Abstract
The MAPs (microtubule-associated proteins) MAP1B and tau are well known for binding to microtubules and stabilizing these structures. An additional role for MAPs has emerged recently where they appear to participate in the regulation of transport of cargos on the microtubules found in axons. In this role, tau has been associated with the regulation of anterograde axonal transport. We now report that MAP1B is associated with the regulation of retrograde axonal transport of mitochondria. This finding potentially provides precise control of axonal transport by MAPs at several levels: controlling the anterograde or retrograde direction of transport depending on the type of MAP involved, controlling the speed of transport and controlling the stability of the microtubule tracks upon which transport occurs.
Keywords: axonal transport, bidirectional movement, microtubule, microtubule-associated protein 1B (MAP1B), mitochondrion, tau
Abbreviations: MAP, microtubule-associated protein
INTRODUCTION
A neuron can be divided into three regions: the cell body (or soma), the axon and the dendrites. The morphological differentiation from a precursor cell to a neuron containing the three indicated regions is regulated by the cytoskeleton [1]. As a component of the cytoskeleton, the microtubules are necessary for the extension and maintenance of neuritic processes. Underlying these processes are the microtubules, which are dynamic structures that are stabilized by MAPs (microtubule-associated proteins) [2]. Importantly, different MAPs are present in axonal and somatodendritic microtubules. In developing neurons, MAP1B and tau are the main MAPs found in axons, whereas another MAP, MAP2, is found in dendrites [3].
MAP1B has been shown to be the first MAP which is expressed in developing neurons ‘in situ’ [4]. The expression of MAP1B is down-regulated during brain development until it essentially disappears from the axon when synaptic contacts are formed between neurons in the central nervous system [5–8]. Tau expression is more complex, where six protein isoforms can be grouped into two classes. One class contains three imperfect repeats composed of a highly conserved tubulin-binding motif followed by a less conserved amino acid sequence, whereas the other class contains an additional fourth repeat. The class with three tubulin-binding domain repeats is predominantly expressed in developing brain, whereas the class with four tubulin-binding domain repeats is generally expressed in adult brain [9–11].
Axonal microtubules serve as tracks for the bidirectional transport of various organelles, including mitochondria and synaptic vesicles between the cell body and the axonal tips. As tracks, microtubules are intrinsically polar structures with two differentiated ends (a plus end and a minus end), and all axonal microtubules have their plus ends pointing towards the axon terminus [12,13]. Two protein superfamilies, the kinesins and the dyneins [14], serve as molecular machines to drive the transport of these organelles along microtubule tracks. Most kinesins move towards the plus end of microtubules and participate in anterograde transport [14]. In contrast, dyneins move towards the opposite (minus) end and participate in retrograde transport [15].
In addition to stabilization of microtubules, it has been suggested that tau protein may also contribute to the regulation of anterograde axonal transport of mitochondria [16–18]. This suggestion opens the idea that MAP1B, as a MAP like tau, might also participate in the regulation of axonal transport of organelles. In the present paper, we now report on our MAP1B studies in axonal transport of mitochondria using cultured neurons from mice deficient in that protein. Our results indicate a novel role for MAP1B in the regulation of the retrograde axonal transport of mitochondria.
EXPERIMENTAL
Animals
Wild-type R1/NMR1 and C57BL/6 mice were used in the present study. The generation of the map1b mutants (R1/NMR1) by gene trapping has been reported previously [19]. The generation of the tau mutants (C57BL/6) have been described previously [20].
Western blotting
Protein mixtures were separated on a 10% (v/v) acrylamide gel to isolate tau and/or a 6% (v/v) acrylamide gel for MAP1B isolation.
The antibodies used were 125 (MAP1B-specific) (diluted 1:20; [21]), 7.51 (tau-specific) (diluted 1:100; a gift from Professor C. M. Wischik, Institute of Medical Sciences, University of Aberdeen, Aberdeen, Scotland, U.K.) and anti-tubulin (diluted 1:2000; Sigma).
