Abstract
Although the function of sleep remains elusive, there is compelling evidence to suggest that sleep plays an important role in learning and memory. A number of studies have now shown that sleep deprivation (SD) results in significant impairment of long-term potentiation (LTP) in the hippocampus. In this study, we have attempted to determine the mechanisms responsible for this impairment. After 72 h SD using the multiple-platform technique, we observed a reduction in the whole-cell recorded NMDA/AMPA ratio of CA1 pyramidal cells in response to Schaffer collateral stimulation. This impairment was specific to sleep deprivation as rats placed over a single large platform, which allowed sleep, had a normal NMDA/AMPA ratio. mEPSCs evoked by local application of a high osmolarity solution revealed no differences in the AMPA receptor function. NMDA currents recorded from outside-out patches excised from the distal dendrites of CA1 cells displayed a reduction in amplitude after SD. While there were no alterations in the glutamate sensitivity, channel open probability or the single channel conductance of the receptor, a crosslinking assay demonstrated that the NR1 and NR2A subunits of NMDA receptors were preferentially retained in the cytoplasm after SD, indicating that SD alters NMDAR surface expression. In summary, we have identified a potential mechanism underlying SD-induced LTP impairment. This synaptic alteration may underlie the cognitive deficits seen following sleep deprivation and could represent a target for future intervention studies.
There is still significant controversy regarding the function of sleep and many theories have been set forth. One that is gaining widespread acceptance is the idea that sleep is involved in learning and memory processes. Support for this theory comes from experiments showing the reorganization of sleep states after learning (Smith & Lapp, 1986; Mandai et al. 1989) and the deleterious effect of sleep deprivation on subsequent learning (Stern, 1971; Karni et al. 1994; Smith, 1995; Youngblood et al. 1997; Ruskin et al. 2004). This idea is further substantiated by multiple demonstrations, across many species, that activity related neuronal firing sequences are replayed during sleep (Pavlides & Winson, 1989; Wilson & McNaughton, 1994; Skaggs & McNaughton, 1996; Kudrimoti et al. 1999; Nadasdy et al. 1999; Maquet et al. 2000, 2003; Dave & Margoliash, 2000; Louie & Wilson, 2001; Poe et al. 2000; Lee & Wilson, 2002). This replay is strongly suggestive of memory trace reactivation during sleep. It has also now been demonstrated in a number of studies that sleep deprivation (SD), induced using a variety of methods, affects long-term potentiation (LTP) of synaptic transmission in the hippocampus, an area crucial for the encoding and storing of memories (Campbell et al. 2001; McDermott et al. 2003; Davis et al. 2003; Romcy-Pereiro & Pavlides, 2004). As synaptic plasticity is believed to underlie memory formation, this may be the mechanism responsible for sleep deprivation-induced cognitive impairments. In this study, we have sought to determine the synaptic mechanisms of the SD-induced inhibition of LTP.
In the CA1 region of the hippocampus, LTP is induced by patterns of activity that activate the NMDA subtype of glutamate receptors. Prolonged depolarization relieves a voltage-dependent Mg2+ block from the channel and allows it to conduct. Calcium influx through these channels is associated with the activation of a number of kinases (Malinow et al. 1989; Malenka et al. 1989; Wang & Kelly, 1995), which ultimately lead to an increase in the postsynaptic response through either an increase in the number of AMPA receptors at the synapse (Hayashi et al. 2000; Andrásfalvy & Magee, 2004) or an increase in channel conductance (Benke et al. 1998; Poncer et al. 2002).
We have found here that 72 h SD, a treatment that reduces LTP at the Schaffer collateral synapse also reduces the NMDA current at this synapse. This effect is not caused by any alteration in NMDAR channel properties, but is associated with an increase in the intracellular pool of the NR1 and NR2A NMDAR subunits in this region. Our data indicate that SD results in a lower density of surface NMDA receptors. We have further found that a modification that enhances NMDAR activity restores the ability of these synapses to undergo potentiation. These observations may be responsible for the reduced ability to induce LTP at these synapses, and by extension, sleep deprivation-induced memory impairments.
