Abstract
Familial Mediterranean fever (FMF) is a recessively inherited autoinflammatory disorder with high carrier frequencies in the Middle East. Pyrin, the protein mutated in FMF, regulates caspase-1 activation and consequently IL-1β production through cognate interaction of its N-terminal PYRIN motif with the ASC adaptor protein. However, the preponderance of mutations reside in pyrin’s C-terminal B30.2 domain. Here we demonstrate direct interaction of this domain with caspase-1. In lysates from cells not expressing ASC, reciprocal GST pull-downs demonstrated the interaction of pyrin with the p20 and p10 catalytic subunits of caspase-1. Coimmunoprecipitations of pyrin and caspase-1 from THP-1 human monocytic cells were consistent with the interaction of endogenous proteins. The C-terminal B30.2 domain of pyrin is necessary and sufficient for the interaction, and binding was reduced by FMF-associated B30.2 mutations. Full-length pyrin attenuated IL-1β production in cells transfected with a caspase-1/IL-1β construct, an effect diminished by FMF-associated B30.2 mutations and in B30.2 deletion mutants. Modeling of the crystal structure of caspase-1 with the deduced structure of the pyrin B30.2 domain corroborated both the interaction and the importance of M694V and M680I pyrin mutations. Consistent with a net inhibitory effect of pyrin on IL-1β activation, small interfering RNA (siRNA)-mediated pyrin knockdown in THP-1 cells augmented IL-1β production in response to bacterial LPS. Moreover, the IL-1 receptor antagonist anakinra suppressed acute-phase proteins in a patient with FMF and amyloidosis. Our data support a direct, ASC-independent effect of pyrin on IL-1β activation and suggest heightened IL-1 responsiveness as one factor selecting for pyrin mutations.
Keywords: autoinflammatory disorder, ASC, siRNA, anakinra, structure
Familial Mediterranean fever (FMF, MIM249100) is the prototype of a group of disorders, termed systemic autoinflammatory diseases, characterized by seemingly unprovoked episodes of inflammation without evidence of high-titer autoantibodies or antigen-specific T cells (1, 2). FMF is recessively inherited and presents with 1- to 3-day attacks of fever accompanied by sterile peritonitis, pleurisy, rash, and/or arthritis (3). During attacks there is a substantial influx of polymorphonuclear leukocytes into the affected tissues. In some patients the ectopic deposition of misfolded fragments of serum amyloid A leads to renal failure and death.
The gene mutated in FMF (designated MEFV for Mediterranean fever) was identified by positional cloning (4, 5), and to date >50 FMF-associated MEFV mutations have been identified (3). FMF carrier frequencies in the range of 1:3 to 1:5 have been documented in a number of populations, including Jews, Arabs, Turks, Armenians, and Italians (3). The fact that these high carrier frequencies are found in multiple populations, with different mutations predominating in the various ethnic groups, strongly favors heterozygote selection over genetic drift as the likely explanation.
The protein product of MEFV, denoted pyrin (or marenostrin), is a 781-aa protein expressed in granulocytes, cytokine-activated monocytes, and serosal and synovial fibroblasts (6–8). A large percentage of FMF-associated pyrin mutations reside in the ≈200-residue C-terminal B30.2 (PRYSPRY, rfp) domain. B30.2 domains in other proteins are thought to mediate protein–protein interactions (9–12), although the precise role of pyrin’s B30.2 domain is unknown. In contrast, the ≈90 aa at the N terminus of pyrin define a motif, variously called the PYRIN domain (13, 14), PYD (15), PAAD (16), or DAPIN (17), that is rarely mutated in FMF, and assumes a six α-helical death-fold structure similar to death domains, death effector domains, and caspase recruitment domains (CARDs). The death-fold conformation permits cognate interactions, and a number of experimental systems have established that pyrin interacts with an adaptor protein denoted apoptosis-associated speck-like protein with a CARD (ASC) through homotypic interaction of their respective PYRIN domains (18, 19). ASC, in turn, plays an important role in regulating cytokine secretion, NF-κB activation, and apoptosis (20–22).
Of particular note, ASC has recently been shown to assemble in macromolecular complexes denoted “inflammasomes” (23–25), which contain one of at least three different members of the NALP (NACHT, leucine-rich repeat, PYRIN) protein family to activate pro-caspase-1 [IL-1β-converting enzyme (ICE)]. In the inflammasome, ASC interacts with one of the NALP proteins through cognate PYRIN domain interactions, and with pro-caspase-1 through homotypic CARD interactions. The inflammasome complex brings two molecules of pro-caspase-1 into close proximity, leading to autocatalysis and the subsequent release of the active catalytic p20 and p10 domains of caspase-1. Caspase-1, in turn, cleaves the 31-kDa precursor form of IL-1β into its biologically active 17-kDa fragment, a potent mediator of fever and inflammation.