Primary culture
Hippocampal neurons were cultured as described by Banker and Cowan [22]. Pregnant wild-type, tau−/− and MAP1B−/− mice were killed at gestational day 18, and the embryos were removed under sterile conditions.
Dissociated hippocampal neurons from the embryos were plated on glass coverslips coated with 1 mg/ml poly(L-lysine) and 20 μg/ml laminin. After incubation for 3 h in medium containing 10% (v/v) horse serum (Gibco), the cells were transferred to N2- and B27-supplemented medium (Gibco).
Immunofluorescence
Cells were fixed 24 h after being plated with 4% (w/v) para-formaldehyde and 4% sucrose for 20 min at 37 °C and the washed with PBS. The cells were then permeabilized with 0.2% Triton X-100 in PBS for 5 h at room temperature (approx. 20 °C) and were incubated with a solution containing 5% (w/v) BSA in PBS for 1 h at room temperature. The cells were incubated with the primary antibodies diluted in PBS and 1% BSA for 1 h at room temperature, before the coverslips were washed three times with PBS. The cells were then exposed to the different antibodies for 1 h at room temperature. Finally, the coverslips were washed three times with PBS and mounted with FluorSave™ (Calbiochem).
Analysis of mitochondrial motility
After 1 day of culture, neurons were incubated with 200 nM of MitoTracker Red CM-H2Xros (Invitrogen), and mitochondrial movement was recorded using a video camera. One image was taken every 30 s. MetaMorph® software (Molecular Devices) was used to analyse the data.
RESULTS
Characterization of the MAP1B−/− mouse
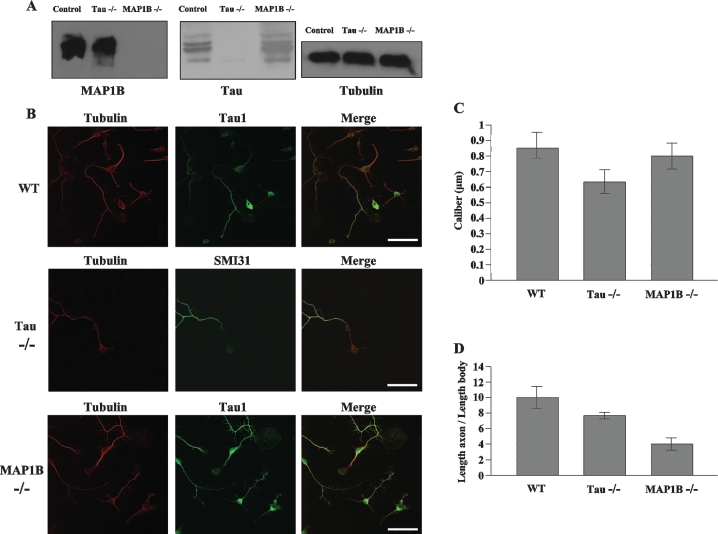
We initially characterized our MAP1B−/− [23] and tau−/− [20] mice using Western blot analysis of brain lysates. Specific antibodies directed against tubulin reacted with tubulin proteins in wild-type, MAP1B−/− and tau−/− mice (Figure 1A). Anti-MAP1B antibodies failed to react with any proteins in lysates from MAP1B−/− mice brains, but did react with MAP1B proteins from the brain lysates of tau−/− and wild-type mice. Anti-tau antibodies failed to react with any proteins in lysates from tau−/− mice brains, but did react with tau proteins from the brain lysates of MAP1B−/− and wild-type mice. These results confirm that MAP1B−/− mice lacked MAP1B proteins and that tau−/− mice lacked tau proteins.
Figure 1. Characteristics of wild-type, tau−/− and MAP1B−/− cultured neurons.