Methods
Animals
Male Sprague-Dawley rats (8–12 weeks) were purchased from Charles River Laboratory. Animals were maintained in a temperature-controlled environment on a 12-h light–dark cycle, with free access to food and water. All procedures were carried out within the guidelines of the LSUHSC Animal Care and Use Committee.
Sleep deprivation
We used the multiple platform method of sleep deprivation (SD). In this technique, rats are placed in a chamber with five small platforms (6.2 cm diameter) surrounded by water as previously described (McDermott et al. 2003). When they enter REM sleep, their muscle tone diminishes and causes them to touch the water, which arouses them. Because the animals can move from platform to platform within the multiplatform chamber, this device has been reported to produce less immobilization stress compared with the widely used single small platform technique (Coenen & Van Luijtelaar, 1985; McDermott et al. 2003). We also tested the impact of a chamber containing a single platform that is large enough for the rats to sleep on but still not large enough for them to walk around on. This Large Platform (LP) device should produce whatever alterations result from non-specific platform effects.
The water reached up to approximately 2 cm below the surface of the platforms and food and water were continuously available. All treatments lasted 72 h as preliminary experiments have shown that the SD-induced impairment of LTP was maximal at 72 h (McDermott et al. 2003).
Slice preparation
Hippocampal slices (400 μm) were prepared using standard procedures that have been previously described (Magee, 1998). Rats were given a lethal dose of a ketamine–zylazine mix and just before death were perfused rapidly through the ascending aorta with an oxygenated salt solution (2°C). After removal of the brain, slices were cut on a Vibratome, incubated in a submerged holding chamber for ∼30 min at 35°C and stored at room temperature (∼22°C) for the remainder of the experiment. Individual slices were transferred as needed from the holding chamber to a submerged recording chamber. Individual neurones were visualized using a Zeiss Axioskop fitted with differential interference contrast (DIC) optics using infrared illumination.
Extracellular recordings
Extracellular recordings were made from stratum radiatum approximately 200 μm from the pyramidal cell body layer. The external solution contained (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, 1.3 MgCl2, 25 dextrose, and 0.01 glycine. The recording electrode was also filled with external solution. A bipolar tungsten stimulating electrode was placed in the middle of stratum radiatum within 200 μm of the recording electrode to stimulate the Schaffer collateral fibres. The amplitude used for the test pulse was approximately half of the maximum. LTP was induced with two 100 Hz stimulus trains of 1 s duration at threshold stimulus with a 30 s interval. A stable baseline was maintained for 10 min before LTP induction and recordings were made for 30 min after.
Outside-out patches
Outside-out patches were excised from apical dendrites approximately 240–280 μm from the soma. Pipettes with a resistance of 5–10 MΩ and an estimated tip diameter of ∼1.5–2 μm contained (mm): 140 KMeSO4, 0.5 EGTA, 10 Hepes, 4 NaCl, 0.28 CaCl2, 4 Mg2ATP, 0.3 Tris2GTP, 14 phosphocreatine. NMDA currents were evoked using a rapid application system as described below. Currents were recorded at −70 mV, filtered at 1 kHz and digitized at 10 kHz.
Fast application
Double-barrelled pipettes fabricated from theta glass tubing were used for fast application of control and agonist solutions to the patch (Andrásfalvy & Magee, 2001). Solutions were perfused through control and agonist barrels (at a rate of 0.3 ml min−1) by means of a multilined peristaltic pump. When patches were pulled they were immediately placed in front of the control barrel. The puffer solution contained (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 10 Hepes, 2.5 CaCl2, 50 dextrose; 5 μm 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxa-line-7-sulfonamide (NBQX: Tocris, Ellisville, MO, USA), 10 μm glycine and 10–1000 μm glutamate were added daily. To apply agonist to the patch, the agonist barrel was moved in front of the patch by means of a piezoelectric element. Puffer solutions containing different concentrations of agonists and/or antagonists were exchanged using solenoid valves. After each patch recording, the application system was tested by rupturing the patch and measuring the open tip current caused by a jump from a 10 to a 100% puffer external solution. The 20–80% exchange time varied between 150 and 250 μs.
