Abstract
The primary somatosensory cortex (S1) contains a map representation of the body surface. We hypothesized that S1 stimulation can successfully substitute for (or be substituted by) direct stimulation of skin receptors. We prepared rabbits for evoking eyelid conditioned responses (CRs) using a trace “shock-air puff” paradigm. In a first series of experiments, animals received a conditioned stimulus (CS, a train of electrical pulses) in the whisker pad or in the S1 areas for vibrissae or for the hind limb. In the three cases, the CS was followed 250 ms from its end by an air puff presented to the cornea as an unconditioned stimulus (US). Learning curves from the three groups presented similar values, although animals stimulated with a central CS acquired their CRs faster. In a second series of experiments, animals were divided into four groups and were presented either centrally or peripherally with the same CS for six conditioning sessions. Then, the CS was switched from central to peripheral, or vice versa, for 5 additional days. Conditioned animals were not able to discriminate between peripheral (vibrissae) stimuli and stimuli presented to the corresponding S1 (vibrissae) area, but they were able to discriminate between CSs presented to S1 (hind limb) and body (vibrissae) regions. The kinetic properties of evoked CRs were not modified by CS switching. It is proposed that S1 allows the construction of somatosensory percepts of the body surface but does not allow distinguishing the central or peripheral location of the evoking stimuli.
Keywords: associative learning, eyelid motor system, rabbits, sensory substitution, trace-conditioning paradigm
The physiological role of the primary somatosensory cortex (S1) seems to be the generation of neural codes coherent with sensory stimuli impinging upon skin receptors (1). Indeed, it has been shown that neuronal responses in the S1 reproduced the actual discriminative behavior of monkeys during the performance of selective vibrotactile discrimination tasks (2). Thus, the neural activities recorded at the different S1 areas seem to correlate with sensory events (3). Accepting that centrally evoked sensory percepts could be similar to those evoked by peripheral stimuli (4, 5), it can be hypothesized that the electrical microstimulation of selected S1 sites can substitute for peripheral skin receptor activation during the acquisition of an associative task, such as the classical conditioning of eyelid responses.
To check the proposed hypothesis, we decided to use here a trace-conditioning paradigm, using peripheral (whisker pad) or central (S1 area for vibrissae or hind limb) electrical stimulation as a conditioned stimulus (CS) and an air puff directed at the cornea (ipsilateral to the whisker pad and contralateral to the stimulated S1 areas) as an unconditioned stimulus (US). The rationale was the following. As opposed to delay conditioning, in which the CS precedes the US in its initiation and coterminates with it, in trace conditioning, there is a time gap between the end of the CS and the beginning of the US. Whereas trace conditioning requires a conscious knowledge (6) and/or explicit memory (7) of the relevant relationships between the CS and the US, delay conditioning does not. It has already been shown that trace classical conditioning is highly sensitive to and/or depends on cerebral cortical activity (8, 9); that the protein product of the early gene fos is expressed in the parietal cortex (10), among other cortical areas, during the acquisition of an eyelid conditioned response (CR) using a trace paradigm; and that the unitary activity of the corresponding S1 areas for vibrissae seems to be active during the acquisition process (11). Moreover, it is well known that electrical stimulation of selected cerebral structures can be successfully used as CS or US (12).
Eyeblink conditioning has been successfully achieved by using vibrotactile (13) or electrical (14) stimulation of the whisker pad. Although mystacial vibrissae are not organized as strictly in rabbits as in rodents, vibrissa representation in the S1 of the former presents a columnar organization, functionally related to inputs arriving from single whiskers (15, 16). Thus, it is possible to identify the corresponding S1 area for the stimulated zone of the whisker pad in rabbits. Moreover, the S1 area for the hind limb is located >5 mm away from the corresponding area for the vibrissae (17).
Animals were prepared for the classical conditioning of eyelid responses by using a trace paradigm. As a CS, we used a train of stimuli (100 ms, 200 Hz) presented peripherally to the central part (row C, column 3) of the rabbit whisker pad or centrally to the S1 areas corresponding to this set of vibrissae or to the hind limb. Because whiskers are finely tuned to selective spatial displacements (3), we decided to use a train of electrical pulses as a CS, because vibration is encoded only by frequency and intensity (4) and would be more easily reproducible by central stimulation than would a complex spatial pattern of vibrissal displacement. Moreover, S1 discrimination seems to depend on a spike count code (18), and both thalamic inputs to the S1 and interneurons located in its layer IV seem to fire at high rates (<200 spikes per second; see refs. 15 and 16). These layer IV interneurons are more sensitive to peripheral stimulation than are spiny neurons but are devoid of direction sensitivity to vibrissal displacements (19). The US was always a puff of air applied to the cornea ipsilateral to the stimulated whisker pad and contralateral to the stimulated S1 areas. We checked whether electrical stimuli applied to S1 would be able to evoke identifiable CRs and whether the selected CS could be switched from S1 to the periphery without any substantial change in the acquired CS–US association. Eyelid responses were recorded with the search-coil technique (20). We also recorded the electromyographic (EMG) activity of orbicularis oculi and vibrissal muscles. A parametric analysis of eyelid CRs was carried out to look for possible differences in their kinematics when evoked either centrally or peripherally.