The role of pyrin in IL-1β activation is controversial. Our own laboratory has provided several lines of evidence that pyrin can inhibit IL-1β activation (19). Transfection of full-length mouse pyrin into the murine RAW monocytic cell line suppresses IL-1β secretion, and peritoneal macrophages from mice expressing a truncated pyrin exhibit increased caspase-1 activation and IL-1β production, relative to WT controls. Moreover, in GST pull-down experiments, mouse ASC bound pyrin preferentially to caspase-1. These findings suggest that pyrin negatively regulates inflammasome activity by competing for ASC. On the other hand, in transfected 293T human embryonic kidney cells, pyrin may actually activate caspase-1 and IL-1β (26).
Whether pyrin inhibits or activates IL-1β through ASC, neither formulation adequately explains the proinflammatory effect and high frequency of mutations in the C-terminal B30.2 domain of pyrin. In this study we investigated the potential interaction of the B30.2 domain of pyrin with caspase-1 at a biochemical, structural, and functional level. We further explored the role of pyrin in IL-1 regulation through knockdown experiments and studies of an FMF patient receiving an IL-1 blocking agent. Taken together, our data provide a possible molecular explanation for the high frequency of pyrin B30.2 mutations in some populations.
Results
Direct Interaction of Pyrin with Caspase-1.
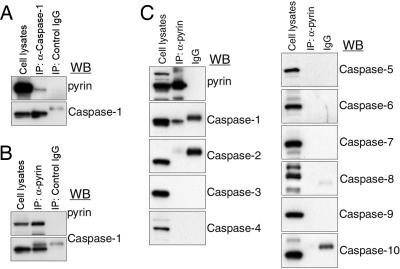
We first examined pyrin–caspase-1 interactions by coimmunoprecipitation assays on lysates from the THP-1 human monocytic leukemia cell line. Immune complexes precipitated with anti-caspase-1 Ab contained pyrin upon Western blot analysis, demonstrating an interaction between the endogenously expressed proteins (Fig. 1A). Conversely, the specificity of the pyrin–caspase-1 interaction was demonstrated by coimmunoprecipitation using anti-pyrin Ab followed by Western blot analysis using Abs specific for caspase-1 to caspase-10 (Fig. 1 B and C). In these immune complexes, only caspase-1 was detected, whereas caspase-2 to caspase-10 were not. However, it was not clear whether the pyrin–caspase-1 interaction is direct, because THP-1 cells, like many other cells expressing both pyrin and caspase-1, also express endogenous ASC, which can interact with both pyrin and caspase-1. Therefore, we also studied NIH 3T3-derived PT67 cells, which do not express ASC even by sensitive RT-PCR (Fig. 2A). In vivo GST pull-down analysis of lysates from PT67 cells cotransfected with GST–pyrin and myc-tagged caspase-1 demonstrated ASC-independent interaction (Fig. 2B, fourth lane).
Fig. 1.
The interaction of human pyrin with caspase-1. (A) Lysates from THP-1 cells expressing endogenous pyrin and caspase-1 were immunoprecipitated with anti-caspase-1 Ab or control IgG. Cell lysates and eluted proteins were subjected to Western blotting with a polyclonal Ab against the N-terminal 374 aa of human pyrin (Upper) or anti-caspase-1 Ab (Lower). IP, immunoprecipitation; WB, Western blot. (B) Lysates from THP-1 cells were immunoprecipitated with anti-pyrin Ab or control IgG. Cell lysates and eluted proteins were analyzed by Western blotting with anti-pyrin Ab (Upper) or anti-caspase-1 Ab (Lower). (C) Lysates from THP-1 cells were immunoprecipitated with anti-pyrin Ab. Lysates, eluted proteins, and control IgG were electrophoresed, followed by Western blotting with anti-pyrin Ab or Abs against caspase-1 to caspase-10.
Fig. 2.
The interaction of pyrin with the catalytic subunits of caspase-1. (A) RT-PCR analysis was performed on total RNA from LPS-treated mouse monocytes (first lane), PT67 cells (second lane), and distilled water control (DW, third lane) by using primers specific to mouse ASC (Upper) and mouse GAPDH (Lower). (B) The full-length, combined p20p10 fragment and p20 subunit of human caspase-1 (all with myc epitope tags) were cotransfected into PT67 cells with GST vector (first three lanes) or GST-fused human pyrin (last three lanes). Lysates were subjected to GST pull-down assay followed by Western blotting with anti-myc and anti-GST Abs (Top and Middle). Expression of caspase-1 is shown in Bottom (cell lysates). (C) Pyrin was cotransfected into PT67 cells with GST vector (first lane) or GST-fused p20 (second lane), p10 (third lane), or the CARD (fourth lane) of caspase-1, and lysates were analyzed as in B.
Binding Domains of Pyrin and Caspase-1.