Synaptic versus extrasynaptic
The origin of receptors in outside out patches is still a matter of debate. Previous attempts to characterize these NMDA receptors concluded that they are mainly synaptic receptors (Andrásfalvy & Magee, 2001). However, outside-out patch currents were more affected than synaptic currents by the loss of the GluR1 subunit of AMPA receptors, which it is thought are localized to the extrasynaptic membrane (Andrásfalvy et al. 2003). This suggests that the patches still contain a proportion of extrasynaptic receptors. Despite that, distance-dependent increases in AMPA receptor density are preserved from synaptic to patch currents (Andrásfalvy & Magee, 2001) suggesting that synaptic changes may be mimicked in the extracellular pool. Although there have been some reports that NMDA receptors in synaptic and extrasynaptic locations differ in their subunit composition, Tovar & Westbrook (2002) demonstrated a lateral movement of receptors into the synapse without any obvious alteration in subunit composition.
Dose–response curve
Varying concentrations of glutamate were used to construct a dose–response curve for activation of NMDA receptors in response to a 10 ms application. Peak current amplitudes were normalized to the current at the maximum concentration and plotted against glutamate concentration. Concentration–response data were fitted by the Hill equation: effect = effectmax/[1 + (EC50/C)n], where C is the agonist concentration, n is the Hill coefficient, and EC50 is the concentration at which the half-maximal response is obtained.
Single-channel conductance
A subsaturating concentration of glutamate (10 μm for 10 ms) was used to isolate single channel activity. An all-points histogram was created for sweeps showing single channel activity and each peak was fitted with a gaussian distribution. Sweeps were made at a range of concentrations and a current–voltage plot constructed. The single channel conductance was calculated from the slope of a straight line fitted to the points of the I–V plot.
MK801 experiments
The anticonvulsant MK-801 (Huettner & Bean, 1988) was used to determine the proportion of NMDA receptors that open during a single saturating (1 mm for 10 ms) application of glutamate. After collection of test traces, 20 μm MK-801 (Tocris, Ellisville, MO, USA) was added to both barrels of the puffer pipette and the patch was stepped once (10 ms) into glutamate. Test traces were again collected after removal of MK-801. As MK-801 is an open channel blocker, the percentage block incurred by this exposure to MK-801 gives an indication of the probability that a liganded channel will open.
Whole-cell recordings
AMPA mEPSCs
Single miniature synaptic events (mEPSCs) were evoked by pressure ejection of a hyperosmotic external solution and recorded locally from the distal dendrites in whole-cell voltage clamp mode (Smith et al. 2003). Recording pipettes (5–7 MΩ), were filled with (mm): 120 mm caesium glutamate, 20 CsCl, 0.5 EGTA, 10 Hepes, 4 NaCl, and 0.28 CaCl2. The external solution contained (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2 and 25 dextrose; 50 μm APV, 10 μm bicuculline (Sigma, St. Louis, MO, USA) and 0.5 μm tetrodotoxin (TTX) (Alomone Labs, Jerusalem, Israel) were added daily. For the external puffer solution, 10 mm Hepes replaced NaHCO3. Series resistance was 15–25 MΩ.
Currents were recorded at −70 mV, filtered at 5 kHz and digitized at 50 kHz. Miniature EPSCs crossing an approximate 2 pA threshold level were selected for further examination using a template fit algorithm written in Igor Pro (WaveMetrics, Inc., Lake Oswego, OR, USA). Events were fitted with a sum of two exponential functions to obtain peak amplitude, rise and decay time constants. Events that had rise-time constants greater than 400 μs were eliminated from analysis since these events were unlikely to be from local synapses (Magee & Cook, 2000). Amplitude histograms were constructed from between 50 and 200 (typically 100–150) unitary events.
NMDA/AMPA ratio
For measurement of NMDA currents Mg2+ was omitted from the external solution and [CaCl2] was increased to 2.5 mm. Slices were preincubated in 0 Mg2+ with 1 mm kynurenic acid for 2 h prior to recording. The total whole-cell EPSC was recorded first before addition of NBQX to isolate the NMDA current. The AMPA component was deduced by subtraction and the NMDA/AMPA ratio calculated.
