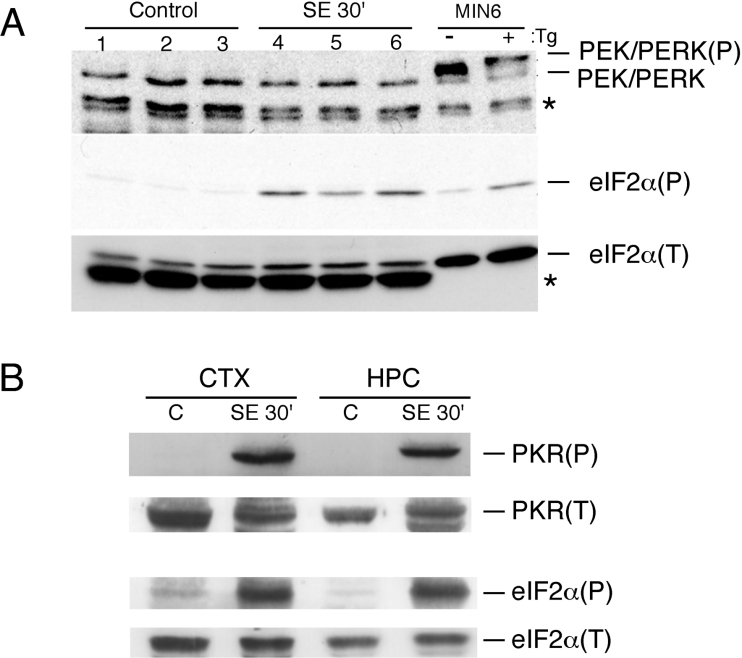
Consistent with our earlier report, eIF2α is highly phosphorylated in the brain of mice subjected to pilocarpine-induced SE (Figure 1A) [11]. In order to determine which eIF2α kinase was activated during SE, we initially investigated the involvement of PEK/PERK, which was previously shown to be activated in the brain ischaemia–reperfusion model [4]. Activation of PEK/PERK can be identified by the slower mobility of the phosphorylated form upon SDS/PAGE. No evidence was found for the activation of PEK/PERK in whole brain extracts (Figure 1A) or in isolated parts (see below). GCN2 activation was also investigated using antibodies directed to the phosphorylated form of this kinase. Although GCN2(P) was detected in whole brain extracts, no evidence was found for GCN2 activation in the hippocampus or cortex (results not shown). Because these are the brain areas most affected by pilocarpine-induced seizures and where eIF2α(P) is highest, GCN2 was ruled out as the main kinase activated during SE. We next studied PKR activation using antibodies that recognize the autophosphorylated form of this kinase. As shown in Figure 1(B), activated PKR was evident in the cortex and hippocampus at 30 min of SE.
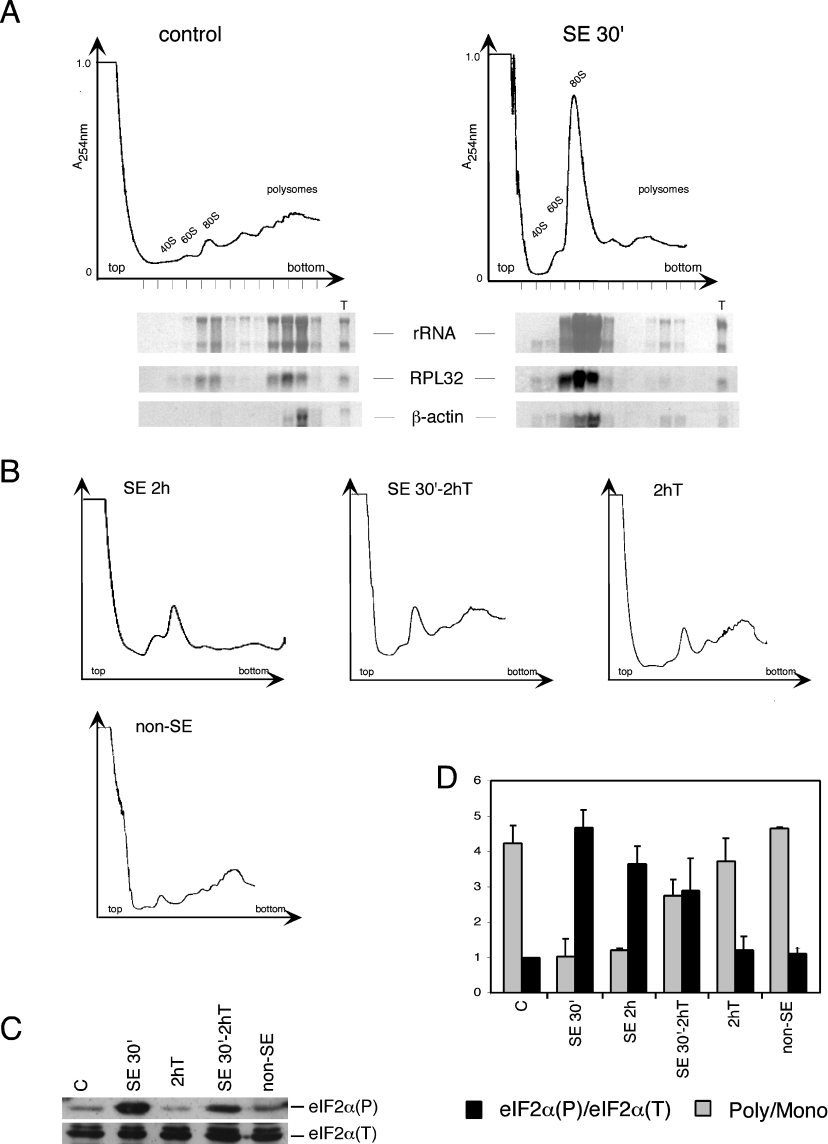
Figure 1. Activation of eIF2α kinases during SE.