Because the N-terminal PYRIN domain of pyrin is a member of the death-fold superfamily that also includes the CARD of caspase-1, it was possible that the binding of pyrin to caspase-1 is mediated by heterotypic interaction between the respective death-fold motifs. Nevertheless, GST pull-down analysis showed that pyrin can be coprecipitated with CARD-deleted caspase-1 and even can be coprecipitated with the p20 subunit of caspase-1 alone (Fig. 2B). For more refined mapping of the caspase-1 binding domain, we cotransfected pyrin with GST-fused p20 or p10 subunits or the CARD. Allowing for differences in p20 and p10 expression, pyrin binds both catalytic subunits but not the CARD of caspase-1 (Fig. 2C).
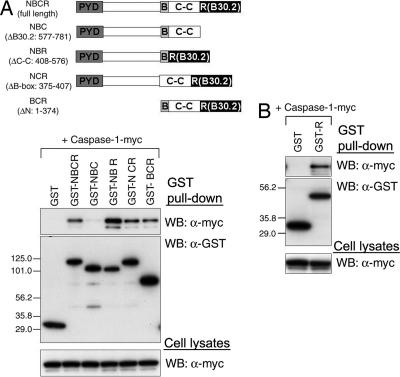
Conversely, to determine the binding domain of pyrin with caspase-1, we performed GST pull-down analysis using various GST-tagged, domain-deleted forms of pyrin and myc-tagged caspase-1. Caspase-1 was coprecipitated with pyrin deletion mutants for the N terminus, coiled-coil domain, or B-box (GST-BCR, GST-NBR, or GST-NCR, respectively), as well as with full-length pyrin (GST-NBCR), but only a small fraction of caspase-1 was coprecipitated with the B30.2 domain deletion mutant (GST-NBC) (Fig. 3A). In contrast, the GST-tagged B30.2 domain alone efficiently coprecipitated caspase-1 (Fig. 3B). These data indicate that the B30.2 domain is both necessary and sufficient for the direct interaction of pyrin with caspase-1.
Fig. 3.
Interaction of the B30.2 domain of human pyrin with caspase-1. (A) Human caspase-1 was cotransfected into PT67 cells with various N-terminal GST-fused pyrin-expressing constructs depicted in the schematic, as indicated. PYD, pyrin domain; B, B-box zinc finger domain; C-C, coiled-coil domain; R, B30.2 domain. Lysates were analyzed as in Fig. 2B. (B) Caspase-1 was cotransfected with GST vector (first lane) or GST-fused B30.2 domain of pyrin. Lysates were analyzed as in Fig. 2B.
Interaction of Caspase-1 with Pyrin Constructs Harboring FMF-Associated Mutations.
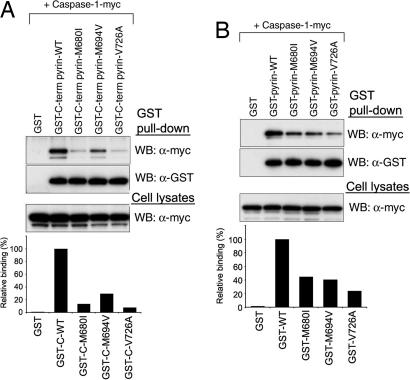
Because many of the major FMF-associated mutations are clustered in the B30.2 domain of pyrin, we considered the possible effect of these mutations on the binding to caspase-1. We first examined the binding by GST pull-down analysis using GST-tagged C-terminal constructs (amino acids 375–781; GST-BCR in Fig. 3A) of WT pyrin or the M680I, M694V, or V726A mutants. The binding of caspase-1 to the mutants was substantially decreased, relative to WT pyrin, as shown on Western blots and corresponding densitometry (Fig. 4A). Similar results were observed in the experiment using GST-tagged full-length constructs of WT pyrin and the three FMF mutants (Fig. 4B).
Fig. 4.
The binding of the B30.2 domain of human pyrin to caspase-1 is reduced by FMF-associated mutations. (A) Myc-tagged human caspase-1 was cotransfected with GST-fused WT or FMF-associated mutant C-terminal (amino acids 375–781) pyrin into PT67 cells and analyzed as in Fig. 2B. Densitometric analysis of α-myc bands, normalized to the α-myc bands in cell lysates, is shown in the histogram below. (B) Myc-tagged caspase-1 was cotransfected with GST-fused WT or FMF-associated mutant full-length pyrin into PT67 cells and analyzed as in A.
Effect of the Pyrin–Caspase-1 Interaction on IL-1β Processing.
A major function of caspase-1 is the proteolytic activation of pro-IL-1β and subsequent secretion of IL-1β. Because pyrin binds to the p20 and p10 catalytic subunits of caspase-1, we hypothesized that pyrin might regulate caspase-1-mediated IL-1β cleavage and secretion. To examine the effect of pyrin on IL-1β processing, we generated a bicistronic construct (pIRES-ICE/IL1β), which expresses both caspase-1 and pro-IL-1β simultaneously. The same amount of pIRES-ICE/IL1β was cotransfected into PT67 cells with an increasing amount of the constructs expressing full-length or B30.2 domain-deleted (NBC) pyrin. Both pyrin and NBC suppressed IL-1β secretion, but full-length pyrin suppressed IL-1β secretion significantly more than NBC (P < 0.0005 at 3 μg of DNA transfected) (Fig. 5A). These results suggest that the effect of the direct interaction of pyrin with caspase-1 is inhibitory.