NMDA potentiation
After collection of NMDA test traces, 10 μm glycine was added and traces were collected for a further 10–15 min. The amount of potentiation was calculated by dividing the average amplitude of the traces after the glycine by the average amplitude of the test traces before the glycine.
Cross-linking assay
Brains were rapidly removed, and hippocampal slices (400 μm) were cut on a Vibratome 3000 and placed in oxygenated artificial cerebrospinal fluid (aCSF). For cross-linking, slices were incubated in ice cold buffer containing 2 mg ml−1 bis(sulfosuccinimidyl) suberate (BS3) (Sigma, St. Louis, MO, USA) for 45 min to label extracellular protein. For measurement of total cellular protein, BS3 was omitted. The reaction was stopped by placing the slices in cold 20 mm Tris (pH 7.6). After washing, the hippocampus was isolated and placed in lysis buffer. The samples were sonicated and aliquots were taken for Western blot analysis. Proteins (10 μg per lane) were separated by SDS-PAGE using a 4–15% gel and transferred to PVDF membrane. Membranes were blocked in PBS containing 0.05% Tween 20 and 5% blocking agent from ECF Western blotting kit (Amersham, Piscataway, NJ, USA) for 1 h. Blots were incubated at 4°C with specific antibodies at 1: 2000 dilution. Antibodies (anti-NMDAR1, anti-NMDAR2A and anti-NMDAR2B) were purchased from Chemicon (Temecula, CA, USA). All other reagents were from the ECF kit. Imaging and quantification were preformed using the Molecular Dynamics, Piscataway, NJ, USA Typhoon 8600 with ImageQuant software. The intracellular protein was reported as a percentage of the total cellular protein.
Data analysis
Data shown represent mean +/− standard error of the mean. Data were compared using ANOVA or student's t test as appropriate.
Results
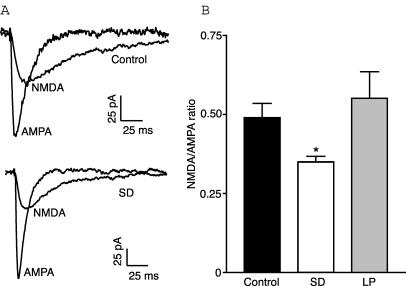
We have previously shown that sleep deprivation results in a significant impairment in the induction of LTP at Schaffer collateral synapses (McDermott et al. 2003). In the experiments outlined here we have attempted to determine the synaptic mechanisms responsible for this impairment. As the NMDA and AMPA subtypes of glutamate receptors are key components involved in the induction and expression of LTP, we first sought to determine whether there were any changes in their function. We examined the NMDA/AMPA ratio recorded from the soma in whole-cell voltage-clamp mode in response to stimulation of the Schaffer collateral pathway. In 0 Mg2+ the NMDA/AMPA ratio recorded in CA1 cells from multi-platform rats subjected to sleep deprivation (SD rats) (0.35 ± 0.2, n= 18) was significantly less than that recorded from control rats (0.5 ± 0.05, n= 19; F2,48= 4.052, P < 0.05, ANOVA) (Fig. 1). This reduction was not seen when rats were placed over a platform large enough to allow sleep (0.55 ± 0.09, n= 14) indicating that it is an effect specific to sleep deprivation. Since these large platform (LP) rats did not have an impairment in LTP either, as reported previously (McDermott et al. 2003), they were not investigated further. These results are indicative of either an increase in AMPA receptor function or a decrease in NMDA receptor function.
Figure 1. NMDA/AMPA ratio is reduced after sleep deprivation.
A, representative traces showing NMDA and AMPA currents from control and sleep deprived rats. B, summary of NMDA/AMPA ratio recorded in control, sleep deprived and large platform rats showing that the NMDA component is significantly reduced in sleep deprived rats relative to both other groups.