Results
Trace Eyelid Conditioning Using Peripheral or Central Stimuli as a CS.
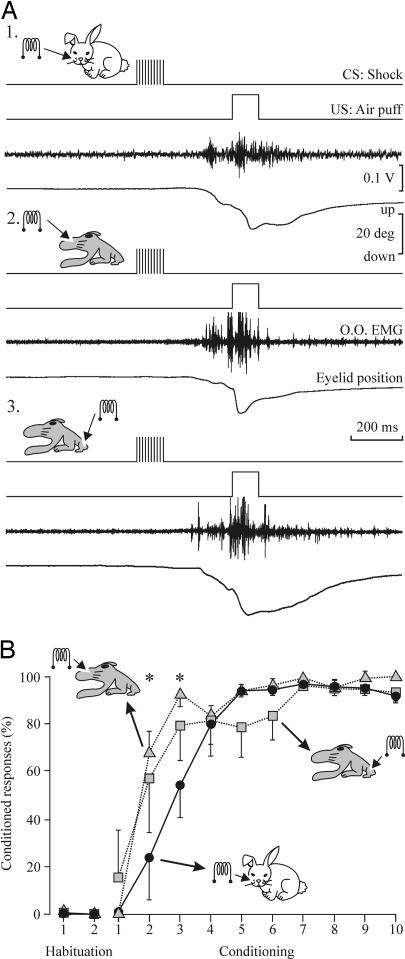
Fig. 1 illustrates the experimental design. Fig. 2 illustrates the evolution through 10 successive classical conditioning sessions of the blink reflex for animals (n = 3) trained with a trace-conditioning paradigm in which the CS was a train of stimuli (200 Hz, 100 ms, and <200 μA) presented to the ipsilateral whisker pad (Fig. 2A1) or the same train (<100 μA) applied to S1 areas related to the C row of vibrissae (n = 3; Fig. 2A2) or to the hind limb (n = 3; Fig. 2A3). The CS in this paradigm was followed 250 ms from its end by a US consisting of an air puff (100 ms, 3 kg/cm2). In three groups of rabbits trained with this trace paradigm, CRs appeared during the second conditioning session and reached criterion (>80% responses per session) by the fourth to sixth conditioning session (Fig. 2B). However, animals presented with a train of electrical stimuli at the corresponding S1 area as a CS reached criterion significantly faster than those in which electrical stimulation of the whisker pad was used as a CS [P < 0.05, F(22, 44) = 1.823]. Once animals were consistently conditioned, they showed an eyelid response in which the CR was integrated with the unconditioned response (Fig. 2A).
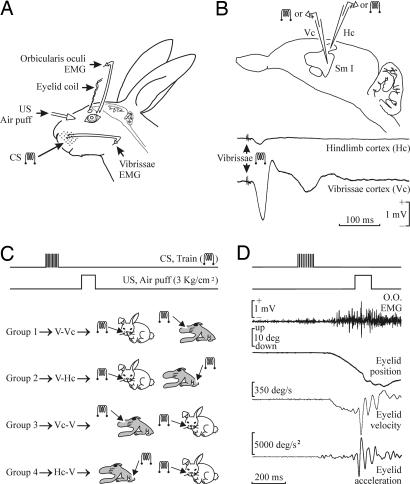
Fig. 1.
Experimental design. (A) We recorded the upper eyelid position and EMG activity of the ipsilateral orbicularis oculi (O.O.) and vibrissal (V) muscles. Peripheral stimuli consisted of the electrical stimulation of the whisker pad as a CS or air puffs presented to the ipsilateral cornea as a US. (B) A diagram of the rabbit S1, illustrating the recording and stimulating sites. Representative examples of the field potentials evoked at the vibrissal (Vc) and hind-limb (Hc) S1 sites by a double-pulse stimulus applied to the contralateral vibrissae. (C) Experimental groups. For the four experimental groups included in Figs. 3 and 4, the CS consisted of a 200-Hz, 100-ms, 1.5 × threshold train presented during the first six conditioning sessions to the left whisker pad (middle part of row C) and then (7th to 11th sessions) to the contralateral S1, the corresponding sites for the C-row vibrissae (group 1) or the hind limb (group 2). For groups 3 and 4, the CS was presented in the reverse order, as illustrated. The US always consisted of a 100-ms 3-kg/cm2 air puff presented to the ipsilateral cornea. (D) Example of a CR recorded during the sixth conditioning session (CS, vibrissa stimulation). Profiles corresponding to O.O. EMG (in mV), eyelid position (in degrees), and acceleration (in degrees per s2) are illustrated.
