Abstract
Protoporphyrin (IX) ferrochelatase catalyses the insertion of ferrous iron into protoporphyrin IX to form haem. These ferrochelatases exist as monomers and dimers, both with and without [2Fe-2S] clusters. The motifs for [2Fe-2S] cluster co-ordination are varied, but in all cases previously reported, three of the four cysteine ligands are present in the 30 C-terminal residues and the fourth ligand is internal. In the present study, we demonstrate that a group of micro-organisms exist which possess protoporphyrin (IX) ferrochelatases containing [2Fe-2S] clusters that are co-ordinated by a group of four cysteine residues contained in an internal amino acid segment of approx. 20 residues in length. This suggests that these ferrochelatases have evolved along a different lineage than other bacterial protoporphyrin (IX) ferrochelatases. For example, Myxococcus xanthus protoporphyrin (IX) ferrochelatase ligates a [2Fe-2S] cluster via cysteine residues present in an internal segment. Site-directed mutagenesis of this ferrochelatase demonstrates that changing one cysteine ligand into serine results in loss of the cluster, but unlike eukaryotic protoporphyrin (IX) ferrochelatases, this enzyme retains its activity. These data support a role for the [2Fe-2S] cluster in iron affinity, and strongly suggest convergent evolution of this feature in prokaryotes.
Keywords: cluster, ferrochelatase, haem, iron–sulphur, mutagenesis, protoporphyrin IX
INTRODUCTION
Protoporphyrin (IX) ferrochelatase (protohaem ferro-lyase, EC 4.99.1.1) catalyses the insertion of a ferrous ion into the tetrapyrrole-derived ring of protoporphyrin IX, to yield protohaem and two protons [1,2]. A comparison of all currently known protoporphyrin (IX) ferrochelatases has revealed the presence of three distinct regions [2]. The first region (I) is present in all eukaryotes and has been identified as an N-terminal organelle targeting motif that is proteolytically cleaved following delivery to the organelle [3,4]. The second, (II), represents the core 330 amino acid residues and is ubiquitous amongst protoporphyrin (IX) ferrochelatases. The third region, (III), is a 30- to 50-amino acid C-terminal sequence that is found mainly in eukaryotic protoporphyrin (IX) ferrochelatases. Region III in animal protoporphyrin (IX) ferrochelatases is involved in dimerization of the enzyme [5], and contains three of the four cysteine ligands to an [2Fe-2S] cluster with the fourth being found in region II [5–8]. Region III of plant protoporphyrin (IX) ferrochelatase is approx. 50 residues and its role is currently unknown [2]. These ferrochelatases do not possess cysteine residues in region III and do not contain [2Fe-2S] clusters. Protoporphyrin (IX) ferrochelatase from the yeast, Saccharomyces cerevisiae, possesses a region III which lacks cysteine residues and does not contain an [2Fe-2S] cluster, whereas the Schizosaccharomyces pombe protoporphyrin (IX) ferrochelatase does possess the necessary cysteine residues and an [2Fe-2S] cluster [9].
The 2.0 Å (1 Å=0.1 nm) crystal structure of human protoporphyrin (IX) ferrochelatase has been solved using anomalous scattering signals generated from bound iron [5]. The asymmetric unit was found to contain a homodimer, with each monomer containing one [2Fe-2S] cluster. Each monomer contains two hydrophobic protrusions which flank the active site cleft and are proposed to be associated with the matrix side of the inner mitochondrial membrane. By contrast, the 1.9 Å crystal structure of Bacillus subtilis protoporphyrin (IX) ferrochelatase revealed a soluble monomeric enzyme that lacks a [2Fe-2S] cluster [10]. More recently a small number of bacteria were identified that possess a C-terminal extension similar to that which is found in eukaryotic protoporphyrin (IX) ferrochelatases, and these also contain a [2Fe-2S] cluster [2,11]. In the present study, we report that yet another set of bacterial protoporphyrin (IX) ferrochelatases exist that possess a [2Fe-2S] cluster, but do not contain a C-terminal extension.
In the present study, we report steady-state kinetic characterization of this group of microbial protoporphyrin (IX) ferrochelatases. Genomic analysis demonstrates that a subset of these enzymes exists that co-ordinate [2Fe-2S] clusters, and that two distinct co-ordination motifs have evolved. Potential roles for the [2Fe-2S] cluster are presented and the evolutionary significance of these observations is discussed.