Fig. 5.
Inhibition of caspase-1-mediated IL-1β processing by human pyrin. (A) pIRES-IL1β/ICE or pIRES-ICE (3 μg) was cotransfected with various amounts of empty vector, WT, or FMF-associated mutant full-length pyrin into PT67 cells. After 48 h, cell culture medium was changed with fresh medium. Cell culture supernatants were collected 3 h later and assayed for IL-1β by ELISA. Data represent the mean ± SE from five complete independent experiments. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005. Western blots demonstrating increasing amounts of the transfected pyrin proteins are shown below. (B) Three micrograms of pIRES-IL1β/ICE, pIRES-IL1β, or pIRES-ICE were cotransfected with 2 μg of empty vector, WT, or FMF-associated mutant full-length pyrin into PT67 cells, and cell culture supernatants were collected and analyzed as in A. In the fifth lane, the caspase-1 inhibitor z-WEHD-fmk was added (at 20 μM) to the culture medium. Data represent the mean ± SE from three replicate experiments. Western blots for the three transfected proteins are shown in Middle. Bottom is a histogram of the amount inhibition of IL-1β in the culture supernatants relative to the inhibition with z-WEHD-fmk.
Because FMF-associated pyrin B30.2 mutants showed reduced binding to caspase-1, we hypothesized that these variants might not inhibit caspase-1 as much as WT pyrin. As shown in Fig. 5B, transfection of full-length WT pyrin inhibited IL-1β in supernatants 75% as efficiently as the caspase-1 inhibitor z-WEHD-fmk. Consistent with our hypothesis, cells transfected with the FMF-associated mutants produced IL-1β levels that were not significantly different from empty vector transfected controls.
A Model for the Interaction of Pyrin with Caspase-1.
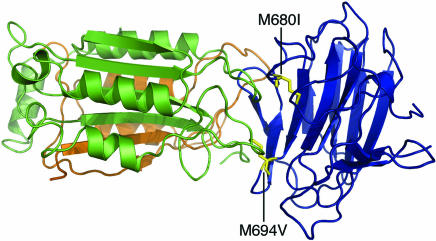
The structure of caspase-1 is well defined (27, 28), and models for the structure of the pyrin B30.2 domain have been recently reported (10–12). We thought to produce a model for the interaction of these structures computationally and analyze this interaction with regard to our experimental data. The two structures used were, for caspase-1, an inhibitor-free structure (Protein Data Bank ID code 1SC1) and, for the pyrin B30.2 domain, a model we generated based on the structure of PRYSPRY. These were subjected to rigid-body docking using the zdock program, and then the top-ranked complexes were refined and reranked by using the program rdock. The highest-scoring complexes used a common binding interface, at the ligand-binding site of caspase-1. The top-ranked model for this interaction was analyzed in more detail. The pyrin residues M694 and M680 were indeed located within the putative binding interface (Fig. 6); the side-chain atoms of both residues were within 5 Å of atoms of the receptor. This finding is in agreement with the potential B30.2/SPRY domain-binding interface identified by mutational analysis (10, 12). Examination of the orientation of caspase-1 in this model revealed also that both the p10 and p20 subunits of caspase-1 contribute to the interaction with pyrin, corroborating our experimental data (Fig. 2). In this modeled interaction, formation of the complex buries 2,761 Å2 of surface as compared with the uncomplexed proteins.
Fig. 6.
A model for the interaction of pyrin with caspase-1. A model for the B30.2 domain of pyrin (blue) was docked to the crystal structure of caspase-1 (p20, green; p10, brown). Two common mutations that cause FMF (M694V and M680I, yellow) are located in the putative binding interface.
Effect of MEFV Small Interfering RNA (siRNA) on IL-1β Secretion.
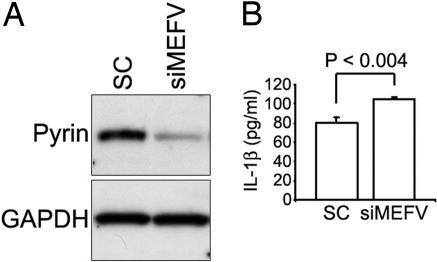
To investigate further the role of human pyrin in the regulation of IL-1β production, we developed double-stranded MEFV siRNAs. Fig. 7A shows the result of transfecting one such MEFV siRNA or a scramble-sequence control siRNA in THP-1 monocytic cells, which endogenously express pyrin, caspase-1, and ASC. The Western blot shows a marked and specific reduction of pyrin protein expression in cells transfected with MEFV siRNA. When these cells were stimulated with bacterial LPS for 4 h, there was a modest but highly significant increase in the secretion of IL-1β relative to cells transfected with the scramble-sequence control. The data suggest that the net effect of pyrin on the combination of ASC-dependent and ASC-independent IL-1β secretion is inhibitory.