Fig. 2.
Learning curves for vibrissal or S1 stimulation (vibrissal or hind-limb areas) as a CS. (A) Representative examples of CRs evoked by the three CSs selected for this study by the sixth conditioning session. (B) Percentage of CRs reaching criterion across the 10 conditioning sessions. The three groups of animals reached asymptotic values (>80% of CRs) by the fourth to sixth sessions. Data represent mean ± SD. Note that the acquisition of the CR is faster with the two types of central CS than for peripheral (vibrissae) CS [∗, P < 0.05, F(22, 44) = 1.823].
Eyelid CRs evoked with the three different stimuli used as a CS in this study were undistinguishable by the evoked eyelid profiles and parametric characteristics. For example, onset latency of CRs decreased through the successive conditioning sessions from a mean value of 227.7 ± 25.2 ms for the first conditioning session to 126.4 ± 17.3 ms for the seventh for the group receiving the CS at the vibrissae. The other two groups presented a similar evolution in the latencies of the evoked CRs during the first and seventh conditioning sessions [S1 vibrissa group, 229.2 ± 20.6 and 131.4 ± 18.6; S1 hind-limb group, 215.5 ± 24.2 and 128.3 ± 21.4; P ≥ 0.32, F(22, 44) = 2.844]. A further proof that the three groups of animals achieved true CRs is that the three groups (n = 3 animals for each type of CS) of pseudoconditioned animals never reached >12 CRs per session. Thus, differences between the conditioned and pseudoconditioned animals were significantly different from the second to tenth conditioning sessions [P < 0.001, F(55, 110) = 20.923]. These results convincingly showed that electrical stimulation of the S1 can be successfully used as a CS, evoking learning curves undistinguishable from those evoked by peripheral stimuli applied to the whisker pad as a CS.
S1 Stimulation Can Substitute for Peripheral Stimuli as CSs in a Trace-Conditioning Paradigm.
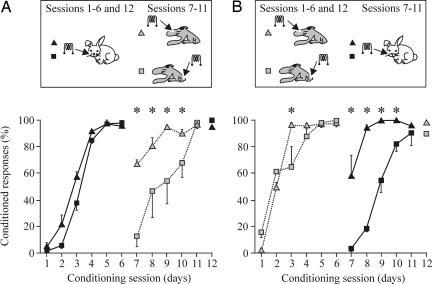
In a second series of experiments, we prepared four experimental groups of animals (n = 4 for each group) in which the CS (peripheral or central) used at the beginning of the conditioning sessions was substituted with the opposite one (central or peripheral) on the seventh session. As illustrated in Fig. 3A, a total of eight animals (groups 1 and 2) were conditioned by using as a CS the already-mentioned train (100 ms, 200 Hz, and 120–190 μA) of stimuli presented to the ipsilateral whisker pad during six conditioning sessions. The two groups of animals reached learning criterion by the fourth session; thus, they were already well conditioned by the sixth. From the seventh to the eleventh conditioning sessions, the peripheral CS was substituted by the same train of stimuli (100 ms, 200 Hz, and 85–100 μA) applied to the S1 area for vibrissae (central part of row C; group 1) or for the hind limb (group 2). During the seventh conditioning session, animals from group 1 presented 67.2% of CRs vs. 14.2% for group 2 (Fig. 3A). From the seventh to ninth sessions, animals from group 1 presented significantly more CRs than did group 2 [Fig. 3A; P < 0.001, F(11, 33) = 4.233]. In this situation, animals stimulated in the S1 vibrissa area (group 1) reached criterion faster than those stimulated in the S1 hind-limb area (group 2).
Fig. 3.
Acquisition of eyelid CRs during peripheral (vibrissae) followed by central (S1 areas for vibrissae or hind limb) CS, or vice versa. (A) Learning curves for groups 1 (black triangles) and 2 (black squares) in which the CS was presented during the first six sessions to the vibrissae and then (sessions 7–11) switched to the S1 area for vibrissae (group 1, gray triangles) or for the hind limb (group 2, gray squares). Data represent mean ± SD. Note that when the two central CSs were substituted by the peripheral CS, the acquisition was significantly faster for the group stimulated at the S1 area for vibrissae [∗, P < 0.001, F(11, 33) = 4.233]. (B) Learning curves for groups 3 (gray triangles) and 4 (gray squares) in which the CS was presented during the first six sessions to the S1 areas for vibrissae (group 3) or for the hind limb (group 4) and then (sessions 7–11) switched to the vibrissae (group 3, black triangles) or for the hind limb (group 4, black squares). Note that when the peripheral CS were substituted by the two central CSs, the acquisition was significantly faster for the group stimulated at the S1 area for vibrissae [∗, P < 0.05, F(11, 33) = 2.913].