EXPERIMENTAL
Cloning and mutagenesis
Mycobacterium tuberculosis and Caulobacter crescentus protoporphyrin (IX) ferrochelatases were cloned and mutated as described previously [11]. Genomic DNA for Pseudomonas aeruginosa (a gift from Dr Wendy Dustman, Department of Microbiology, University of Georgia, Athens, GA, U.S.A.), Porphyromonas gingivalis (a gift from Dr James Travis, Department of Biochemistry and Molecular Biology, University of Georgia, Athens, GA, U.S.A.), and Azotobacter vinelandii (a gift from Dr William Lanzilotta, Department of Biochemistry and Molecular Biology, University of Georgia, Athens, GA, U.S.A.) was prepared [12]. Genomic DNA from Myxococcus xanthus was obtained from Dr Larry Shimkets (Department of Microbiology, University of Georgia, Athens, GA, U.S.A.). The genomic DNA of Bdellovibrio bacteriovorus was obtained from Dr John Iandolo (Department of Microbiology and Immunology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, U.S.A.). Pseudomonas putida genomic DNA was purchased from American Type Culture Collection (A.T.C.C., Manassas, VA, U.S.A.). The cDNAs for M. xanthus, A. vinelandii, P. putida, P. aeruginosa, P. gingivalis, and Bd. bacteriovorus protoporphyrin (IX) ferrochelatases were amplified via PCR from genomic DNA using primers to introduce restriction sites for cloning into the expression vector pTrcHisA (Invitrogen), which contains an N-terminal His6-tag for ease of purification of the recombinant protein. Mutagenesis was performed using a QuikChange® kit (Stratagene), to create each of the M. xanthus (C213S, C219S, C220S, C230S and Δ209–230) and P. putida (D207C, K202T/D207C, K202T/ΔV204/D207C and Δ202–225) mutants. The Escherichia coli hemH deletion mutant [13] was rendered chemically competent and transformed using each of the expression plasmids.
Protein purification
Proteins were purified as described previously [11]. Yields of M. xanthus and P. putida protoporphyrin (IX) ferrochelatases were increased by including 1% Triton X-100 in the solubilization buffer. Protein concentration was determined using calculated extinction coefficients based on the amino acid sequence. These values were 48.8, 54.9, 53.5, 43.7, 43.0 mM−1·cm−1 for M. xanthus, P. putida, C. crescentus, M. tuberculosis and Bd. bacteriovorus respectively.
Spectroscopy
UV absorption spectra were measured with a Cary 50 spectrophotometer (Varian). Spectra were recorded in ferrochelatase solubilization buffer [50 mM Tris/Mops (pH 8.0), 100 mM KCl and 1.0% (w/v) sodium cholate].
Substrate preparation
Mesoporphyrin IX (Porphyrin Products, Logan, UT, U.S.A.) was solubilized with a few drops of 30% NH4OH and then diluted with a 2% (v/v) solution of Triton X-100 immediately before use.
Ferrochelatase assays
Iron chelation was measured using a continuous assay, by monitoring the depletion of mesoporphyrin IX absorbance at 496 nm using an extinction coefficient of 7.5 mM−1·cm−1 [14]. This was performed using a Cary 50 spectrophotometer at 25 °C. The reactions were started by the addition of enzyme, which was pre-incubated at 25 °C, and the depletion of mesoporphyrin IX was monitored for 3 min. Where substrate inhibition was not present, fixed concentrations of 17 μM mesoporphyrin IX and 50 μM Fe2+ were used when the other substrate was being varied. Where inhibition was observed at high concentrations of Fe2+, the ferrous iron concentration was fixed at 25 μM when mesoporphyrin IX was varied. The concentration of iron was ranged from 5–50 μM, and mesoporphyrin IX was ranged from 5–30 μM. All assays contained 0.5 μM protein, 50 mM Tris/MOPS (pH 8.0), 1% (v/v) Tween-20 and 5 mM 2-mercaptoethanol. The steady-state rates were estimated using linear regression of the time-course at the start of the reaction. V versus [S] curves were fitted to the Michaelis–Menten equation using non-linear regression (Sigmaplot 8).