Fig. 7.
Knockdown of endogenous pyrin in THP-1 cells. Cells were transfected with MEFV siRNA or a scrambled sequence dsRNA control and after 72 h were stimulated with 100 ng/ml LPS for 4 h. (A) Western blot of cell lysates probed with Abs to pyrin or GAPDH. (B) ELISA of culture supernatants for IL-1β. Data represent the mean ± SE for three replicate cultures. Data are from a representative experiment from six independent transfections.
Role of IL-1β Inhibition in FMF.
Although colchicine is the standard treatment for FMF, there are a few patients who do not respond or cannot tolerate its side effects. We recently treated one such FMF patient with the M694V/M694V genotype and systemic amyloidosis with anakinra, the IL-1 receptor antagonist, as adjunctive therapy. Monitoring of acute-phase reactants (Fig. 8, which is published as supporting information on the PNAS web site) demonstrated control of both the serum amyloid A and C reactive protein over several months, except when drug was discontinued during treatment of an infection.
Discussion
In this manuscript we provide evidence for a previously unrecognized, ASC-independent pathway by which pyrin, the protein mutated in FMF, regulates caspase-1 activation. Our data indicate that the C-terminal B30.2 domain of pyrin binds and inhibits the catalytic activity of the p20 and p10 caspase-1 subunits and that three common FMF-associated B30.2 mutations attenuate this effect (Fig. 9, which is published as supporting information on the PNAS web site). Computational docking of the crystal structure of caspase-1 (27, 28) with the modeled structure of the pyrin B30.2 domain (10–12) supports the likelihood of this interaction. Moreover, knockdown experiments corroborate a net inhibitory effect of pyrin on IL-1β processing, and clinical data also substantiate the importance of IL-1β in the pathogenesis of FMF.
IL-1β is a major mediator of fever and systemic inflammation (29, 30), and therefore it is not surprising that there is redundancy in the regulation of its production and activity. The naturally occurring IL-1 receptor antagonist is structurally similar to IL-1β and binds the IL-1 receptor without stimulating the accessory receptor necessary for effective signaling (31). Another highly regulated step is the caspase-1-dependent cleavage of pro-IL-1β into its biologically active 17-kDa fragment. Caspase-1 is thought to undergo autocatalysis by an “induced proximity” mechanism (32) through N-terminal CARD interactions. This process of caspase-1 activation can be mediated by RIP2/RICK/CARDIAK (33–35), Nod1 (36), and CARD12/CLAN/Ipaf (37–39) and also occurs on macromolecular scaffolds termed inflammasomes (23–25).
Under various experimental conditions (19, 26), pyrin itself can either inhibit or accentuate caspase-1 activity through the interaction of its N-terminal death-fold with ASC (19, 26), a key molecule in the inflammasome (23–25). This article demonstrates that pyrin also modulates caspase-1 activation through a second interaction involving the binding of its C-terminal B30.2 domain to the catalytic domains of caspase-1. The B30.2 domain is found in >500 different proteins of various functions and is thought to mediate protein–protein interactions (9–12). The B30.2 domain, in turn, comprises an ≈139-aa C-terminal SPRY segment (named after the dual-purpose splA kinase and the ryanodine receptor) and an ≈61-aa N-terminal PRY subdomain.
Recently, the structure of the SPRY domain of SSB-2 was determined by NMR (10), and the structures of a second SPRY domain and a B30.2 domain were solved by crystallography (11, 12). All three analyses indicated a β-sandwich structure formed by antiparallel β-sheets in the SPRY domain and showed that the residues equivalent to the M694V and M680I mutations in the pyrin B30.2 domain fall in distinct loops comprising a common binding surface. The computational docking studies reported here indicate that this surface of pyrin’s B30.2 domain can in fact interact with caspase-1 and that FMF-associated mutations disrupt this interaction. Our modeling did not predict a significant role for the V726A mutation in abrogating the pyrin–caspase-1 interaction. However, this mutation has been associated with a milder phenotype than the other two variants (3, 4), and it is possible that the effects we see in our biochemical and functional assays are the result of structural perturbations that are more apparent in vitro than in vivo.
Our knockdown data indicate that the overall effect of pyrin on IL-1β processing in human monocytes, taking into account both ASC-dependent and ASC-independent pathways, is inhibitory. We had previously proposed an ASC-dependent inhibitory effect of pyrin on IL-1β processing based on studies of mouse pyrin. Consistent with this hypothesis, others had shown in a 293T transfection system that human pyrin inhibits the interaction of ASC with cryopyrin and that pyrin inhibits the interaction of human ASC with caspase-8 (40, 41).