In addition, we conditioned eight more animals (groups 3 and 4) using central stimulation of S1 areas for vibrissae (group 3) or hind limb (group 4) as a CS during the first six sessions (Fig. 3B). Here again, animals reached criterion by the third to fourth conditioning sessions. By the seventh session, central S1 stimulation was substituted by the above-mentioned train presented to the whisker pad as a CS (groups 3 and 4). During the seventh conditioning session, animals included in group 3 presented a mean of 57.1% CRs vs. 3.7% presented by group 4. Indeed, from the 7th to 10th sessions, the percentage of CRs presented by animals belonging to group 3 was significantly larger than that from group 4 [Fig. 3B; P < 0.05, F(11, 33) = 2.913]. As illustrated in Fig. 3B, animals belonging to group 3, receiving their CS at the S1 area corresponding to the vibrissae, regained learning criterion faster than those from group 4, which received their central stimulation at the hind limb S1 area.
A recall (12th) session was carried out for the four groups, presenting again as a CS the one presented during the first six sessions. As illustrated in Fig. 3, all animals seemed to retain the previously acquired CS–US association.
We decided to analyze whether the transfer of CS from peripheral to central (groups 1 and 2) or vice versa (groups 3 and 4) not only modified the probability of evoking CRs but also changed their kinematics, depending on the intrinsic coherence of the substitution (i.e., S1 areas for vibrissae or hind limb vs. peripheral vibrissae). Fig. 4 illustrates the evolution of the latency and peak amplitude of evoked CRs for the four experimental groups. For example, it is known that the latency of evoked CRs decreases steadily with training (21, 22). This is what happened during the first six sessions for animals included in groups 1 and 2 presented with a train of stimuli at the whisker pad as a CS (Fig. 4A1). Latency values were >200 ms during the first conditioning session and decreased to <150 ms by the sixth session. When (from the seventh session on) the CS was switched to the S1, only those animals stimulated at the S1 area corresponding to the vibrissae (group 1) presented CR latencies that followed the previous trend. Thus, the latency values during the seventh conditioning session were 136.1 ± 5.2 ms for group 1 and 220.3 ± 23.1 ms for group 2. In fact, latency values evoked by the two central CSs were significantly different for sessions 7–10 [P < 0.01, F(11, 33) = 2.294]. A similar trend was observed regarding the peak amplitude of CRs collected from groups 1 and 2 before and after CS switches (Fig. 4A2), although the statistically significant differences were found only for sessions seven and eight. The latency of CRs evoked by a central (S1) train of stimuli used as CS (groups 3 and 4) decreased across conditioning sessions (one to six sessions) with similar values and slope (Fig. 4B1), whereas the peak amplitude of the evoked responses increased steadily (Fig. 4B2). Latency and peak amplitude values of CRs evoked during the seventh conditioning session, when the CS was presented (peripherally) to the vibrissae, were in accordance and continuity with those collected during the preceding sixth session, but only for group 3 of animals, namely those previously stimulated in the S1 area for the vibrissae (Fig. 4 B1 and B2). Mean latency and peak amplitude values collected from the four groups during the recall (12th) session indicate that performance achieved during the first six conditioning sessions was not erased by the new CS–US association acquired during sessions seven to eleven (Fig. 4).
Fig. 4.
Quantitative analysis of CR evolution through conditioning sessions for the four experimental groups. (A) The experimental design for groups 1 and 2 is illustrated in the Inset at the top. Time histograms for the latency (1, in ms) and peak amplitude (2, in degrees) of CRs during the sessions (1–6) in which the CS was presented to the vibrissae (group 1, black triangles; group 2, black squares) followed by the sessions (7–11) in which the CS was presented to the S1 area for vibrissae (group 1, gray triangles) or to the hind limb (group 2, gray squares). Data represent mean ± SD. Significant differences in latency [∗, P < 0.01, F(11, 33) = 2.294] and amplitude [∗, P ≤ 0.05, F(11, 33) = 2.310] after the CS switch are indicated. (B) The experimental design for groups 3 and 4 is illustrated at the top. Time histograms for the latency (1, in ms) and peak amplitude (2, in degrees) of CRs during the sessions (1–6) in which the CS was presented to the S1 area for vibrissae (group 3, black triangles) or for the hind limb (group 4, black squares) followed by the sessions (7–11) in which the CS was presented to the vibrissae (group 3, gray triangles; group 4, gray squares). Significant differences in latency [∗, P < 0.01, F(11, 33) = 2.965] and amplitude [∗, P < 0.01, F(11, 33) = 4.407] after the CS switch are indicated.