RESULTS
As part of an effort to elucidate the function of the [2Fe-2S] cluster in protoporphyrin (IX) ferrochelatases, our laboratory performs sequence analysis of genomes as they become available in order to identify potential haem-synthetic enzymes. A TIGR (The Institute for Genome Research) BLAST screen using the genome of M. xanthus yielded a sequence in contig 581 which had reasonable similarity to known protoporphyrin (IX) ferrochelatase sequences. Pile-up analysis of this putative ferrochelatase sequence with several cluster containing protoporphyrin (IX) ferrochelatases, both eukaryotic and bacterial, revealed that the M. xanthus gene did not contain the required C-terminal extension found in protoporphyrin (IX) ferrochelatases that possess a [2Fe-2S] cluster. However, M. xanthus protoporphyrin (IX) ferrochelatase does possess a 22 amino acid insertion which contains four cysteine residues (Figure 1).
Figure 1. Multiple sequence alignment of protoporphyrin (IX) ferrochelatases.
The black shading indicates a 90% sequence identity and the grey shading indicates where 90% of the residues are functionally similar. The solid boxes highlight potential conserved cysteine residues in the subset of ferrochelatases containing the ‘cysteine-rich insertion’. *, the cysteine ligands to the iron-sulphur cluster in human ferrochelatase. The broken-line boxes indicate the primary sequence of human ferrochelatase that is shaded in blue in Figure 5.
A BLAST search using the M. xanthus sequence yielded additional microbial protoporphyrin (IX) ferrochelatases with the insertion. Among these were Bdellovibrio bacteriovorus, Pseudomonas syringae, Pseudomonas putida, Azotobacter vinelandii, Pseudomonas aeruginosa and Porphyrymonas gingivalis. Interestingly, three of these enzymes (from A. vinelandii, Bdellovibrio bacteriovorus and P. syringae) contained four cysteine residues in the insertion, whereas the other three contained one to three of the four cysteine residues (Figure 1).
Mutagenesis
Recombinant protoporphyrin (IX) ferrochelatase from M. xanthus, P. putida and P. aeruginosa was expressed in E. coli and purified. UV spectra for these enzymes showed the presence of a [2Fe-2S] cluster in the M. xanthus enzyme but not in the P. putida or P. aeruginosa enzymes. The enzyme from P. gingivalis was expressed but precipitated immediately after purification, making it unsuitable for further analysis. The A. vinelandii enzyme was not expressed by this system.
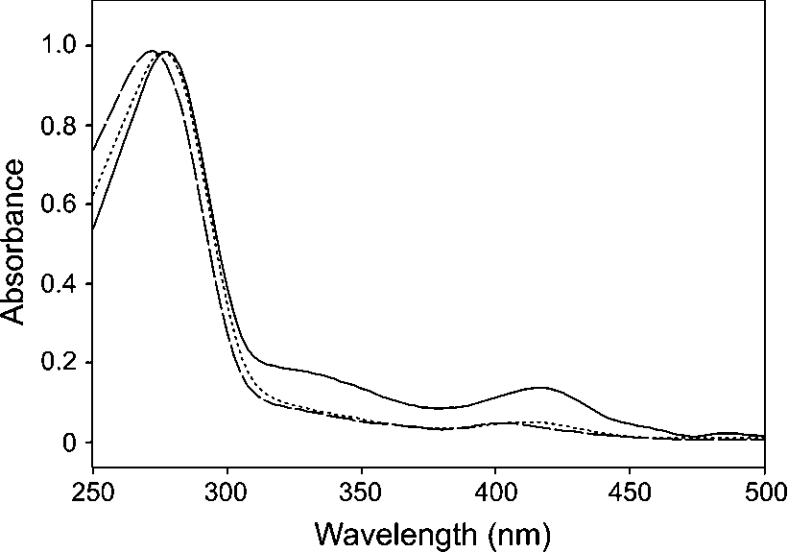
To confirm that the four cysteine residues postulated as ligands to the [2Fe-2S] cluster are involved in cluster assembly, site-directed mutagenesis of the M. xanthus enzyme was performed to convert each individual cysteine residue (C213, C219, C220 and C230) into a serine residue. The absorption band at 330 nm and absorption peaks at approx. 415 and 550 nm that are characteristic of the [2Fe-2S] centre [8,15], are absent from the C213S, C219S (Figure 2, panel A), C220S and C230S spectra, indicating the loss of the cluster. Despite the absence of the cluster, each of these mutants was able to support the growth of the E. coli ΔhemH strain [a strain lacking the E. coli protoporphyrin (IX) ferrochelatase enzyme]. Additional site-directed mutagenesis of the M. xanthus enzyme was performed to remove the cluster-ligating insertion. The resulting mutant, Δ209–230, retains enough activity to complement E. coli ΔhemH, but was unstable after purification, preventing any biochemical analysis. The C219S mutant was selected for kinetic analysis, as described below.