(A) Western-blot analyses of brain extracts derived from control (wild-type), MAP1B−/− and tau−/− mice. Samples were analysed with antibodies recognizing MAP1B (125) and tau (7.51) proteins. For the loading control, anti-tubulin antibody was used. (B) Double immunofluorescence analysed by confocal microscopy with an antibody recognizing specific axonal proteins. Hippocampal neurons from wild-type (WT) and MAP1B−/− mice were incubated with anti-tau1 antibody, and hippocampal neurons from tau−/− mice were incubated with anti-neurofilament antibody SMI31. All of the neurons were stained with an antibody recognizing tubulin protein. Scale bar, 50 μm. (C) The range of axon calibre of hippocampal neurons (n=10) is between 0.74 and 0.94 μm for wild-type (WT) mice, between 0.56 and 0.72 μm for tau−/− mice and between 0.72 and 0.89 μm for MAP1B−/− mice. (D) The range of axon length of hippocampal neurons (n=10) is between 8.2 and 11.8 μm for wild-type (WT) neurons, between 7.5 and 8.1 μm for tau−/− mice and between 3.2 and 4.8 μm for MAP1B−/− mice.
Morphology of hippocampal neurons from wild-type and MAP1B−/− mice
Hippocampal neuronal cultures from E18 (embryonic day 18) embryos were established as described previously [24]. After 1 day in culture to reach stage II, axonogenesis started to be observed. When compared with cultures of wild-type mice that express MAP1B proteins, a significant decrease in axonal length was observed in MAP1B−/− neurons (Figures 1B and 1C), whereas the axon diameter was found to be similar for processes from neurons that either lacked or contained MAP1B proteins (Figure 1C). As a control, the morphology of tau−/− neuronal cultures was examined under the same conditions (Figures 1B–1D) and, as reported previously [20], a decreased axonal length was also observed in tau−/− neuronal cultures. These results suggest that the lack of MAPs, either MAP1B or tau, is associated with decreased growth of axonal length in primary neuronal cultures.
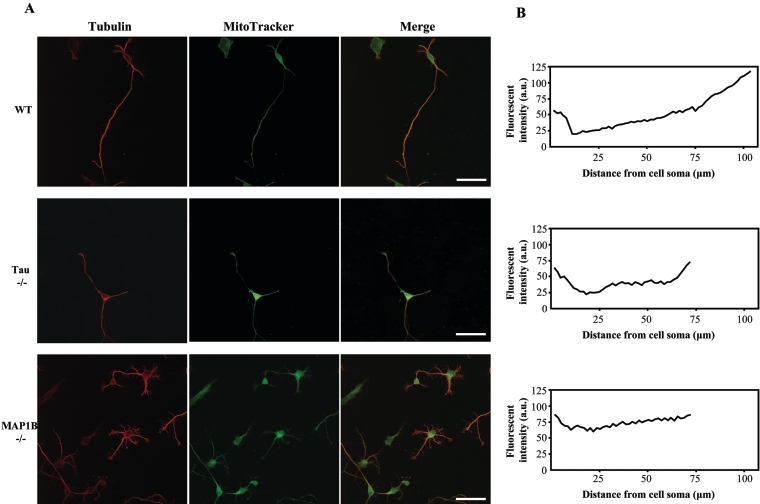
To study the microtubule–MAP complex as a functional unit, we investigated the association between mitochondria and this complex. As shown in Figure 2, we found a higher proportion of mitochondria at the distal end of axons than at the ends proximal to the growing axon in our neuronal cultures. Although this bias for more mitochondria at the distal end of axons was maintained in tau−/− neuronal cultures, a different pattern was observed for MAP1B−/− neurons, where slightly more intense staining of mitochondria was observed in the proximal region of the growing axon. Combined with our previous report [25] showing that dispersion of Golgi complex was observed in MAP1B−/− neurons, this new result suggests that retrograde axonal transport of mitochondria may be affected in MAP1B−/− neurons.
Figure 2. Mitochondrial localization in wild-type, tau−/− and MAP1B−/− neurons.
(A) Hippocampal neurons from wild-type (WT), tau−/− and MAP1B−/− mice analysed by double immunofluorescence showing tubulin and MitoTracker distribution. Scale bar, 50 μm. (B) Immunofluorescence intensity (from MitoTracker results) at distances of the cell soma up to 100 μm (wild-type) or 75 μm (tau−/− and MAP1B−/−) are indicated. a.u., arbitrary units.