(A) Immunoblots of whole brain extracts of three control animals and three animals subjected to 30 min of continuous SE (SE 30′) using antibodies directed to PEK/PERK (upper panel), to the phosphorylated form of eIF2α [eIF2α(P)] or the total eIF2α [eIF2α(T)]. The asterisks indicate non-specific bands. (B) Immunoblots of extracts prepared from the cortex (CTX) and hippocampus (HPC) of control animals (C) and of animals subjected to 30 min of SE using antibodies that recognize the phosphorylated form of PKR [PKR(P)] or total PKR [PKR(T)] (upper panels) and antibodies against total eIF2α and against the phosphorylated form of eIF2α (lower panels). The samples shown in (B) represent pools obtained from four animals each. The same gel was employed for the PKR and for the eIF2α blots. These immunoblots are representative of at least three independent experiments.
PEK/PERK substitutes for PKR
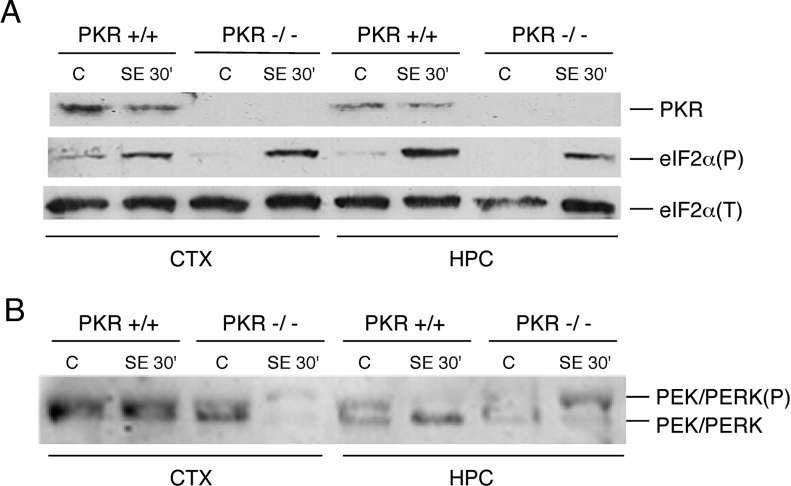
The involvement of PKR in the phosphorylation of eIF2α during SE was further investigated by the use of PKR−/− animals [32]. These mice and their isogenic wild-type strain 129Sv enter SE with a higher dose of pilocarpine when compared with the Swiss albino mice. Surprisingly, the PKR−/− animals subjected to SE displayed similar levels of eIF2α(P) in the cortex and hippocampus as the PKR+/+ mice (Figure 2A). Several reports have suggested that eIF2α kinases may compensate for each other under certain stress conditions [25,26]. Thus we investigated whether in the absence of PKR, another eIF2α kinase could be activated during SE. In PKR−/− animals subjected to SE, we found that PEK/PERK was activated in the cortex and hippocampus as determined by the slower electrophoretic mobility that characterizes the phosphorylated and activated form of this kinase (Figure 2B). Confirming the results shown in Figure 1(A), PEK/PERK was not activated in the wild-type animals. These results thus suggest a dynamic relationship between the two eIF2α kinases, whereby PEK/PERK can serve as a secondary kinase in the cortex and hippocampus that elicits SE-induced eIF2α phosphorylation in the absence of the primary kinase, PKR.
Figure 2. Activation of PEK/PERK in PKR-deficient mice.
(A) Extracts prepared from the indicated brain parts of PKR+/+ and PKR−/− mice, control and after 30 min of SE, were subjected to immunoblotting using antibodies against total PKR, and against total eIF2α or the phosphorylated form of eIF2α. (B) Immunoblot of the same extracts as in (A) employing antibodies directed towards total PEK/PERK. The upper band represents the phosphorylated form of this eIF2α kinase.