Figure 2. UV absorption spectra of protoporphyrin (IX) ferrochelatases.
Absorption spectra of wild-type (solid line) and C219S mutant (dashed line) ferrochelatases from M. xanthus, and C341S mutant (dotted line) ferrochelatase from C. crescentus. The peak heights have been normalized for clarity.
The P. putida enzyme, which lacks a [2Fe-2S] cluster, contains three of the four cluster-ligating cysteine residues. In an attempt to create a [2Fe-2S] cluster in this enzyme, the amino acid in the position of the fourth cysteine ligand, which in P. putida is an aspartic acid, was mutated to cysteine. This recombinant enzyme (D207C) did not form a [2Fe-2S] cluster. To further mimic the sequence of the internal cluster-containing enzymes of M. xanthus and A. vinelandii, Lys202 of the P. putida enzyme was mutated into a threonine residue in the previously mutated D207C enzyme. This enzyme (K202T/D207C) also did not form a [2Fe-2S] cluster. A third mutation, deletion of the valine residue at site 204 (ΔV204/K202T/D207C) to mimic the spacing of the M. xanthus enzyme, as well as the conserved residues at Lys202 and Asp207, also did not assemble a cluster, suggesting that additional, currently unidentified residues spatially close to the cluster are essential for assembly or stability. Deletion of the P. putida insertion resulted in an enzyme without the ability to rescue the growth of the hemH mutant of E. coli. This enzyme was not amenable to purification.
Protoporphyrin (IX) ferrochelatase from C. crescentus, which has previously been described [11], does not contain the cysteine-rich insert, but possesses a cysteine-rich C-terminal extension similar to that of the human enzyme (Figure 1). Attempts to produce a catalytically active C. crescentus enzyme that lacks the [2Fe-2S] cluster were made by mutating putative cluster-ligands into serine residues (C328S, C332S, C339S and C341S) [11]. In the current study the C341S enzyme was purified and subjected to kinetic characterization, as described below, to determine if the loss of the cluster elicited different effects compared with a protoporphyrin (IX) ferrochelatase that contains the cysteine-rich insert (M. xanthus C219S). The data shown in Figure 2 demonstrates that the C341S mutation results in the loss of the [2Fe-2S] cluster. The absorption spectrum of the C. crescentus wild-type enzyme resembles the M. xanthus wild-type spectrum shown in Figure 2, so was omitted for clarity.
Kinetic analysis
Wild-type protoporphyrin (IX) ferrochelatases from M. xanthus, Bd. bacteriovorus, P. putida, M. tuberculosis and C. crescentus were purified and subjected to preliminary kinetic analysis, along with two cluster-knockout mutant protoporphyrin (IX) ferrochelatases: M. xanthus C219S and C. crescentus C341S. Table 1 shows the kinetic parameters obtained when all of these purified enzymes were subjected to kinetic analysis. The data in Figure 3 shows the V versus [Fe2+] curves for M. xanthus wild-type and C219S mutant enzymes. Both are fitted to single rectangular hyperbolae, although the data points above 25 μM Fe2+ for the mutant enzyme were omitted from the fitting. This M. xanthus C219S mutant enzyme is subject to iron substrate inhibition, and exhibits a larger Km value for Fe2+ than the wild-type. Figure 4 shows the V versus [Fe2+] curves for C. crescentus wild-type and C341S mutant enzymes. Both data-sets are fitted to single rectangular hyperbolae. The mutant enzyme appears to possess a lower Km value for Fe2+ than that of the wild-type.
Table 1. Kinetic parameters for various protoporphyrin (IX) ferrochelatases.
| Enzyme | Insertion | Cluster | kcat (min−1) | Kmmeso (μM) | KmFe (μM) |
|---|---|---|---|---|---|
| M. xanthus wild-type | Yes | Yes | 4.9±0.3 | 9.6±2.5 | 6.5±1.7 |
| M. xanthus C219S | Yes | No | 2.0±0.4 | 12.7±2.6 | 13.9±3.0 |
| Bd. Bacteriovorus wild-type | Yes | Yes | 4.4±0.3 | 8.0±2.1 | 28.0±2.9 |
| P. putida wild-type | Yes | No | 11.7±1.4 | 8.9±2.6 | 20.0±3.9 |
| C. crescentus wild-type | No | Yes | 16.0±1.2 | 8.7±2.3 | 11.0±2.3 |
| C. crescentus C341S | No | No | 3.8±0.1 | 4.7±1.0 | 2.8±0.5 |
Figure 3. V versus [Fe2+] curves for M. xanthus wild-type (■) and C219S mutant (●) protoporphyrin (IX) ferrochelatases.