Faster mitochondrial retrograde transport was found in developing MAP1B−/− neurons
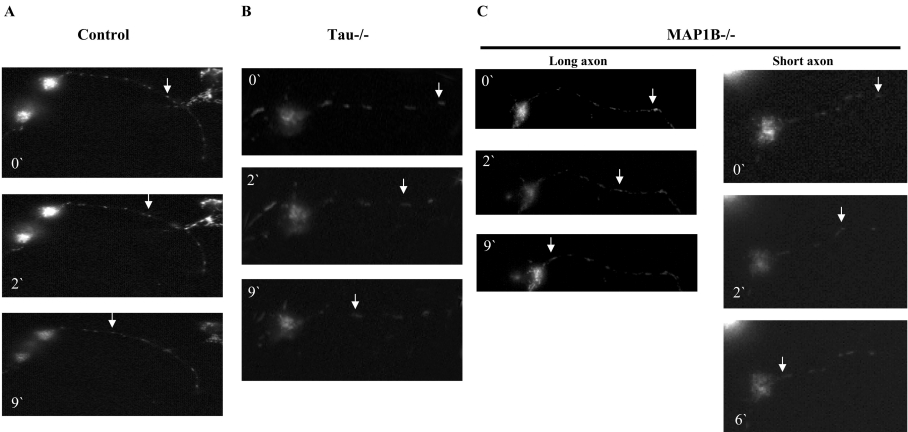
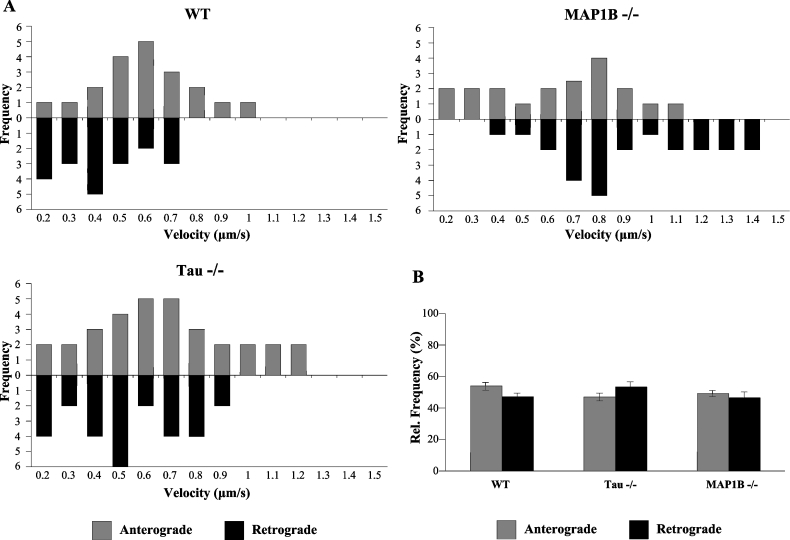
Video imaging of mitochondria on axons has shown that three possibilities can take place: no movement, anterograde movement and retrograde movement. One combination of these movement types is known as saltatory movement which involves frequent stops and starts in either the anterograde or the retrograde direction [26]. Since tau is known to participate in mitochondrial transport, we also wanted to measure the participation of MAP1B in mitochondrial movement in axons. As shown in the still pictures from time-lapse imaging of stained mitochondria in axons, we compared the velocities of mitochondrial movement in neuronal cultures from wild-type, tau−/− and MAP1B−/− mice. Figure 3 is an example of that type of analysis. Figure 3(A) shows axonal retrograde movement of a single mitochondrion through the axon of a neuron isolated from the brain of a wild-type mouse. Figure 3(B) indicates the axonal transport found in a neuron from a tau−/− mouse, tested as an additional control. Figure 3(C) shows the axonal transport in two different neurons lacking MAP1B. These two neurons have a different axonal length. Using these pictures, the velocities of movement of individual mitochondria that did not stop or ‘pause’ were measured and plotted as a histogram in Figure 4(A). In comparison with wild-type neurons, the highest velocities of mitochondrial movement in the anterograde fashion are enhanced in tau−/− neurons, whereas the highest rates of mitochondrial movement in the retrograde fashion are enhanced in MAP1B−/− neurons. Similarly, the average velocities of all mitochondria analysed show increased anterograde velocities in tau−/− neurons and increased retrograde velocities in MAP1B−/− neurons. Although the fraction of mitochondria moving in the anterograde and the fraction moving in the retrograde directions are similar between all three genotypes (Figure 4B), the mitochondria with the fastest velocities of movement are found in MAP1B−/− or tau−/− neurons. Similarly, the fraction of mitochondria that do not constantly move because they stop or pause is significantly decreased in MAP1B−/− or tau−/− neurons when compared with wild-type controls (Table 1). These results suggest that MAPs are associated with regulating the velocities of mitochondrial movement along the microtubule and that the lack of MAP1B increases velocities in the retrograde direction, whereas the lack of tau increases velocities in the anterograde direction.