AMPA miniature EPSCs
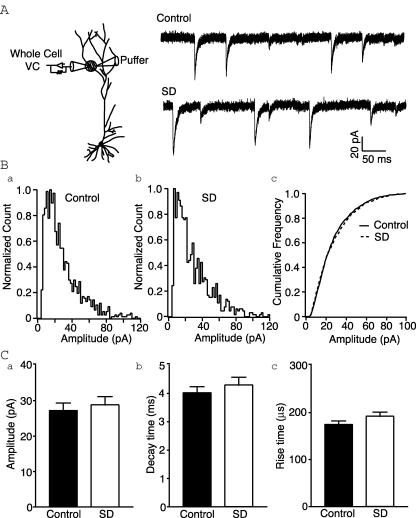
In order to investigate AMPAR function, we recorded locally evoked AMPA miniature EPSCs from the distal dendrites (approx. 240–280 μm from soma). This region receives dense, almost exclusive Schaffer collateral innervation. Mean mEPSC amplitudes were similar to previous reports (Smith et al. 2003) and were not significantly different between the two groups (control versus SD: 27.2 ± 2.1 pA (n= 11) versus 28.8 ± 2.3 pA (n= 8); P= 0.62, t test; Fig. 2). Similarly, rise (174.5 ± 7.5 versus 192.1 ± 9.0 μs; P= 0.15, t test) and decay time constants (4.0 ± 0.2 versus 4.3 ± 0.3 ms; P= 0.44, t test) were not affected by sleep deprivation (Fig. 2). This excludes any effect of AMPAR function on the altered NMDA/AMPA ratio. Thus it would appear that the SD-induced change in the NMDA/AMPA ratio is due to altered NMDAR function. This is expected given the prominent role of NMDARs in LTP induction.
Figure 2. AMPA receptor function is unaffected by sleep deprivation.
A, left, schematic of recording configuration. Right, representative recordings of hypertonically evoked synaptic activity from distal dendrites in control and sleep deprived rats. B, normalized frequency histograms of mEPSC amplitudes from CA1 cells of control (a) and sleep deprived (b) rats showing similar distributions. c, normalized cumulative frequency distributions for mEPSC amplitudes. C, no differences are detected in mEPSC amplitude (a), decay time constants (b) or rise time constants (c) between cells from control and sleep deprived rats.
NMDA current amplitude
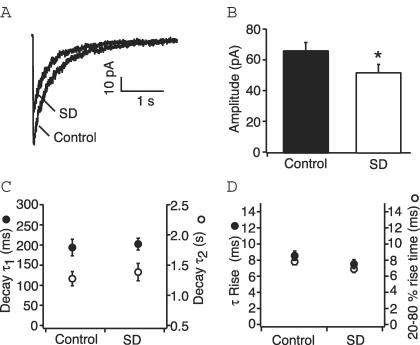
The NMDA current has a very slow decay time and synaptic currents are much smaller than their AMPA counterparts making the study of NMDA mEPSCs extremely difficult. Therefore, in order to investigate NMDAR function we pulled outside-out patches from the distal CA1 apical dendrites ranging from 240 to 280 μm from the soma. Patches were voltage-clamped at −70 mV, and NMDA currents were evoked by rapidly applying glutamate in the presence of 5 μm NBQX and 10 μm glycine. All recordings were made in the absence of external magnesium. A comparison of amplitudes of these NMDA currents evoked by a saturating concentration of glutamate (250 μm for 10 ms) revealed a significant reduction in the patches from sleep deprived animals compared to controls (control v. SD: 66.7 ± 4.7 pA (n= 52) versus 52.5 ± 4.6 pA (n= 30); P < 0.05, t test; Fig. 3).
Figure 3. Properties of NMDA currents in patches from CA1 distal dendrites of control and sleep deprived rats.
A, representative NMDA currents from outside-out patches from control and sleep deprived rats in response to a 10 ms application of glutamate (250 μm). Traces are averages of 8–10 responses. B, bar graph plotting the mean amplitude for control and sleep deprived rats. Responses are significantly attenuated after sleep deprivation. *P < 0.05. C, mean values for the fast (left axis, •) and slow (right axis, ○) decay time constants of NMDA currents from control and sleep deprived rats show no significant difference. D, similarly sleep deprivation does not affect the rise time constant (left axis, •) or 20–80% rise time (right axis, ○) for the NMDA current.