Both datasets were fitted to single rectangular hyperbolae, but only the datapoints below 25 μM Fe2+ were used for the regression analysis of the C219S mutant data. The kcat and KappFe values for the wild-type enzyme were 4.9±0.3 min−1 and 6.5±1.7 μM respectively. The kcat and KappFe values for the C219S mutant enzyme were 2.0±0.4 min−1 and 13.9±3.0 μM respectively.
Figure 4. V versus [Fe2+] curves for C. crescentus wild-type (■) and C341S mutant (●) protoporphyrin (IX) ferrochelatases.
Both datasets were fitted to single rectangular hyperbolae. The kcat and KappFe values for the wild-type enzyme were 16.0±1.2 min−1 and 11.0±2.3 μM respectively. The kcat and KappFe values for the C431S mutant enzyme were 3.8±0.1 and 2.8±0.5 μM respectively.
DISCUSSION
Above is reported the discovery of a new class of bacterial protoporphyrin (IX) ferrochelatases possessing internal insertions of approx. 20 amino acids, some of which contain a sufficient number of cysteine residues to ligate a [2Fe-2S] cluster. Among the enzymes containing the insertion, there are only six cysteine residues that are relatively conserved: the four within the insertion (in the enzymes which possess the four) and two additional cysteine residues at positions equivalent to human Pro334 and His341, found in all of the insertion-containing enzymes. Since Pro334 and His341 residues are located in the active site spatially distant from any other cysteine residues, the cysteine residues occupying the analogous position in these bacterial ferrochelatases were not considered to be likely [2Fe-2S] cluster ligands. Site-directed mutagenesis of the four cysteine residues in the insertion in M. xanthus protoporphyrin (IX) ferrochelatase confirmed that the residues in the insertion were in fact responsible for co-ordination of the cluster.
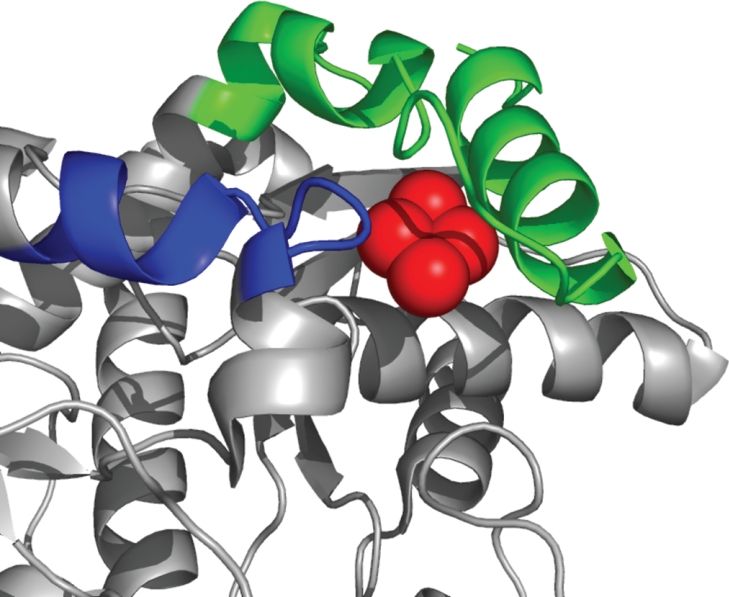
Until the previous finding of [2Fe-2S] clusters in a limited number of bacterial protoporphyrin (IX) ferrochelatases [5], it was believed that this feature was restricted to higher animal protoporphyrin (IX) ferrochelatases. Although differences exist in the spacing between the three terminal cysteine residues, the general motif for cluster ligation found previously has been via four cysteines with one cysteine located near the middle of the primary sequence and the other three located within an approx. 30 residue C-terminal extension. By analogy with the human tertiary structure, it would be expected that this newly identified cluster resides in the same spatial location as does the [2Fe-2S] cluster in human protoporphyrin (IX) ferrochelatase (Figure 5). Although final determination of the exact orientation will require crystallographic data, it is highly unlikely that the spatial position will vary significantly, given the clear conservation of structure found between human [5], yeast [15a], and bacterial [10] protoporphyrin (IX) ferrochelatases reported to date.