Figure 3. Retrograde mitochondrial transport in hippocampal neurons.
The movement of a single mitochondrion, at different times, is indicated for neurons from wild-type mice (A), tau−/− mice (B) and MAP1B−/− mice (C), in a long axon and in a short axon. Sample images from a video recording are shown. By looking at this video, it is possible to indicate that we were looking at the same (single) mitochondrion.
Figure 4. Anterograde and retrograde mitochondrial movements in neurons from wild-type, tau−/− and MAP1B−/− mice.
(A) Anterograde and retrograde velocity distribution of mitochondrial movement in neurons from wild-type (WT), tau−/− and MAP1B−/− mice. (B) Relative (Rel.) frequency of anterograde and retrograde mitochondrial movements in neurons from wild-type (WT), tau−/− and MAP1B−/− mice. Different numbers of mitochondria (40 mitochondria for wild-type, 60 for tau−/− and 44 for MAP1B−/− mice) were analysed. Data are summarized in Table 1.
Table 1. Mitochondria transport in wild-type (40 samples), tau−/− (60 samples) and MAP−/− (44 samples) neurons.
Results are also shown in Figure 4. For the frequency of anterograde and retrograde movements, up to 100 mitochondria from each sample were tested, and the variation of the results is shown. Additionally, pausing was measured in 100 mitochondria from each sample.
| Wild-type | Tau−/− | MAP1B−/− | |
|---|---|---|---|
| Number of mitochondrial anterograde movements | 20 | 32 | 20 |
| Number of mitochondrial retrograde movements | 20 | 28 | 24 |
| Anterograde velocity (μm/s) | 0.60 | 0.68 | 0.60 |
| Retrograde velocity (μm/s) | 0.43 | 0.53 | 0.90 |
| Anterograde velocity range (μm/s) | 0.2–1.0 | 0.2–1.2 | 0.2–1.1 |
| Retrograde velocity range (μm/s) | 0.2–0.7 | 0.2–0.9 | 0.4–1.4 |
| Anterograde relative frequency | 56±5% | 49±6% | 51±4% |
| Retrograde relative frequency | 44±7% | 51±5% | 49±5% |
| Pausing | 29±5% | 6±2% | 2±1% |
For tau−/− mouse, our results support the previous observation indicating that tau mainly acts as an inhibitor of organelle-motor attachment [17]. Thus, in the absence of tau, an increase in the number of mitochondria bound to motor proteins is to be expected. Our data on ‘pausing’ indicate that it is the case for tau, as expected, but also in the case of MAP1B−/−.
Mitochondrial movement does not depend on axonal length
We have indicated above that the average length of the axon in cultured neurons after 1 day of culture is shorter in MAP1B−/− neurons compared with wild-type neurons. Thus we have compared the mitochondrial movement in MAP1B−/− neurons containing axons of a different length. Figure 3(C) shows that there are essentially no differences in mitochondrial velocity in axons of different length from MAP1B−/− neurons. Similar results to those in Table 1 were observed (not shown) for the transport in short and long axons in MAP1B−/− neurons.