Levels of eIF2α phosphorylation in brain parts following SE onset
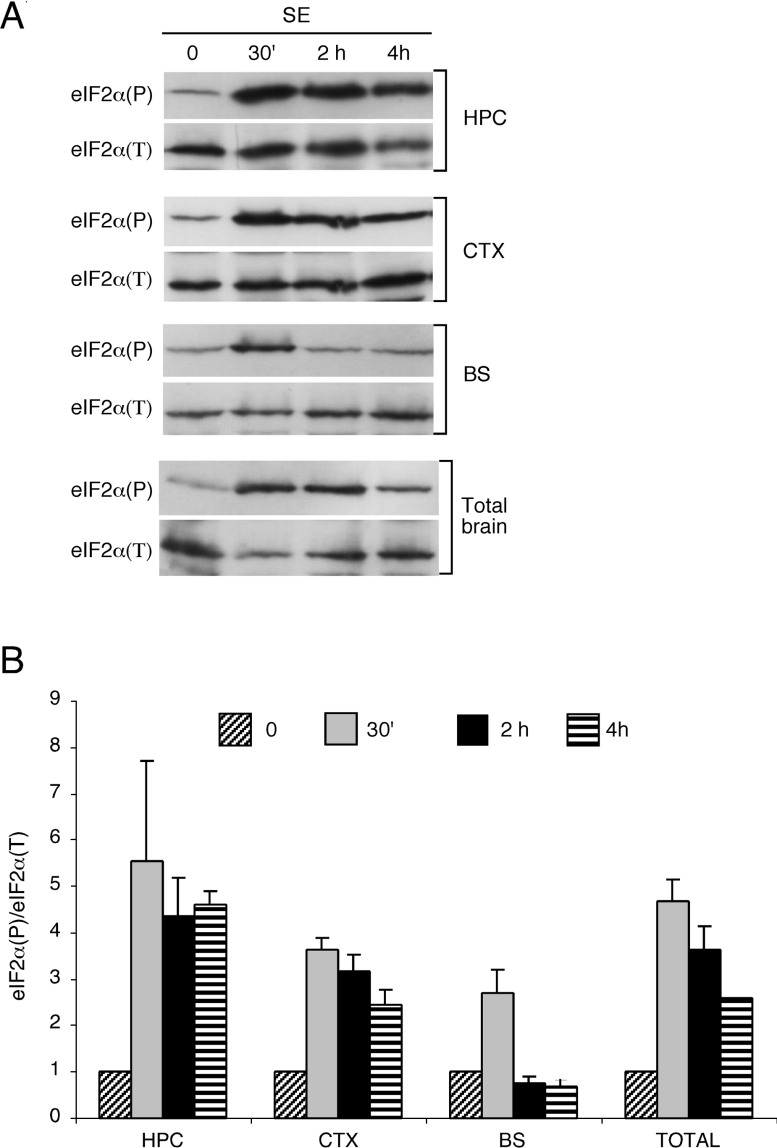
The duration of continuous SE determines whether animals will later develop spontaneous epileptic seizures and is correlated to the extent of cell death. The levels of eIF2α(P) were then studied at later time points of continuous SE in extracts of the hippocampus, cortex and the brain stem, a region not directly related to TLE (Figure 3). At 30 min of SE, the levels of eIF2α(P) reached a peak in all regions, with the hippocampus and cortex showing the highest eIF2α(P) levels compared with the brain stem. However, the duration of eIF2α(P) phosphorylation differed significantly, with eIF2α(P) levels in the hippocampus, the region most affected in this epilepsy model, remaining elevated for up to 4 h of continuous SE stress. In the brain stem, eIF2α(P) returned to control levels within 2 h of continuous SE. Phosphorylation of eIF2α(P) in the cortex was moderately diminished following the extended SE duration. Finally, in total brain extracts, the levels of phosphorylation followed an intermediary pattern, with maximum eIF2α(P) at 30 min of SE, followed by a 40% reduction by 4 h of this stress.
Figure 3. Levels of eIF2α(P) in different brain tissues prepared from mice subjected to SE.
(A) Lysates were prepared from the cortex (CTX), hippocampus (HPC), brain stem (BS) and whole brain (Total brain) from control animals (C) or mice after 30 min (30′), 2 h and 4 h of sustained SE. Equal amounts of each lysate were analysed by immunoblots using antibodies that specifically recognize the phosphorylated form of eIF2α or total eIF2α. (B) Quantification of eIF2α(P). Band intensities obtained from three independent experiments with four animals per group each were quantified by Kodak Digital Science software and normalized assuming a value of 1 to the ratio of eIF2α(P)/eIF2α obtained from control animals. Results are means±S.D.
Induction of GADD34 expression during SE
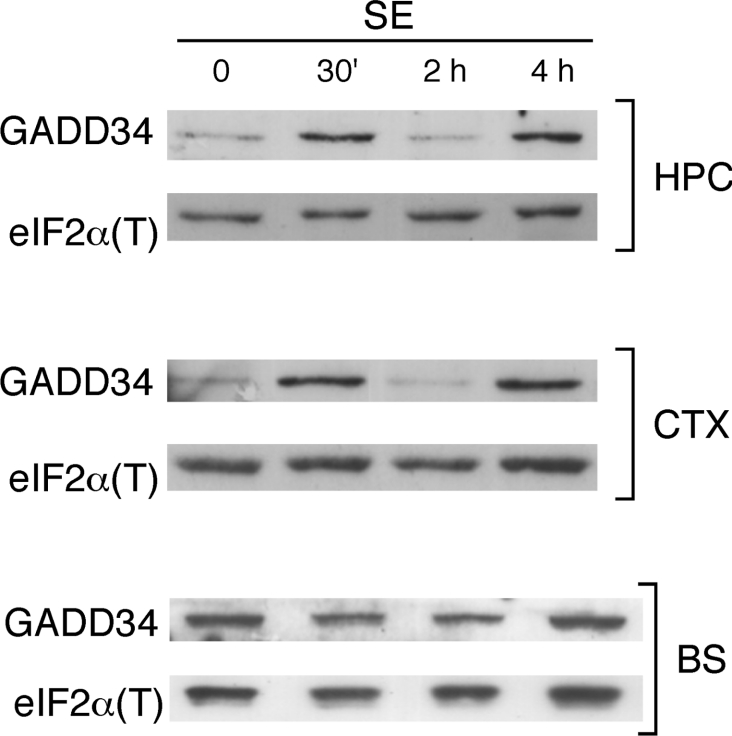
GADD34 is a regulatory subunit of PP1 (protein phosphatase 1) that targets PP1 to dephosphorylate eIF2α. Its transcriptional induction during the ISR triggered by eIF2α(P) serves as a negative feedback mechanism to alleviate the inhibition of protein synthesis that results from the activation of the eIF2α kinases [33]. The levels of GADD34 were then determined at the same time points of continuous SE for each of the brain parts (Figure 4). Interestingly, GADD34 showed a biphasic expression profile in the cortex and hippocampus, with a peak at 30 min of SE and another peak at 4 h of SE. In the brain stem, on the other hand, GADD34 levels did not change in response to SE, and its basal level was higher when compared with the other two brain regions. These results taken together with the pattern of eIF2α(P) suggest a dynamic stress response that may involve both the regulation of eIF2α kinases and phosphatases. Importantly, the increase in GADD34 by 30 min of SE supports the notion that the increased eIF2α(P) levels in SE in the hippocampus and cortex are due to elevated activity of a kinase, and not to the decreased activity of a phosphatase.