Figure 5. Ribbon diagram of the human protoporphyrin (IX) ferrochelatase structure showing the spatial orientation of the [2Fe-2S] cluster and the cysteine residues co-ordinating it.
The blue shading indicates the region of the human enzyme that corresponds to the cysteine rich insertion in some bacterial protoporphyrin (IX) ferrochelatases (broken-line box in Figure 1). The C-terminal extension found in human, but not these bacterial enzymes, is shown in green. The iron-sulphur cluster is shown in red.
The kinetic data obtained were for one bacterial C-terminal cluster-ligated protoporphyrin (IX) ferrochelatase: C. crescentus, and two internal insertion cluster-containing protoporphyrin (IX) ferrochelatases: Bd. bacteriovorus and M. xanthus. In addition, cluster-lacking C→S mutants for C. crescentus and M. xanthus were examined along with the P. putida wild-type enzyme, which has an internal insert, but lacks a [2Fe-2S] cluster. Although it was possible to produce and characterize stable mutant enzymes that lacked the normal [2Fe-2S] cluster (e.g. M. xanthus C219S and C. crescentus C341S), it has not yet proven possible to convert a non-cluster containing enzyme into a cluster-containing one. Attempts to convert the P. putida enzyme by introducing a fourth cysteine residue and changing the surrounding residues and spacing to resemble cluster-containing protoporphyrin (IX) ferrochelatases were unsuccessful. Interestingly, complete removal of the 22 amino acid residue insertion, which converts the protein into a more typical bacterial protoporphyrin (IX) ferrochelatase primary sequence, results in the production of an unstable protein.
Although it might be assumed that the variations in spacing of the terminal three cysteine residues found in previously reported bacterial protoporphyrin (IX) ferrochelatases arose through evolution by random mutagenesis of an ancestral cluster-containing bacterial ferrochelatase, the origin of the currently described clusters that are ligated by four cysteines in an internal insertion may best be attributed to convergent evolution. This fact makes clear the importance of the [2Fe-2S] cluster to the protoporphyrin (IX) ferrochelatases which possess it and argues that it is not just a randomly incorporated feature, but serves a significant in vivo function.
The kinetic data presented in Table 1 point to a potential difference in function between these two subsets of microbial ferrochelatases. The M. xanthus C219S mutant enzyme, which lacks the cluster, exhibits a larger apparent Km value for iron than that of the wild-type enzyme, as well as being subject to inhibition by ferrous ion concentrations greater than 25 μM. This suggests that the presence of the [2Fe-2S] cluster enhances iron binding at lower Fe2+ concentrations, and assists the productive binding at higher concentrations. Kinetic measurements were performed on two additional insert-containing protoporphyrin (IX) ferrochelatases from Bd. bacteriovorus and P. putida. The [2Fe-2S] cluster-containing Bd. bacteriovorus enzyme possesses a similar kcat value to that of M. xanthus protoporphyrin (IX) ferrochelatase, although the KmFe value is 4-fold larger than that of the M. xanthus enzyme. The cluster-less P. putida enzyme has a much larger kcat value than the other members of this family possessing the [2Fe-2S] cluster. However, this enzyme only possesses three cysteine residues in the insert, and therefore does not co-ordinate a [2Fe-2S] cluster. The KmFe of the P. putida enzyme is far higher than that of the M. xanthus wild-type enzyme. This is consistent with the theory that the [2Fe-2S] cluster enhances iron binding in this subset of protoporphyrin (IX) ferrochelatases. By contrast, the C. crescentus C341S mutant enzyme has a lower apparent Km value for iron. This suggests that this cluster (not bound by an internal four cysteine insertion) may serve to down-regulate the incorporation of Fe2+ into protoporphyrin IX. Furthermore, the C. crescentus wild-type enzyme has a much larger kcat value than protoporphyrin (IX) ferrochelatases containing the cysteine-rich insert, and removal of the cluster causes a 4-fold reduction in the kcat value. However, removal of the cluster from the M. xanthus enzyme causes no significant decrease in the catalytic rate. Steady-state kinetic analysis was performed on the M. tuberculosis enzyme to support the data from the C. crescentus protoporphyrin (IX) ferrochelatase. However, this enzyme exhibits a much lower kcat value compared with the wild-type C. crescentus enzyme, as was previously described [11]. There are no data to explain this unusually low enzymatic activity.