DISCUSSION
Brain microtubules contain some associated proteins that contribute to the stabilization of microtubules needed to maintain neuron morphology. In the axon of a developing neuron, there are two of these MAPs, MAP1B and tau. For tau, it has been found not only that it could stabilize in vitro-assembled microtubules, but also that it can compete for microtubule binding with protein motors that are involved in the movement of organelles, such as mitochondria (in non-neurons and neurons), mainly by disturbing their anterograde transport [16,18]. Mitochondria can move in both anterograde and retrograde directions in one axon [27]. For the anterograde movement, kinesin-1 family (the nomenclature used is the standardized kinesin nomenclature [28]) have been involved for mitochondrial transport [15]. For the retrograde transport, dynein [29] is probably involved, since the velocity of mitochondrial retrograde transport is consistent with that of dynein driving movements, but not with alternative motors such as kinesin-13 [26]. Mitochondria change direction frequently and very rapidly, suggesting that it could not be an easy exchange from one motor to another for the opposite direction. Axonal transport of MAPs such as tau has been also studied [30,31].
The proper functioning of neurons depends upon their ability to form synapses and to then supply those structures with the proper organelles, energy sources and molecules that support synaptic transmission. Maintaining the supply of synaptic components requires that they be transported from the cell body to the synapse and back again, through specialized neuritic processes that include axons and dendrites. One of the key features in the formation of these processes is the presence of microtubules, which can provide a structural frame, a motive force and a track upon which supplies and additional components can be moved. These inherently dynamic microtubule structures must then be stabilized with MAP1B and tau to provide scaffolds for the maintenance of morphology of the neuritic processes and for reliable tracks upon which synaptic components can be moved.
While the movement of mitochondria in both anterograde and retrograde directions could be explained by unidirectional movement of kinesins alone or dynein alone bound to the mitochondria and to the microtubule, the frequent and rapid change of directions of movement would imply that an exchange of one motor for another on a rapid time scale would be required. Such an implication would further predict that anterograde mitochondrial movement is regulated independently of retrograde mitochondrial movement, although this point is under discussion [32,33]. However, our work and that of others (reviewed in [3]) also shows that MAP1B and tau are bound to the microtubule tracks, and we now show that removal of MAP1B or tau results in increased velocity of retrograde or anterograde movement of mitochondria respectively. This result suggests that a new mechanism could contribute to the observed movement of mitochondria in axons where MAP1B and/or tau could compete with kinesin and/or dynein motors for binding or positioning on the microtubule tracks. Depending upon the density of MAPs and motors in spatial proximity to one another, rates of movement could be finely regulated and, if needed, could be stopped completely, a situation consistent with the observed movement of mitochondria in axons. While the mechanisms underlying a complete change in the vector of direction remains poorly understood, the postulate that MAPs could temporarily stop movement for long enough to permit an exchange of motors that drive mitochondrial movement is plausible. Alternatively, if multiple kinesin and dynein motors are attached to a single mitochondrion, then the well-positioned presence of selected MAPs may encourage detachment of motors from the microtubule tracks that would permit a net change in the vector of direction.
Thus we suggest that tau protein could regulate anterograde transport, whereas MAP1B could regulate retrograde transport. However, the motor that regulates retrograde transport, dynein, is first transported anterogradely to the axonal ends before it moves to the minus end of microtubules playing the role of the motor for retrograde transport [23]. Thus a defect in anterograde transport could indirectly affect retrograde transport. This could explain the slight effect found for retrograde transport in tau−/− neurons.