Figure 4. Expression of GADD34 during SE.
Immunoblots of extracts of the cortex (CTX), hippocampus (HPC) and brain stem (BS) from control animals and from animals after 30 min (30′), 2 h and 4 h of sustained SE, using anti-GADD34 antibodies. The bottom part of the same gel was employed for the immunoblots for normalization with eIF2α.
Protein synthesis inhibition during SE
Translational inhibition has been previously shown to occur in the brain of animals during seizures induced by electroshock or during SE induced by kainic acid; however, no biochemical basis for this event has been provided [34–36]. The extent of eIF2α(P) determines protein synthesis initiation rates. Thus we decided to evaluate whether in the pilocarpine model, where extensive eIF2α(P) is observed in the brain, protein synthesis was halted during SE. At 30 min of SE stress, all brain regions characterized showed extensive eIF2α(P), and we therefore asked whether this phosphorylation results in lowering of translation initiation. Polysomal profiles prepared by sucrose gradient centrifugation are the standard by which blocks in initiation of protein synthesis are determined [37]. As shown in Figure 5(A), there was a significant inhibition of translation initiation in the 30 min-SE brains, as indicated by the dissociation of polysomes and an increase in the monosome (80 S) peak. Northern-blot analysis of fractions obtained from the gradient showed that there is a marked reduction of translation of standard mRNAs, RPL32 and actin. While the abundance of these transcripts did not change, their association with translating ribosomes was significantly diminished. This profile is diagnostic of inhibition of translation initiation caused by eIF2α(P).
Figure 5. Inhibition of translation initiation during SE.
(A) Polysomal profiles of total extracts prepared from brains of control animals and of animals subjected to 30 min of SE following pilocarpine administration. The A254 profile of the gradient is illustrated, with free 40 and 60 S subunits, 80 S ribosomes and polysomes indicated. RNAs isolated from the fractions from these gradients are shown below, with the rRNA visualized directly on the membranes by Methylene Blue staining. Levels of the mRNAs encoding RPL32 and β-actin were detected on the same membranes by Northern blotting using sequential hybridization with 32P-labelled probes and detected on Typhoon. ‘T’ indicates total RNA (5 μg) used as control for the hybridizations. (B) Polysomal profiles of brain extracts from animals subjected to 2 h of continuous SE (SE 2h), animals subjected to 30 min of pilocarpine-induced SE followed by 2 h of thionembutal (SE 30′-2h T), animals treated with only thionembutal for 2 h (2h T), and animals that did not enter SE after pilocarpine administration (non-SE). (C) Immunoblot of whole brain extracts of the indicated groups of animals, using antibodies directed to total eIF2α and to eIF2α(P). (D) Quantitative analysis of polysome profiles and of eIF2α(P). The ratio of polysomes to monosomes was calculated by measuring the areas occupied by the 80 S peak and by polysomes (2-mers to n-mers). The ratio of eIF2α(P)/eIF2α(T) was calculated as described in Figure 3. Results are means±S.D. for at least three independent experiments with four animals per group.
In order to address whether the levels of eIF2α(P) correlate with the rates of protein synthesis, we performed polysome analysis of total brain from animals that were submitted to 2 h of continuous SE, and to 30 min SE followed by a 2 h recovery period with thionembutal, an anaesthetic that alleviates the acute crisis (Figure 5B). The polysome profiles were then compared with the extent of eIF2α phosphorylation in the whole brain (Figures 5C and 5D). As noted earlier, there is still a significant amount of eIF2α(P) in whole brain lysates following continuous SE for 2 h, albeit diminished relative to the 30 min SE (Figure 3). Accordingly, the profile indicates a decrease in the 80 S monosomes, with an apparent increase in the 40–60 S region, suggesting a resumption of the formation of initiation complexes. However, the formation of polysomes by 2 h of SE is still decreased. In the group treated with thionembutal, there was a reduction of eIF2α(P) levels and a polysome profile indicative of the increase in polysomes, and thus of protein synthesis (Figures 5B and 5D). The administration of thionembutal alone does not elicit eIF2α(P), although it causes a small reduction in translation initiation as judged by some increase in free ribosome levels as compared with the control polysome profile (Figures 5A, 5B and 5D). Animals that, although injected with pilocarpine, did not enter SE were also investigated for polysome profile and eIF2α(P) at a time point corresponding to approx. 30 min of SE (Figures 5B–5D). Brains prepared from these non-SE animals showed low levels of eIF2α(P) and enhanced translation initiation, as measured by the polysomes/monosomes ratio. In each of the treatment groups, there was a clear correlation between the extent of eIF2α(P) and the polysome profiles. The higher the levels of eIF2α(P), the more extensive the dissociation of polysomes due to reduced translation initiation.