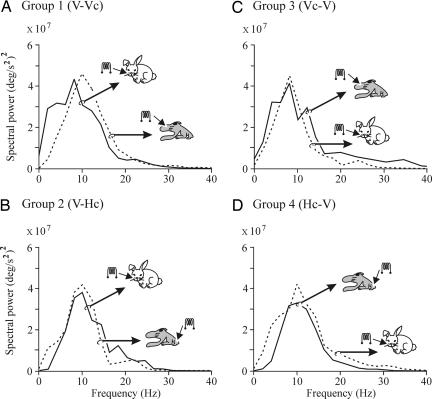
Recently, it has been shown that eyelid CRs in the rabbit present a dominant (8- to 12-Hz) oscillation, suggesting that a common neural integrator underlies their generation and/or performance (20). We have checked here the oscillatory characteristics of CRs evoked by the three different CSs used in this study. As illustrated in Fig. 5, CRs collected from the sixth conditioning session in the four experimental groups presented nonsignificantly different power spectra, even when compared with the power spectra of CRs collected from the eleventh session, i.e., well after switching from a peripheral to central CS or vice versa (P ≥ 0.1, χ2-distributed test; correlation coefficient ≥0.996, P ≤ 0.005, Pearson test). In all of these experimental situations, the dominant oscillation frequency of eyelid responses presented values ≈10 Hz, suggesting that the generation of CRs is not affected by CS presented at peripheral somatosensory receptors or directly at the S1.
Fig. 5.
Frequency domain analyses of eyelid CRs evoked by peripheral and central stimuli used as CS. Histograms showing the mean power spectra of acceleration profiles computed from CRs evoked by CS-alone presentations. Each power spectrum was averaged from ≥12 records. Records were collected from the 6th (continuous lines) or 11th (dotted lines) conditioning sessions for the indicated experimental groups. The CS evoking each record is also indicated. No significant differences were detected between each pair of power spectra (P ≥ 0.1, χ2-distributed test; correlation coefficient ≥0.996, P ≤ 0.005, Pearson test). V, vibrissae; Hc and Vc, hind-limb and vibrissa S1 cortices.
Discussion
The present results indicate that electrical stimulation of S1 areas can be successfully used as a CS able to evoke CRs undistinguishable from those evoked by a similar CS presented directly to skin receptors. Moreover, rabbits acquiring an associative learning using a trace-conditioning paradigm are unable to differentiate between the peripheral or central presentation of the same CS, i.e., their subjective experience was similar for both stimuli (1). These results also suggest that a similar sensory percept is evoked when animals are stimulated in the S1 area for vibrissae as when stimulated directly on the whisker pad (4, 5). In this regard, it has been convincingly shown that neural responses in the S1 encode the observed performance of behaving monkeys during vibrotactile discrimination tasks, and that the electrical microstimulation of the same S1 areas can substitute for the direct stimulation of the corresponding Meissner’s corpuscles located at the finger tips and sensitive to frequencies (<50 Hz) subjectively perceived as a flutter (2). Finally, the direct stimulation of selective (vibrissae or hind limb) S1 areas apparently allows the detection of stimulus location in space, as recently shown in humans (23).
The acquisition rate, kinematics, and frequency-domain properties of evoked CRs were significantly less disturbed by the sudden change (external toward internal and vice versa) in the site where the CS was applied when both were presented to the corresponding (whisker pad and S1 areas for vibrissae) sites in the somatosensory pathway than when CSs were presented to a noncorresponding site, namely the small S1 area for the hind limb. Results obtained during the recall session carried out with the four experimental groups suggest that animals were able to retain CS–US associative strength regardless of the modifications introduced in the location of the stimuli (internal vs. external, S1 area for the vibrissae vs. S1 area for the hind limb). These results are indicative of the presence of multiple distributed forms of associative learning not restricted to small sets of cortical synaptic circuits (24). Nevertheless, according to data illustrated in Figs. 3 and 4, the present results cannot be considered the result of a generalization process between centrally and peripherally applied stimuli (12, 18). The large distance (in millimeters) between the cortical S1 selected in this study and the even larger separation of the corresponding receptor sites at the animal’s skin preclude these results of being considered as a mere generalization process.