More recently, Yu et al. (26), using a 293T cell-based ASC–caspase-1 transfection system similar to the two previous studies, but stably expressing a more physiologic level of ASC, did not observe that pyrin inhibits cryopyrin-induced caspase-1 activation. Moreover, under these experimental conditions WT pyrin actually induced ASC-dependent caspase-1 activation and IL-1β secretion, but FMF-associated mutations did not increase or decrease this effect. It is noteworthy that another laboratory, also using the 293T transfection system, observed a potentiating effect of pyrin on caspase-1 activation when ASC was present, but an inhibitory effect of pyrin when pyrin was coexpressed with caspase-1 and IL-1β but without ASC (42).
Human embryonic kidney 293T cells may afford a number of advantages in their ease of transfection and passage in tissue culture. However, even if they are stably transfected with physiologic levels of ASC, they may not recapitulate all of the relevant interactions of endogenous proteins in leukocytes. In our previous work we demonstrated that transfection of MEFV into human U937 myeloid cells, which endogenously express ASC, pro-caspase-1, and IL-1β, inhibited LPS-induced IL-1β production relative to cells transfected with empty vector (19). In the present study we used THP-1, another human monocytic cell line that endogenously expresses all of the relevant molecular machinery of IL-1β secretion. Using siRNA specific for MEFV we observed dramatic and specific reduction of pyrin expression and a concomitant increase in LPS-induced IL-1β secretion.
Our data are consistent with a model in which WT pyrin inhibits caspase-1 activation, perhaps as part of a homeostatic loop induced by proinflammatory stimuli. By this formulation, FMF-associated mutations have less of an inhibitory effect and may have been selected in human history because of the resulting increase in innate immune responses. The beneficial effect of anakinra in an otherwise refractory FMF patient heightens interest in the role of pyrin in the regulation of cytokine processing.
Materials and Methods
GST Pull-Down Assay, Coimmunoprecipitation, and Western Blots.
To perform coimmunoprecipitation of endogenous pyrin and caspase-1, THP-1 cells were lysed with Mild lysis buffer (CytoSignal) and incubated with a polyclonal Ab against the N-terminal 374 aa of human pyrin or anti-caspase-1 Ab (Santa Cruz Biotechnology), which were crosslinked to protein A agarose beads (Pierce) for 16 h at 4°C. After washing the beads, bound proteins were eluted and subjected to Western blotting. For in vivo GST pull-down, WT, FMF-associated mutant, and domain-deleted MEFV and genes encoding each domain of caspase-1 were cloned into a mammalian GST expression vector (pEGST) and pcDNA3.1-myc/His (Invitrogen). Each GST-fused expression construct for pyrin and caspase-1 was transiently cotransfected into RetroPack PT67 cells (CLONTECH) with myc-tagged caspase-1 and pyrin expression constructs, respectively, using Lipofectamine 2000 reagent (Invitrogen). After 24 h, cells were lysed with Strong lysis buffer (CytoSignal) and incubated with GST beads (Amersham Pharmacia) for 16 h at 4°C. Cell lysates or eluted samples obtained from GST pull-down and coimmunoprecipitation were subjected to Western blotting. Western blots were performed by using primary Abs: anti-pyrin Ab; anti-caspase-1, -4, -5, and -6 Abs (Santa Cruz Biotechnology); anti-caspase-2, -3, -7, -8, -9, and -10 Abs (R & D Systems); anti-GST Ab (Amersham Pharmacia); and anti-myc Ab (Santa Cruz Biotechnology).
RT-PCR.
RT-PCR analysis was performed on total RNA from PT67 cells and LPS-treated mouse monocytes using a forward primer (5′-ATGGGGCGGGCACGAGATG-3′) and a reverse primer (5′-GCTCTGCTCCAGGTCCATCAC-3′) for the detection of mouse ASC expression and mouse G3PDH control amplimer set (CLONTECH).
Measurement of IL-1β Secretion.
Both or each of the genes encoding IL-1β and caspase-1 were subcloned into the bicistronic mammalian expression vector pIRES (CLONTECH) and denoted pIRES-IL1β/ICE, pIRES-IL1β, or pIRES-ICE. These constructs were cotransfected into PT67 cells with various amounts of pMEFV-Myc and pNBC-Myc or 2 μg of mutant pyrin constructs (pM680I-Myc, pM694V-Myc, and pV726A-Myc), as indicated. After 48 h, cell culture medium was changed with fresh medium, and cell culture supernatants were collected 3 h later for IL-1β ELISA (R & D Systems), performed according to the manufacturer’s instructions.
siRNA Studies.
siRNA duplex (sequence CTCTGCTGGTCACCTACTA) corresponding to pyrin was designed by Dharmacon. The negative control scramble-sequence siRNA was purchased from Ambion. THP-1 cells were transfected with siRNA (100 nM) by using Lipofectamine 2000 (Invitrogen), incubated for 72 h, and then cultured an additional 4 h with LPS (100 ng/ml). Cell lysates and supernatants were then collected for Western blotting and IL-1β ELISA, respectively.