Another well-characterized mechanism regulating translation initiation involves phosphorylation of 4E-BP1, which has been described to be altered in the brain ischaemia–reperfusion model [31]. 4E-BP1 directly binds to and sequesters the cap-binding protein, eIF4E. 4E-BP1 phosphorylation, mediated through the mTOR (mammalian target of rapamycin) pathway, abolishes this interaction, thus liberating eIF4E to participate in translation initiation. 4E-BP1 can be phosphorylated at multiple sites. As judged from the distinct mobilities of the different phosphorylated forms of 4E-BP1 in SDS/PAGE, no alteration in the phosphorylation status of 4E-BP1 was observed in the brains of SE animals compared with controls, suggesting that this mechanism does not modulate translation in response to SE stress (results not shown).
Translation initiation and eIF2α(P) levels are not affected during SE in C57BL/6 mice
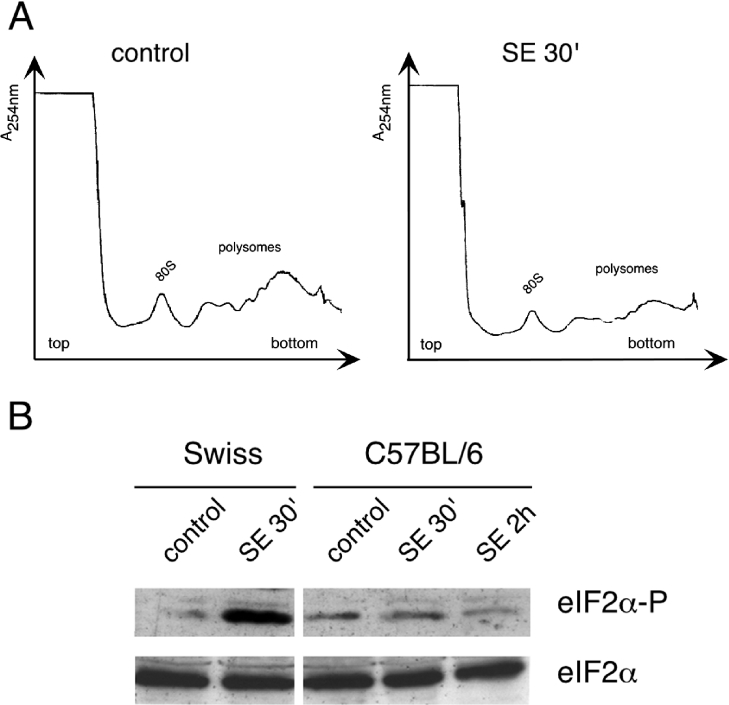
C57BL/6 mice have been described to show little neuronal damage incurred by SE induced by a variety of convulsant agents and are resistant to the excitotoxicity of glutamate [38,39]. We therefore analysed this strain of mice for both brain polysomal profiles and eIF2α(P) levels during SE. Although these animals enter SE, the pilocarpine dose needed was higher than the dose used for the Swiss mice, as has been described earlier [40]. No detectable inhibition of translation initiation was observed in the brain of C57BL/6 mice during SE, as indicated by the normal polysome profiles after 30 min of continuous SE (Figure 6A). In addition, we did not detect significant increases in eIF2α(P) at 30 min or at 2 h of sustained SE (Figure 6B). These results further support the idea that induced eIF2α(P) and accompanying translational control contribute to neural damage and ensuing changes associated with SE.
Figure 6. eIF2α(P) and translational control are not induced in C57BL/6 mice that are resistant to SE-induced neural damage.
(A) Polysome profiles of total extracts prepared from brains of C57BL/6 control animals and of animals subjected to 30 min of SE following pilocarpine administration. The position of the 80 S peak and the polysomes are indicated on the two profiles. (B) Immunoblots of total brain extracts from Swiss and C57BL/6 mice subjected to the indicated treatments, using antibodies that specifically recognize total eIF2α and eIF2α(P).
DISCUSSION
We have shown in the present study that translation inhibition coincident with activation of PKR and phosphorylation of eIF2α were elicited in the brain soon after animals enter SE induced by pilocarpine. Phosphorylation of eIF2α was sustained at high levels in the hippocampus and cortex. The accompanying long-lasting reduction in protein synthesis may be a central feature in the ensuing neuronal death in these critical regions. PKR activation has been recently suggested to be involved in several additional neurodegenerative disorders, such as Alzheimer's disease, amyotrophic lateral sclerosis and Huntington's disease (reviewed in [12]). The mechanism that contributes to the activation of PKR under these varied conditions is not known. During viral infection of cells, viral-derived dsRNA activates PKR by associating with two dsRNA-binding domains in PKR, and thus enhancing eIF2α phosphorylation and blocking cellular translation required for viral replication and proliferation [41]. At present, there is no evidence to support the involvement of dsRNA ligands in PKR regulation during SE or other neuropathologies. Interestingly, the protein PACT(RAX) (where RAX is the murine orthologue of the human PACT) was found to bind to PKR and to mediate its activation under stress conditions, including serum starvation and peroxide or arsenite treatments [42,43]. The mechanisms regulating PACT and its interactions with PKR are not well understood. Therefore PACT as well as other regulatory ligands that bind directly to PKR may be integral to the activation of PKR in the SE model.