To a certain extent, sensory substitution can compensate after the failure of a given sensory system (25). In this regard, an increase in the relative strength of somatosensory inputs from neck muscle proprioceptors to compensate for a missing vestibular input has been reported (26), and crossmodal plasticity seems to be evoked in the congenitally blind using electrotactile stimulation of the tongue (27). A possibility to be checked experimentally is that in cases of unimodal plasticity, such as the results presented here, central stimulation of the appropriate S1 sites with the corresponding neural codes should be able to compensate for circuitry changes evoked by the loss of peripheral receptors. Because the vibrissal receptor system allows a complex set of putative perceptions, depending upon individual vibrissal displacements (3, 19), here we used a stimulus that was probably “read” as vibration when presented both at the whisker pad and to the selected S1 areas. Swadlow and coworkers (15, 16, 19) have reported the presence of putative inhibitory interneurons in layer IV of S1 areas for vibrissae. These neurons are able to fire at a high rate (200 spikes per second), are more sensitive to peripheral stimuli than are pyramidal neurons, are devoid of direction sensitivity to vibrissal displacements, and are easily activated by thalamic (ventroposterior medial) neurons (19, 28). This population of cells is susceptible to activation by the CS used here and could be involved in the central subjective perception of vibration (>50 Hz) signals undistinguishable from the direct stimulation of the whisker pad with the same CS.
The present results further confirm the involvement of S1 areas in the acquisition of classically conditioned eyelid responses. As already shown, c-Fos is selectively expressed in the S1 of conscious rabbits during the acquisition of trace conditioning paradigms (10). Although cortical lesions do not completely prevent the appearance of CRs, they do affect their proper timing and performance. As shown here, the kinetic properties of CRs were modified not by the central vs. peripheral location of the CS but by their different location in the real or subjective space of the conditioned animal. Moreover, oscillatory properties of evoked eyelid CRs were not modified by the different CSs used in this study, indicating that the neural oscillator determining the dominant frequency of eyelid reflex and CRs is located somewhere along the efferent motor pathway (29).
Methods
Subjects.
Experiments were carried out on 34 adult rabbits (New Zealand White albino) weighing 2.3–2.7 kg on arrival from an authorized supplier (Iffa Credo). All experimental procedures were carried out in accordance with the guidelines of the European Union Council (86/609/EU) and following the Spanish regulations (BOE 67/8509–8512) for the use of laboratory animals in chronic experiments.
Surgery.
Animals were anesthetized with a ketamine–xylazine mixture (Ketaminol, 50 mg/ml; Rompun, 20 mg/ml; and atropine sulfate, 0.5 mg/kg). The anesthesia dosage was 0.85 ml/kg and was maintained by i.v. perfusion of the mixture at a flow rate of 10 mg/kg per hr. A five-turn coil (3 mm in diameter) was implanted into the center of the left upper eyelid, close to the lid margin. Coils were made of Teflon-coated stainless-steel wire (A-M Systems, Everett, WA) with an external diameter of 50 μm and weighed 10–15 mg. Animals were also implanted with recording bipolar hook electrodes in the left orbicularis oculi muscle and in the lateral whisker pad. A pair of stimulating electrodes was implanted in the center of the whisker pad (row C, column 3). These three electrodes were made of the same wire as the coils and bared ≈1 mm at the tip. A silver electrode (1 mm in diameter) was attached to the skull as a ground.
In selected animals, a 4 × 4-mm window was drilled in the parietal bone, centered on the right S1 areas for the vibrissae [row C, anteroposterior (AP) = −1.7 mm, lateral (L) = 7 mm, depth (D) = 2.5 mm from bregma] and the hind limb (AP = 0 mm, L = 1 mm, D = 2.5 mm; see refs. 17 and 30). Two 50-μm tungsten bipolar stimulating electrodes were implanted in the selected sites. The final location of these stimulating electrodes was decided according to the extracellular field potentials evoked by the electrical stimulation of the whisker pad (Fig. 1B) or the peroneal nerve. The latter was stimulated with bipolar electrodes implanted transiently at the ankle level. The dura mater surface was protected with an inert plastic cover and the window closed with acrylic resin. Terminals of lid coil, EMG, and stimulating and ground electrodes were soldered to two nine-pin sockets. All of these connectors were covered with acrylic resin, and the whole system was attached to the skull with the aid of three small screws fastened and cemented to the bone (Fig. 1).
Recording and Stimulation Procedures.
Recording sessions began 2 weeks after surgery. Each rabbit was placed in a Perspex restrainer specially designed for limiting the animal’s movements (20). The box was placed on the recording table and surrounded by a black cloth. The recording room was kept softly illuminated, and a 50-dB background white noise was switched on during the experiments. For all subjects, the first two sessions consisted of adapting the rabbit to the restrainer and to the experimental conditions. Animals were presented at random with the stimulus used as CS (see below). Data shown in Fig. 2 were obtained from classical conditioning of eyelid responses using a trace paradigm as described below. A total of 18 animals were used in this series; the remaining 16 animals were divided into four groups (Fig. 1C), and data collected from these four groups are presented in Figs. 3–5.