Computational Analysis of the Interaction of Pyrin with Caspase-1.
The modeller program (version 6v2) (43) was used to produce homology models of the pyrin B30.2 domain based on the structure of PRYSPRY (Protein Data Bank ID code 2FBE) as a template. An alignment for the sequences of these proteins was obtained by using the program fugue (44). Twenty-five models were then created, and the model with lowest modeller objective function was used for docking with the receptor. The zdock (45) program was used to perform rigid-body docking of the pyrin B30.2 domain model as the ligand, with caspase-1 as the receptor, producing 2,000 possible interactions ranked according to their structural and electrostatic complementarity. The C-terminal residues projecting from the p10 subunit of caspase-1 (V292 to D297) were removed because this region is likely to be flexible. The alanine mutation at position 285 was converted to the native cysteine. The highest-scoring initial 200 models were subjected to molecular mechanics refinement and redocking using the rdock approach (46). The interaction energy in the refined models was evaluated by using a scoring function including desolvation and electrostatic terms. The buried surface area was calculated from the difference in solvent-accessible surface areas of the uncomplexed and complexed structures by using the program naccess (version 2.1.1) (47).
Supplementary Material
Abbreviations
- FMF
familial Mediterranean fever
- CARD
caspase recruitment domain
- ICE
IL-1β-converting enzyme
- siRNA
small interfering RNA.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Galon J., Aksentijevich I., McDermott M. F., O’Shea J. J., Kastner D. L. Curr. Opin. Immunol. 2000;12:479–486. doi: 10.1016/s0952-7915(00)00124-2. [DOI] [PubMed] [Google Scholar]
- 2.Stojanov S., Kastner D. L. Curr. Opin. Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 3.Kastner D. L., Aksentijevich I. Arthritis and Allied Conditions. In: Koopman W. J., Moreland L. W., editors. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 1411–1461. [Google Scholar]
- 4.International FMF Consortium. Cell. Vol. 90. 1997. pp. 797–807. [DOI] [PubMed] [Google Scholar]
- 5.French FMF Consortium. Nat. Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 6.Centola M., Wood G., Frucht D. M., Galon J., Aringer M., Farrell C., Kingma D. W., Horwitz M. E., Mansfield E., Holland S. M., et al. Blood. 2000;95:3223–3231. [PubMed] [Google Scholar]
- 7.Matzner Y., Abedat S., Shapiro E., Eisenberg S., Bar-Gil-Shitrit A., Stepensky P., Calco S., Azar Y., Urieli-Shoval S. Blood. 2000;96:727–731. [PubMed] [Google Scholar]
- 8.Diaz A., Hu C., Kastner D. L., Schaner P., Reginato A. M., Richards N., Gumucio D. L. Arthritis Rheum. 2004;50:3679–3689. doi: 10.1002/art.20600. [DOI] [PubMed] [Google Scholar]
- 9.Henry J., Mather I. H., McDermott M. F., Pontarotti P. Mol. Biol. Evol. 1998;15:1696–1705. doi: 10.1093/oxfordjournals.molbev.a025896. [DOI] [PubMed] [Google Scholar]
- 10.Masters S. L., Yao S., Willson T. A., Zhang J. G., Palmer K. R., Smith B. J., Babon J. J., Nicola N. A., Norton R. S., Nicholson S. E. Nat. Struct. Mol. Biol. 2006;13:77–84. doi: 10.1038/nsmb1034. [DOI] [PubMed] [Google Scholar]
- 11.Grutter C., Briand C., Capitani G., Mittl P. R., Papin S., Tschopp J., Grutter M. G. FEBS Lett. 2006;580:99–106. doi: 10.1016/j.febslet.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 12.Woo J. S., Imm J. H., Min C. K., Kim K. J., Cha S. S., Oh B. H. EMBO J. 2006;25:1353–1363. doi: 10.1038/sj.emboj.7600994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertin J., DiStefano P. S. Cell Death Differ. 2000;7:1273–1274. doi: 10.1038/sj.cdd.4400774. [DOI] [PubMed] [Google Scholar]
- 14.Fairbrother W. J., Gordon N. C., Humke E. W., O’Rourke K. M., Starovasnik M. A., Yin J. P., Dixit V. M. Protein Sci. 2001;10:1911–1918. doi: 10.1110/ps.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F., Hofmann K., Tschopp J. Curr. Biol. 2001;11:R118–R120. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 16.Pawlowski K., Pio F., Chu Z., Reed J. C., Godzik A. Trends Biochem. Sci. 2001;26:85–87. doi: 10.1016/s0968-0004(00)01729-1. [DOI] [PubMed] [Google Scholar]
- 17.Staub E., Dahl E., Rosenthal A. Trends Biochem. Sci. 2001;26:83–85. doi: 10.1016/s0968-0004(00)01717-5. [DOI] [PubMed] [Google Scholar]