In the brain ischaemia–reperfusion model of neuronal stress, extensive eIF2α phosphorylation and neuronal degeneration through necrosis or apoptosis are observed in the same regions as in TLE. Whereas PEK/PERK activation has been shown to occur soon after reperfusion in the ischaemia–reperfusion model, we found no evidence for the activation of this kinase in the early stages of SE when eIF2α(P) are highest. Although both models of stress result in similar cellular damage, the neurochemical events involved in each stress are strikingly dissimilar [44]. During ischaemia, metabolic rate ceases, with a rapid and extensive decrease in tissue energy state, thus lowering the availability of ATP. After reperfusion, concomitant with the rise in ATP, there is extensive oxidative damage, a condition suggested to contribute to the activation of PEK/PERK. On the other hand, during SE, regions considered vulnerable to epileptic damage show markedly augmented metabolic rates (200–300% of control), with cerebral energy state upheld, even at later stages of SE. Oxidative damage is known to occur through glutamate excitotoxicity later in SE. Interestingly, in the Swiss albino mice, we detected the activation of PEK/PERK and the unfolded protein response in the hippocampus and cortex at 8 h following SE onset (results not shown), which may be due to oxidative stress. Thus metabolic differences between SE and ischaemia–reperfusion may account for the different kinases activated in the same brain areas.
Our findings that, in the absence of PKR, PEK/PERK was activated in SE support the model of compensatory regulatory mechanisms involving eIF2α kinases. In response to SE, PEK/PERK may be activated at low, undetectable levels in the hippocampus and cortex, or in a small subpopulation of neurons in these regions. In the absence of PKR, this response may be exacerbated. In view of our results, it will certainly be important to study the consequences of SE induction in PKR−/− animals, regarding both the extent of neuronal degeneration and their susceptibility to recurrent epileptic seizures. Furthermore, we found that SE stress can induce GCN2 in other portions of the brain not directly linked with TLE (results not shown). Thus there are clear tissue variations in which a given environmental stress can activate each member of the eIF2α kinase family. These variations may reflect the degree to which cell types in organisms are exposed to an environmental stress and the differences among tissues in their susceptibility to these stresses. Furthermore, there appear to be variations in the expression of each eIF2α kinase among cell types, as exemplified by the preferential expression of HRI in erythroid cells and the high levels of PEK/PERK in secretory tissues, such as the pancreas. Differences in eIF2α kinase levels could alter the basal levels of eIF2α phosphorylation, as well as the degree to which eIF2α is phosphorylated in response to a given stress condition.
In the hippocampus, the most vulnerable area in this model of epilepsy, we demonstrated sustained high eIF2α(P) levels during SE. In areas not directly affected by SE, such as the brain stem, eIF2α(P) levels were lower and returned rapidly to basal levels. The immunohistochemistry data shown in our previous report [11] appear to reveal only the population of neurons with highest eIF2α(P) in the most affected areas; it is likely that this technique is unable to resolve the lower rates of phosphorylation observed in other brain areas, which were detected in the present study by Western blots, a much more sensitive and quantitative approach.
The pattern of eIF2α(P) and GADD34 levels in the brain parts suggests a dynamic stress response that may involve both regulation of eIF2α kinases and phosphatases. In response to stress, eIF2α(P) enhances ATF4 expression, leading to downstream expression of CHOP [C/EBP (CCAAT/enhancer-binding protein) homologous protein]/GADD153 and ATF3, bZIP (basic leucine zipper protein) transcription activators that trigger elevated expression of GADD34 and subsequent eIF2α(P) dephosphorylation in a negative feedback loop [33]. It is interesting to note that the increase in GADD34 observed at 30 min of SE in the hippocampus and cortex occurs at a time point when protein synthesis is severely inhibited as judged from polysome profiles and eIF2α(P) levels. The GADD34 mRNA has two upstream open reading frames and phosphorylation of eIF2α early during SE may result in a transient translational induction of a pre-existing small pool of this message. The reduction of GADD34 observed at 2 h of SE suggests that this protein may be labile in the brain subjected to SE, while the increase at 4 h could be accounted for by the transcriptional downstream response mediated through ATF4.
The phosphorylation state of eIF2α correlates well with the inhibition of protein synthesis as revealed by the polysome profiles, both in extent and in the recovery kinetics, strongly suggesting that impairment in ternary complex formation is the main cause of the translational shut off observed in SE. Also, our results with the C57BL/6 mice strain further corroborate the idea that eIF2α(P) is a major contributor to translation inhibition during SE.