In the present study, we have indicated a possible role for MAP1B in retrograde mitochondrial transport. Recently, it has been indicated that C190RF5, a homologue of MAP1B, could affect mitochondrial transport in non-neurons [34], or that another homologue of MAP1B, futsch protein, leads to axonal transport defects [35]. For retrograde mitochondrial transport, dynein may be involved, and it has been indicated that MAP1B could interfere with dynein motor functions through its binding to LIS1 (lissencephaly-related protein 1) [25] or by an EB1 (end-binding protein 1)-like function [36].
Also in the present study, we have found that MAP1B could regulate, in a negative way, the axonal retrograde movement of mitochondria, in the opposite way to tau, that mainly prevents axonal anterograde transport [18,37].
By taking into account the previous observations, we suggest that, in a developing neuron, the anterograde transport of mitochondria is favoured. This is suggested by the following. (i) The main tau isoform present in the developing neuron is tau containing only three tubulin-binding sequences (tau 3R). This tau isoform will interfere less with axonal transport than that containing four tubulin-binding sequences (tau 4R). (ii) The retrograde axonal transport will be down-regulated by the presence of MAP1B. A possible relationship between the increase in anterograde and the decrease in retrograde transport with axonogenesis was suggested previously [38].
Afterwards, once neuron maturation occurs and synaptogenesis takes place, MAP1B is no longer expressed and tau 4R expression increases [1]. In this situation, axonal anterograde transport should slow down, whereas retrograde transport should increase. In summary, axonal MAPs such as MAP1B and tau not only may have a structural role as with proteins involved in microtubule stabilization, but also appear to have an additional role in regulating mitochondrial axonal transport.
Acknowledgments
This work is supported by PNCYT (Plan Nacional de Ciencia y Tecnología), CM (Comunidad de Madrid) and FIS (Fondo de Investigación Sanitaria) grants to J.A., and by NIH (National Institutes of Health) grant AG 15307 to M.P.V. We thank Dr J. J. Lucas for critical reading and correction of this manuscript before submission. E.M.J.-M. was awarded with a fellowship from Spanish MCYT (Ministerio de Ciencia y Tecnología).
References
- 1.Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu. Rev. Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison T., Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 3.Avila J., Dominguez J., Díaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int. J. Dev. Biol. 1994;38:13–25. [PubMed] [Google Scholar]
- 4.Tucker R. P., Garner C. C., Matus A. In situ localization of microtubule-associated protein mRNA in the developing and adult rat brain. Neuron. 1989;2:1245–1256. doi: 10.1016/0896-6273(89)90309-7. [DOI] [PubMed] [Google Scholar]
- 5.Binder L. I., Frankfurter A., Rebhun L. I. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann. N.Y. Acad. Sci. 1986;466:145–166. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- 6.Díaz-Nido J., Serrano L., López-Otín C., Vandekerckhove J., Avila J. Phosphorylation of a neuronal-specific β-tubulin isotype. J. Biol. Chem. 1990;265:13949–13954. [PubMed] [Google Scholar]
- 7.Garner C., Garner A., Huber G., Kozak K. S., Matus A. Molecular cloning of microtubule-associated protein 1 A (MAP1A) and microtubule-associated protein 5 (MAP1B): identification of distinct genes and their differential expression in developing brain. J. Neurochem. 1990;55:146–154. doi: 10.1111/j.1471-4159.1990.tb08832.x. [DOI] [PubMed] [Google Scholar]
- 8.Tucker R. P., Matus A. I. Microtubule-associated proteins characteristic of embryonic brain are found in the adult mammalian retina. Dev. Biol. 1988;130:423–434. doi: 10.1016/0012-1606(88)90338-7. [DOI] [PubMed] [Google Scholar]
- 9.Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 10.Kosik K. S. Tau: structure and function. In: Avila J., Brandt R., Kosik K. S., editors. Brain Microtubule Associated Proteins: Modifications in Disease. Chur: Harwood Academic Publishers; 1997. pp. 43–52. [Google Scholar]