Iron–sulphur clusters have been identified in other branches of tetrapyrrole metabolism. A 2Fe-2S centre has recently been shown to be present in sirohydrochlorin ferrochelatase from Arabidopsis thaliana (At-SirB) [16], whereas some cobaltochelatases appear to harbour [4Fe-4S] centres [17]. The At-SirB sirohydrochlorin ferrochelatase was shown to contain a [2Fe-2S], which is proposed to be co-ordinated by three C-terminal cysteine residues and a conserved central cysteine residue, reminiscent of the mammalian protoporphyrin ferrochelatases. However, this ferrochelatase is very unstable and is degraded rapidly in an aerobic environment, unlike human protoporphyrin ferrochelatase, which gradually disappears over a period of 24 h [8]. The cobaltochelatase, CbiX, is required for vitamin B12 biosynthesis, and possesses a C-terminal MXCXXC motif, which provides two of the four cysteine ligands to the [4Fe-4S] cluster [17]. Disruption of the Fe-S centre via mutagenesis of the cysteine ligands did not result in a reduction in catalysis. This observation is reminiscent of the results discussed in the present study, and could reflect another non-catalytic role for iron-sulphur clusters in ferrochelatases. Since the chelation rate was independent of the redox state of the cluster, the authors suggest that the redox centre does not act as a sensor, but may be present to interact with another protein, possibly involved in the delivery of cobalt to the active site. This is an interesting possibility, and is significant to the data in the present study, especially as loss of the cluster can have a significant effect on the Km values for iron.
Since the clusters of the enzymes examined in the present study are not reduced by dithionite, it seems unlikely that the [2Fe-2S] cluster acts as a redox switch to modulate iron chelatation. Furthermore, these clusters, unlike the eukaryotic protoporphyrin (IX) ferrochelatase [2Fe-2S] clusters, are very stable and are not destroyed by NO (T. A. Dailey, unpublished work). At present, there are no comprehensive studies published on the effect of cluster redox state on eukaryotic protoporphyrin (IX) ferrochelatase activity. However, given the differences in the physical properties of [2Fe-2S] clusters, it would not be unexpected if during evolution, a different role for the cluster in higher eukaryotes came into being. Indeed, the sensitivity of the animal cluster to NO results in diminished in vivo activity [18] and the lack of enzyme activity in the absence of the cluster means that cellular iron stores may influence the terminal step in haem biosynthesis [15]. Neither of these possibilities seem to be valid for bacterial systems. Nonetheless, it is clear that the presence of the [2Fe-2S] cluster in some bacterial protoporphyrin (IX) ferrochelatases creates ways in which cellular iron metabolism and/or other cellular/environmental factors can have an impact on haem synthesis. It is apparent that regulation of microbial iron and haem metabolism is complex and may be interrelated [20] and that the presence of the [2Fe-2S] cluster in some microbial protoporphyrin (IX) ferrochelatases may represent another piece of this puzzle of evolution.
Acknowledgments
This work was funded by the National Institutes of Health (grant DK32303 to H. A. D.).
References
- 1.Dailey H. A. Ferrochelatase. In: Hausinger R. P., Einchorn G. L., Marzilli L. G., editors. Mechanisms of Metallocenter Assembly. New York, U.S.A.: VCH Inc.; 1996. pp. 77–98. [Google Scholar]
- 2.Dailey H. A., Dailey T. A., Wu C. K., Medlock A. E., Wang K. F., Rose J. P., Wang B. C. Ferrochelatase at the millennium: structures, mechanisms and 2Fe-2S clusters. Cell. Mol. Life Sci. 2000;57:1909–1926. doi: 10.1007/PL00000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karr S. R., Dailey H. A. The synthesis of murine ferrochelatase in vitro and in vivo. Biochem. J. 1988;254:799–803. doi: 10.1042/bj2540799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad A. R. K., Dailey H. A. Effect of cellular location on the function of ferrochelatase. J. Biol. Chem. 1995;270:18198–18200. doi: 10.1074/jbc.270.31.18198. [DOI] [PubMed] [Google Scholar]