- 18.Richards N., Schaner P., Diaz A., Stuckey J., Shelden E., Wadhwa A., Gumucio D. L. J. Biol. Chem. 2001;276:39320–39329. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 19.Chae J. J., Komarow H. D., Cheng J., Wood G., Raben N., Liu P. P., Kastner D. L. Mol. Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 20.Masumoto J., Taniguchi S., Ayukawa K., Sarvotham H., Kishino T., Niikawa N., Hidaka E., Katsuyama T., Higuchi T., Sagara J. J. Biol. Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasula S. M., Poyet J. L., Razmara M., Datta P., Zhang Z., Alnemri E. S. J. Biol. Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 22.Stehlik C., Fiorentino L., Dorfleutner A., Bruey J. M., Ariza E. M., Sagara J., Reed J. C. J. Exp. Med. 2002;196:1605–1615. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinon F., Burns K., Tschopp J. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 24.Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F., Tschopp J. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Yu J. W., Wu J., Zhang Z., Datta P., Ibrahimi I., Taniguchi S., Sagara J., Fernandes-Alnemri T., Alnemri E. S. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 27.Wilson K. P., Black J. A., Thomson J. A., Kim E. E., Griffith J. P., Navia M. A., Murcko M. A., Chambers S. P., Aldape R. A., Raybuck S. A., et al. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 28.Romanowski M. J., Scheer J. M., O’Brien T., McDowell R. S. Structure (London) 2004;12:1361–1371. doi: 10.1016/j.str.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello C. A. J. Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello C. A. Crit. Care Med. 2005;33:S460–S462. doi: 10.1097/01.ccm.0000185500.11080.91. [DOI] [PubMed] [Google Scholar]
- 31.Arend W. P., Malyak M., Guthridge C. J., Gabay C. Annu. Rev. Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 32.Salvesen G. S., Dixit V. M. Proc. Natl. Acad. Sci. USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inohara N., del Peso L., Koseki T., Chen S., Nunez G. J. Biol. Chem. 1998;273:12296–12300. doi: 10.1074/jbc.273.20.12296. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy J. V., Ni J., Dixit V. M. J. Biol. Chem. 1998;273:16968–16975. doi: 10.1074/jbc.273.27.16968. [DOI] [PubMed] [Google Scholar]
- 35.Thome M., Hofmann K., Burns K., Martinon F., Bodmer J. L., Mattmann C., Tschopp J. Curr. Biol. 1998;8:885–888. doi: 10.1016/s0960-9822(07)00352-1. [DOI] [PubMed] [Google Scholar]
- 36.Yoo N. J., Park W. S., Kim S. Y., Reed J. C., Son S. G., Lee J. Y., Lee S. H. Biochem. Biophys. Res. Commun. 2002;299:652–658. doi: 10.1016/s0006-291x(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 37.Damiano J. S., Stehlik C., Pio F., Godzik A., Reed J. C. Genomics. 2001;75:77–83. doi: 10.1006/geno.2001.6579. [DOI] [PubMed] [Google Scholar]
- 38.Geddes B. J., Wang L., Huang W. J., Lavellee M., Manji G. A., Brown M., Jurman M., Cao J., Morgenstern J., Merriam S., et al. Biochem. Biophys. Res. Commun. 2001;284:77–82. doi: 10.1006/bbrc.2001.4928. [DOI] [PubMed] [Google Scholar]
- 39.Poyet J. L., Srinivasula S. M., Tnani M., Razmara M., Fernandes-Alnemri T., Alnemri E. S. J. Biol. Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 40.Dowds T. A., Masumoto J., Chen F. F., Ogura Y., Inohara N., Nunez G. Biochem. Biophys. Res. Commun. 2003;302:575–580. doi: 10.1016/s0006-291x(03)00221-3. [DOI] [PubMed] [Google Scholar]
- 41.Masumoto J., Dowds T. A., Schaner P., Chen F. F., Ogura Y., Li M., Zhu L., Katsuyama T., Sagara J., Taniguchi S., et al. Biochem. Biophys. Res. Commun. 2003;303:69–73. doi: 10.1016/s0006-291x(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 42.Stehlik C., Lee S. H., Dorfleutner A., Stassinopoulos A., Sagara J., Reed J. C. J. Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 43.Sali A., Blundell T. L. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 44.Shi J., Blundell T. L., Mizuguchi K. J. Mol. Biol. 2001;310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- 45.Chen R., Li L., Weng Z. Proteins. 2003;52:80–87. doi: 10.1002/prot.10389. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Chen R., Weng Z. Proteins. 2003;53:693–707. doi: 10.1002/prot.10460. [DOI] [PubMed] [Google Scholar]
- 47.Hubbard S. L, Thornton J. M. London: University College London; 1993. naccess, version 2.1.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.