- 11.Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988;239:285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- 12.Baas P., Heidemann S. R. Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites. J. Cell Biol. 1986;103:917–927. doi: 10.1083/jcb.103.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baas P., Buster D. W. Slow axonal transport and the genesis of neuronal morphology. J. Neurobiol. 2004;58:3–17. doi: 10.1002/neu.10281. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa N., Takemura R. Molecular motors in neuronal development, intracellular transport and diseases. Curr. Opin. Neurobiol. 2004;14:564–573. doi: 10.1016/j.conb.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Hirokawa N., Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 16.Stamer K., Vogel R., Thies E., Mandelkow E., Mandelkow E. M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinczek B., Ebneth A., Mandelkow E. M., Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- 18.Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J. Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury K., Bonaldo P., Torres M., Stoykova A., Gruss P. Evidence for the stochastic integration of gene trap vectors into the mouse germline. Nucleic Acids Res. 1997;25:1531–1536. doi: 10.1093/nar/25.8.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson H., Ferreira A., Eyster M. V., Ghoshal N., Binder L. I., Vitek M. P. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- 21.Ulloa L., Diaz-Nido J., Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 1993;12:1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Billault C., Demandt E., Wandosell F., Torres M., Bonaldo P., Stoykova A., Chowdhury K., Gruss P., Avila J., Sanchez M. P. Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels. Mol. Cell. Neurosci. 2000;16:408–421. doi: 10.1006/mcne.2000.0880. [DOI] [PubMed] [Google Scholar]
- 24.Dotti C. G., Banker G. A., Binder L. I. The expression and distribution of the microtubule-associated proteins tau and microtubule-associated protein 2 in hippocampal neurons in the rat in situ and in cell culture. Neuroscience. 1987;23:121–130. doi: 10.1016/0306-4522(87)90276-4. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Mateos E. M., Wandosell F., Reiner O., Avila J., Gonzalez-Billault C. Binding of microtubule-associated protein 1B to LIS1 affects the interaction between dynein and LIS1. Biochem. J. 2005;389:333–341. doi: 10.1042/BJ20050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollenbeck P. J., Saxton W. M. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollenbeck P. J. The pattern and mechanism of mitochondrial transport in axons. Front. Biosci. 1996;1:d91–d102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence C. J., Dawe R. K., Christie K. R., Cleveland D. W., Dawson S. C., Endow S. A., Goldstein L. S., Goodson H. V., Hirokawa N., Howard J., et al. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paschal B., Shpentner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utton M. A., Connell J., Asuni A. A., van Slegtenhorst M., Hutton M., de Silva R., Lees A. J., Miller C. C., Anderton B. H. The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. J. Neurosci. 2002;22:6394–6400. doi: 10.1523/JNEUROSCI.22-15-06394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utton M. A., Noble W. J., Hill J. E., Anderton B. H., Hanger D. P. Molecular motors implicated in the axonal transport of tau and α-synuclein. J. Cell Sci. 2005;118:4645–4654. doi: 10.1242/jcs.02558. [DOI] [PubMed] [Google Scholar]
- 32.Martin M., Iyadurai S. J., Gassman A., Gindhart J. G., Jr, Hays T. S., Saxton W. M. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L., Vo A., Liu G., Mckeehan W. L. Distinct structural domains within C190RF5 support association with stabilize microtubules and mitochondrial aggregation and genome destruction. Cancer Res. 2005;65:4191–4201. doi: 10.1158/0008-5472.CAN-04-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettencourt da Cruz A., Schwarzel M., Schulze S., Niyyati M., Heisenberg M., Kretzschmar D. Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila. Mol. Biol. Cell. 2005;16:2433–2442. doi: 10.1091/mbc.E04-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Mateos E. M., Paglini G., Gonzalez-Billault C., Caceres A., Avila J. End binding protein-1 (EB1) complements microtubule-associated protein-1B during axonogenesis. J. Neurosci. Res. 2005;80:350–359. doi: 10.1002/jnr.20453. [DOI] [PubMed] [Google Scholar]
- 37.Baas P. W., Qiang L. Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol. 2005;15:183–187. doi: 10.1016/j.tcb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Ligon L., Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 2000;427:551–561. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]