- 5.Wu C. K., Dailey H. A., Rose J. P., Burden A., Sellers V. M., Wang B. C. The 2.0 Å structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 2001;8:156–160. doi: 10.1038/84152. [DOI] [PubMed] [Google Scholar]
- 6.Crouse B. R., Sellers V. M., Finnegan M. G., Dailey H. A., Johnson M. K. Site-directed mutagenesis and spectroscopic characterization of human ferrochelatase: identification of residues coordinating the 2Fe-2S cluster. Biochemistry. 1996;35:16222–16229. doi: 10.1021/bi9620114. [DOI] [PubMed] [Google Scholar]
- 7.Sellers V. M., Wang K. F., Johnson M. K., Dailey H. A. Evidence that the fourth ligand to the 2Fe-2S cluster in animal ferrochelatase is a cysteine – characterization of the enzyme from Drosophila melanogaster. J. Biol. Chem. 1998;273:22311–22316. doi: 10.1074/jbc.273.35.22311. [DOI] [PubMed] [Google Scholar]
- 8.Dailey H. A., Finnegan M. G., Johnson M. K. Human ferrochelatase is an iron-sulfur protein. Biochemistry. 1994;33:403–407. doi: 10.1021/bi00168a003. [DOI] [PubMed] [Google Scholar]
- 9.Medlock A. E., Dailey H. A. Examination of the activity of carboxyl-terminal chimeric constructs of human and yeast ferrochelatases. Biochemistry. 2000;39:7461–7467. doi: 10.1021/bi000134p. [DOI] [PubMed] [Google Scholar]
- 10.Al Karadaghi S., Hansson M., Nikonov S., Jonsson B., Hederstedt L. Crystal structure of ferrochelatase: the terminal enzyme in heme biosynthesis. Structure. 1997;5:1501–1510. doi: 10.1016/s0969-2126(97)00299-2. [DOI] [PubMed] [Google Scholar]
- 11.Dailey T. A., Dailey H. A. Identification of 2Fe-2S clusters in microbial ferrochelatases. J. Bacteriol. 2002;184:2460–2464. doi: 10.1128/JB.184.9.2460-2464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson K. Preparation of Genomic DNA from Bacteria. In: Ausubel F. A., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K., editors. Current Protocols in Molecular Biology. New York, U.S.A.: Greene Publishing and Wiley Interscience; 1994. pp. 241–245. [Google Scholar]
- 13.Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia-coli K12. J. Mol. Biol. 1991;219:393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- 14.Falk J. E. Amsterdam, Netherlands: Elsevier; 1964. Porphyrins and metalloporphyrins: their general, physical and coordination chemistry, and laboratory methods. [Google Scholar]
- 15.Sellers V. M., Johnson M. K., Dailey H. A. Function of the 2Fe-2S cluster in mammalian ferrochelatase: A possible role as a nitric oxide sensor. Biochemistry. 1996;35:2699–2704. doi: 10.1021/bi952631p. [DOI] [PubMed] [Google Scholar]
- 15a.Karlberg T., Lecerof D., Gora M., Silvegren G., Labbe-Bois R., Hansson M., Al-Karadaghi S. Metal binding to Saccharomyces cerevisiae ferrochelatase. Biochemistry. 2002;41:13499–13506. doi: 10.1021/bi0260785. [DOI] [PubMed] [Google Scholar]
- 16.Raux-Deery E., Leech H. K., Nakrieko K. A., McLean K. J., Munro A. W., Heathcote P., Rigby S. E. J., Smith A. G., Warren M. J. Identification and characterization of the terminal enzyme of siroheme biosynthesis from Arabidopsis thaliana – a plastidlocated sirohydrochlorin ferrochelatase containing a 2Fe-2S center. J. Biol. Chem. 2005;280:4713–4721. doi: 10.1074/jbc.M411360200. [DOI] [PubMed] [Google Scholar]
- 17.Leech H. K., Raux E., McLean K. J., Munro A. W., Robinson N. J., Borrelly G. P. M., Malten M., Jahn D., Rigby S. E. J., Heathcote P., Warren M. J. Characterization of the cobaltochelatase CbiX(L) – evidence for a 4Fe-4S center housed within an MXCXXC motif. J. Biol. Chem. 2003;278:41900–41907. doi: 10.1074/jbc.M306112200. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y. M., Bergonia H. A., Muller C., Pitt B. R., Watkins W. D., Lancaster J. R. Loss and degradation of enzyme-bound heme induced by cellular nitric-oxide synthesis. J. Biol. Chem. 1995;270:5710–5713. doi: 10.1074/jbc.270.11.5710. [DOI] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20.Qi Z. H., O'Brian M. R. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Mol. Cell. 2002;9:155–162. doi: 10.1016/s1097-2765(01)00431-2. [DOI] [PubMed] [Google Scholar]