Eyelid movements were recorded with the magnetic field search-coil technique (20). The EMG activity of the selected muscles and field potentials evoked at S1 areas were recorded with Grass P511 differential amplifiers with a bandwidth of 0.1 Hz to 10 kHz (Grass-Telefactor, West Warwick, RI). Air puffs were applied through the opening of a plastic pipette (3 mm in diameter) attached to a metal holder fixed to the animal’s nine-pin socket (dual-channel air-puff device; Biomedical Engineering, Thornwood, NY). Electrical stimulation of the whisker pad and of the selected areas of S1 was achieved across an isolation unit. Single (cathodal, square, 50-μs, <200-μA pulses) and train (200 Hz) stimuli were programmed with a CS-220 stimulator (Cibertec, Madrid).
Classical Conditioning.
Classical conditioning of eyelid movements was achieved by a trace-conditioning paradigm. For this, animals were presented with a train (100 ms, 200 Hz) as CS, followed 250 ms later by an air puff (100 ms, 3 kg/cm2) as US. CSs applied to the whisker pad were presented on the same (left) side as the US, whereas CSs applied to S1 (vibrissal or hind-limb) areas were presented to the contralateral (right) side. CSs applied to the whisker pad or to the S1 area for vibrissae were adjusted to 1.5 × threshold for evoking an EMG response from vibrissal intrinsic muscles. CSs applied to the S1 area for the hind limb were adjusted to the same value as selected for the vibrissal S1 area.
The conditioning session consisted of 66 trials separated at random by intervals of 50–70 s. Six of the 66 trials were test trials in which the CS was presented alone. The daily conditioning session lasted for ≈80 min, and each animal was trained for 10 (Fig. 2) or 12 (Figs. 3 and 4) successive days. An animal was considered conditioned when it was able to produce 80% of CRs per session to the CS–US-paired presentation.
Pseudoconditioning sessions also consisted of 66 trials, separated at random by intervals of 50–70 s. For each trial, the CS was presented unpaired in relation to the US, the only restriction being that no more than two CS or US trials occurred sequentially (20). The total training per session for pseudoconditioning was the same as for conditioning.
Histology.
At the end of the recording sessions, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with saline and 4% paraformaldehyde. The proper location of the lid recording-coil and EMG electrodes was then checked. To confirm the final location of the electrodes implanted in S1, the brain was removed and cut into slices (50 μm), and the relevant cortical areas were processed for Toluidine blue staining.
Data Collection and Analysis.
The horizontal and vertical positions of the upper eyelid, the unrectified EMG activity of the recorded muscles, and 1-V rectangular pulses corresponding to CSs and USs presented during conditioning sessions were stored digitally on an eight-channel videotape recording system. Data were transferred later through an analog-to-digital converter (CED 1401-plus; CED, Cambridge, U.K.) to a computer for quantitative off-line analysis. Data were sampled at 1,000 (for eyelid position) or 4,000 (for EMG and field potential recordings) Hz, with an amplitude resolution of 12 bits. Computer programs (spike2 and sigavg from CED) were used to determine eyelid position, velocity, and acceleration and to display EMG activity of the muscles. These programs also allowed the quantification, with the aid of cursors, of the onset latency, latency to the peak, and amplitude and peak velocity of the eyelid displacement, and the onset latency, peak amplitude, and area of the rectified EMG activity of the orbicularis oculi muscle. A systematic average of recorded EMG and eyelid position recording was carried out for all habituation, conditioning, and recall sessions. The power spectra of eyelid movements were calculated from the corresponding acceleration (20, 31). Briefly, acceleration recordings were divided into segments of 1,024 s, starting at CS presentation. Segments containing CRs were selected exclusively from those obtained during the presentation of the CS alone.
Statistical analyses were carried out by using the spss package (SPSS, Chicago) for a statistical significance level of P = 0.05. Unless otherwise indicated, mean values were calculated from ≥100 measurements, collected from a minimum of two animals. Statistical differences of mean values were determined by two-way (group and session) ANOVA. Peaks of power spectra were tested with the χ2-distributed test for spectral density functions. Pearson correlation coefficients were determined for each pair of power spectra (Fig. 5).
Acknowledgments
We thank Ms. Mónica Cadenas and Ms. María Esteban for technical assistance and Mr. Roger Churchill for editorial help. This work was supported by Spanish Ministerio de Ciencia y Tecnología Grants BFU2005-01924 and BFU2005-02512 and Junta de Andalucía Grant CTS-168.