The implication that high levels of eIF2α(P) lead to protein synthesis shut off in hippocampal neurons is apparently at odds with data on the expression of IEG (immediate early gene) proteins in the hippocampus in experimental models of epilepsy, such as c-Jun and c-Fos [45,46]. The expression of these proteins at early SE (30 min), however, has been observed in the hippocampal granule cells where little eIF2α(P) was detected by immunohistochemistry [11]. The increase in the synthesis of these proteins observed in the CA1 and CA3 regions of the hippocampus seems to follow the rates of general eIF2α(P) dephosphorylation that we have described here. It is also possible that these mRNAs can be translated under conditions of moderate eIF2α(P) levels, mediated either by a re-initiation mechanism similar to ATF4 or by IRESs (internal ribosomal entry sites) activated by low levels of ternary complex [47]. Interestingly, the chicken c-Jun mRNA has been shown to contain an IRES that mediates cap-independent translation initiation [48], and the 5′-UTR (5′-untranslated region) of mouse c-Jun mRNA contains features that are similar to the chicken c-Jun mRNA.
The work described here provides a biochemical basis for the inhibition of protein synthesis observed during SE. The activation of PKR adds to the biochemical events that have been described to occur during SE. Importantly, the extent and duration of eIF2α phosphorylation as determined here for the different brain regions as an early response elicited in SE have relevant implications regarding the activation of ISR downstream targets that may ultimately determine cell fate, as shown for a variety of other stress conditions. In the case of SE, they may relate directly to the ensuing neuronal degeneration and the development of recurrent epileptic seizures.
Acknowledgments
We thank Drs Antonis Koromilas and Nahum Sonenberg (McGill University) for anti-PKR(P) antibodies, and Luiz Fernando Reis (Ludwig Institute for Cancer Research, São Paulo, Brazil) for the PKR−/− animals. This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grants to B.A.C. and L.E.A.M.M. and by an NIH grant to R.C.W. L.S.C. and C.B.J. were recipients of doctoral fellowships and C.M.P. of a post-doctoral fellowship from FAPESP.
References
- 1.Kaufman R. J., Scheuner D., Schroder M., Shen X., Lee K., Liu C. Y., Arnold S. M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman R. J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D. Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R., Azam S., Sullivan J. M., Owen C., Cavener D. R., Zhang P., Ron D., Harding H. P., Chen J. J., Han A., et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J. Neurochem. 2001;77:1418–1421. doi: 10.1046/j.1471-4159.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- 5.Anthony T. G., McDaniel B. J., Byerley R. L., McGrath B. C., Cavener D. R., McNurlan M. A., Wek R. C. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 6.Gietzen D. W., Ross C. M., Hao S., Sharp J. W. Phosphorylation of eIF2α is involved in the signaling of indispensable amino acid deficiency in the anterior piriform cortex of the brain in rats. J. Nutr. 2004;134:717–723. doi: 10.1093/jn/134.4.717. [DOI] [PubMed] [Google Scholar]
- 7.Ryu E. J., Harding H. P., Angelastro J. M., Vitolo O. V., Ron D., Greene L. A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peel A. L., Bredesen D. E. Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice. Neurobiol. Dis. 2003;14:52–62. doi: 10.1016/s0969-9961(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 9.Petrov T., Rafols J. A., Alousi S. S., Kupsky W. J., Johnson R., Shah J., Shah A., Watson C. Cellular compartmentalization of phosphorylated eIF2α and neuronal NOS in human temporal lobe epilepsy with hippocampal sclerosis. J. Neurol. Sci. 2003;209:31–39. doi: 10.1016/s0022-510x(02)00461-6. [DOI] [PubMed] [Google Scholar]
- 10.Abbott C. M., Proud C. G. Translation factors: in sickness and in health. Trends Biochem. Sci. 2004;29:25–31. doi: 10.1016/j.tibs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Carnevalli L. S., Pereira C. M., Longo B. M., Jaqueta C. B., Avedissian M., Mello L. E., Castilho B. A. Phosphorylation of translation initiation factor eIF2α in the brain during pilocarpine-induced status epilepticus in mice. Neurosci. Lett. 2004;357:191–194. doi: 10.1016/j.neulet.2003.12.093. [DOI] [PubMed] [Google Scholar]
- 12.Peel A. L. PKR activation in neurodegenerative disease. J. Neuropathol. Exp. Neurol. 2004;63:97–105. doi: 10.1093/jnen/63.2.97. [DOI] [PubMed] [Google Scholar]
- 13.Hinnebusch A. G. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N., Hershey J. W. B., Mathews M. B., editors. Translational Control of Gene Expression. Plainview: Cold Spring Harbor Laboratory Press; 2000. pp. 185–243. [Google Scholar]
- 14.Vattem K. M., Wek R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu P. D., Harding H. P., Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 17.Dever T. E. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 18.Deng J., Harding H. P., Raught B., Gingras A. C., Berlanga J. J., Scheuner D., Kaufman R. J., Ron D., Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr. Biol. 2002;12:1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H. Y., Wek R. C. Phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J. Biol. Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman R. J. Double-stranded RNA-activated protein kinase PKR. In: Sonenberg N., Hershey J. W. B., Mathews M. B., editors. Translational Control of Gene Expression. Plainview: Cold Spring Harbor Laboratory Press; 2000. pp. 503–527. [Google Scholar]