Abbreviations
- CS
conditioned stimulus
- CR
conditioned response
- EMG
electromyographic
- S1
primary somatosensory cortex
- US
unconditioned stimulus.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lafuente V. de, Romo R. Nat. Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 2.Romo R., Salinas E. Annu. Rev. Neurosci. 2001;24:107–137. doi: 10.1146/annurev.neuro.24.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Wilent W. B., Contreras D. Nat. Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- 4.Romo R., Hernández A., Zainos A., Salinas E. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 5.Salzman C. D., Britten K. H., Newsome W. T. Nature. 1990;346:174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- 6.Clark R. E., Squire R. L. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum H. Nat. Neurosci. 1999;2:775–776. doi: 10.1038/12137. [DOI] [PubMed] [Google Scholar]
- 8.Weible A. P., McEchron M. D., Disterhoft J. F. Behav. Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- 9.Woody C. D., Brozek G. J. Neurophysiol. 1969;32:717–726. doi: 10.1152/jn.1969.32.5.717. [DOI] [PubMed] [Google Scholar]
- 10.Gruart A., Morcuende S., Martínez S., Delgado-García J. M. Neuroscience. 2000;100:719–730. doi: 10.1016/s0306-4522(00)00325-0. [DOI] [PubMed] [Google Scholar]
- 11.Wikgren J., Ruusuvirta T., Korhonen T. Neurosci. Lett. 2003;341:119–122. doi: 10.1016/s0304-3940(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 12.Doty R. W., Rutledge L. T. J. Neurophysiol. 1959;22:428–435. doi: 10.1152/jn.1959.22.4.428. [DOI] [PubMed] [Google Scholar]
- 13.Das S., Weiss C., Disterhoft J. F. Behav. Neurosci. 2001;115:731–736. doi: 10.1037//0735-7044.115.3.731. [DOI] [PubMed] [Google Scholar]
- 14.Troncoso J., Múnera A., Delgado-García J. M. Learn. Mem. 2004;11:724–726. doi: 10.1101/lm.81204. [DOI] [PubMed] [Google Scholar]
- 15.Swadlow H. A. J. Neurophysiol. 1990;63:1477–1498. doi: 10.1152/jn.1990.63.6.1477. [DOI] [PubMed] [Google Scholar]
- 16.Swadlow H. A., Beloozerova I. N., Sirota M. G. J. Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- 17.Gould H. J., III J. Comp. Neurol. 1986;243:207–233. doi: 10.1002/cne.902430206. [DOI] [PubMed] [Google Scholar]
- 18.Loucks R. B. J. Psychol. 1935;1:5–44. [Google Scholar]
- 19.Alonso J. M., Swadlow H. A. J. Neurophysiol. 2005;94:26–32. doi: 10.1152/jn.01281.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gruart A., Schreurs B. G., Domínguez del Toro E., Delgado-García J. M. J. Neurophysiol. 2000;83:836–852. doi: 10.1152/jn.2000.83.2.836. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein A. L. J. Gen. Psychol. 1934;10:173–197. [Google Scholar]
- 22.Smith M. C. J. Comp. Physiol. Psychol. 1968;66:679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- 23.Harris J. A., Karlov L., Clifford W. G. J. Neurosci. 2006;26:948–952. doi: 10.1523/JNEUROSCI.4318-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman D. E., Brecht M. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 25.Bach-y-Rita P. Ann. N.Y. Acad. Sci. 2004;1013:83–91. doi: 10.1196/annals.1305.006. [DOI] [PubMed] [Google Scholar]
- 27.Ptito M., Moesgaard S. M., Gjedde A., Kupers R. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- 26.Strupp M., Arbosow V., Dietrich M., Sautier W., Brandt T. Brain. 1998;121:677–685. doi: 10.1093/brain/121.4.677. [DOI] [PubMed] [Google Scholar]
- 28.Hartings J. A., Temereanca S., Simons D. J. J. Neurophysiol. 2000;83:2791–2801. doi: 10.1152/jn.2000.83.5.2791. [DOI] [PubMed] [Google Scholar]
- 29.Delgado-García J. M., Gruart A. Cerebellum. 2002;1:289–308. doi: 10.1080/147342202320883597. [DOI] [PubMed] [Google Scholar]
- 30.Girgis M., Shih-Chang W. St. Louis: Green; 1981. A New Stereotaxic Atlas of the Rabbit Brain. [Google Scholar]
- 31.Domingo J. A., Gruart A., Delgado-García J. M. J. Neurophysiol. 1997;78:2518–2530. doi: 10.1152/jn.1997.78.5.2518. [DOI] [PubMed] [Google Scholar]