- 21.Chen J. J. Heme-regulated eIF2α kinase. In: Sonenberg N., Hershey J. W. B., Mathews M. B., editors. Translational Control of Gene Expression. Plainview: Cold Spring Harbor Laboratory Press; 2000. pp. 529–546. [Google Scholar]
- 22.Lu L., Han A. P., Chen J. J. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey M., Cao C., Dar A. C., Tamura T., Ozato K., Sicheri F., Dever T. E. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Dar A. C., Dever T. E., Sicheri F. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H. Y., Wek S. A., McGrath B. C., Lu D., Hai T., Harding H. P., Wang X., Ron D., Cavener D. R., Wek R. C. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H. Y., Wek S. A., McGrath B. C., Scheuner D., Kaufman R. J., Cavener D. R., Wek R. C. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell. Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan K., Narasimhan J., Wek R. C. Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics. 2004;168:1867–1875. doi: 10.1534/genetics.104.031443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McInerney G. M., Kedersha N. L., Kaufman R. J., Anderson P., Liljestrom P. Importance of eIF2α phosphorylation and stress granule assembly in Alphavirus translation regulation. Mol. Biol. Cell. 2005;16:3753–3763. doi: 10.1091/mbc.E05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltzis D., Qu L. K., Papadopoulou S., Blais J. D., Bell J. C., Sonenberg N., Koromilas A. E. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 2004;78:12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalheiro E. A., Santos N. F., Priel M. R. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–1019. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin de la Vega C., Burda J., Nemethova M., Quevedo C., Alcazar A., Martin M. E., Danielisova V., Fando J. L., Salinas M. Possible mechanisms involved in the down-regulation of translation during transient global ischaemia in the rat brain. Biochem. J. 2001;357:819–826. doi: 10.1042/0264-6021:3570819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y. L., Reis L. F., Pavlovic J., Aguzzi A., Schafer R., Kumar A., Williams B. R., Aguet M., Weissmann C. Deficient signaling in mice devoid of doublestranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novoa I., Zeng H., Harding H. P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasterlain C. G. Effects of epileptic seizures on brain ribosomes: mechanism and relationship to cerebral energy metabolism. J. Neurochem. 1977;29:707–716. doi: 10.1111/j.1471-4159.1977.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 35.Kiessling M., Xie Y., Kleihues P. Regional impairment of protein synthesis in the rat brain during bicuculline-induced seizures. Brain Res. 1984;296:1–13. doi: 10.1016/0006-8993(84)90506-7. [DOI] [PubMed] [Google Scholar]
- 36.Dwyer B. E., Wasterlain C. G., Fujikawa D. G., Yamada L. Brain protein metabolism in epilepsy. Adv. Neurol. 1986;44:903–918. [PubMed] [Google Scholar]
- 37.Cigan A. M., Foiani M., Hannig E. M., Hinnebusch A. G. Complex formation by positive and negative translational regulators of GCN4. Mol. Cell. Biol. 1991;11:3217–3228. doi: 10.1128/mcb.11.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauwecker P. E. Genetic basis of kainate-induced excitotoxicity in mice: phenotypic modulation of seizure-induced cell death. Epilepsy Res. 2003;55:201–210. doi: 10.1016/s0920-1211(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G. M., II, Wenzel H. J., Robbins C. A., Sosunov A. A., Schwartzkroin P. A. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–561. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 40.Borges K., Gearing M., McDermott D. L., Smith A. B., Almonte A. G., Wainer B. H., Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp. Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman R. J. The double-stranded RNA-activated protein kinase PKR. In: Sonenberg N., Hershey J. W. B., Mathews M. B., editors. Translational Control of Gene Expression. Plainview: Cold Spring Harbor Laboratory Press; 2000. pp. 503–527. [Google Scholar]
- 42.Patel C. V., Handy I., Goldsmith T., Patel R. C. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 43.Bennett R. L., Blalock W. L., May W. S. Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J. Biol. Chem. 2004;279:42687–42693. doi: 10.1074/jbc.M403321200. [DOI] [PubMed] [Google Scholar]
- 44.Siesjö B. K., Wielock T. Epileptic brain damage: pathophysiology and neurochemical pathology. In: Delgado-Escueta A. V., Ward A. A. Jr, editors. Advances in Neurology. New York: Raven Press; 1986. pp. 813–847. [PubMed] [Google Scholar]
- 45.Beer J., Mielke K., Zipp M., Zimmermann M., Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- 46.Mielke K., Brecht S., Dorst A., Herdegen T. Activity and expression of JNK1, p38 and ERK kinases, c-Jun N-terminal phosphorylation, and c-jun promoter binding in the adult rat brain following kainate-induced seizures. Neuroscience. 1999;91:471–483. doi: 10.1016/s0306-4522(98)00667-8. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez J., Yaman I., Merrick W. C., Koromilas A., Wek R. C., Sood R., Hensold J., Hatzoglou M. Regulation of internal ribosome entry site-mediated translation by eukaryotic initiation factor-2α phosphorylation and translation of a small upstream open reading frame. J. Biol. Chem. 2002;277:2050–2058. doi: 10.1074/jbc.M109199200. [DOI] [PubMed] [Google Scholar]
- 48.Sehgal A., Briggs J., Rinehart-Kim J., Basso J., Bos T. J. The chicken c-Jun 5′ untranslated region directs translation by internal initiation. Oncogene. 2000;19:2836–2845. doi: 10.1038/sj.onc.1203601. [DOI] [PubMed] [Google Scholar]