Kinetics
The kinetic properties of NMDA receptors are notably slower than those of other non-NMDA glutamate receptors and are an important determinant of calcium influx. There is considerable variation in the rates of decay of NMDA currents depending on the subunits present. The rise time course of the current evoked by a 250 μm, 10 ms glutamate pulse could be described by a single rising exponential function. The rise time constant from control patches was 8.54 ± 0.6 ms, and the 20–80% rise time was 7.8 ± 0.4 ms (n= 52). Patches from sleep deprived animals did not show any appreciable differences in the rise time constant (7.5 ± 0.6 ms, P= 0.13, t test) or 20–80% rise time (6.9 ± 0.4 ms, P= 0.23, t test) (n= 30). The decay phase for the same current was best described by two exponentials having time constants of 193.5 ± 20.6 ms (65.4 ± 3.6%) and 1.27 ± 0.12 s (34.6 ± 3.6%) (n= 14). There were no differences in the decay rates (τ1= 202.3 ± 14.6 ms, P= 0.73, t test; τ2= 1.38 ± 0.14 s, P= 0.62, t test) or distribution (τ1= 65.7 ± 2.6%; τ2= 34.3 ± 2.6%; P= 0.94, t test) (n= 30) for NMDA currents from sleep deprived animals. This strongly suggests that there is no alteration in subunit composition of the NMDA receptors.
There are a number of factors which contribute to the final amplitude of the NMDA current – the affinity of the receptors for glutamate, the single channel conductance, the probability that the receptors will open once glutamate has bound, and the density of receptors in the membrane. We have proceeded to investigate each of these possibilities separately.
Agonist affinity
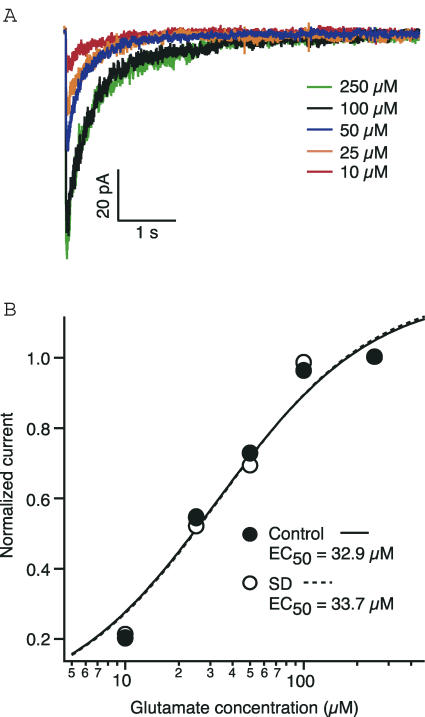
Reduced synaptic and patch NMDA currents could be accounted for by changes in the agonist affinity of the receptor. In order to determine whether glutamate affinity was affected by sleep deprivation, we examined the dose–response relationship for NMDA patch currents from control and sleep deprived rats. Increasing concentrations of glutamate (10–250 μm, 10 ms pulse) were applied to patches excised from the (240–280 μm distal from soma) apical dendrites of CA1 pyramidal cells. All peak current values were normalized to the peak response for the maximum concentration and then plotted against concentration (Fig. 4). After being fitted with a binding isotherm, dose–response curves did not show any shift (P= 0.55, paired t test), with the EC50 of the control patches being equal to that of patches from SD rats (32.9 μm, n= 18 and 33.7 μm, n= 10, respectively). Initial reports on the dose–response relationship for NMDA receptors calculated the EC50 for glutamate to be between 1 and 10 μm (Patneau & Mayer, 1990), However, a subsequent report has determined this to be a function of exposure time (Chen et al. 2001). Our calculation of an EC50 of approximately 33 μm is within the expected range for a 10 ms exposure (Chen et al. 2001). These data suggest that the agonist affinity of NMDA receptors for glutamate is not altered by sleep deprivation.
Figure 4. The affinity of NMDA receptors for glutamate is not altered by sleep deprivation.
A, representative traces from control rats showing the NMDA response to a range of glutamate concentrations. B, dose–response curves for glutamate activation of NMDA receptors from control and sleep deprived rats (application duration 10 ms). The data points are normalized with respect to the maximum current.
Single channel conductance
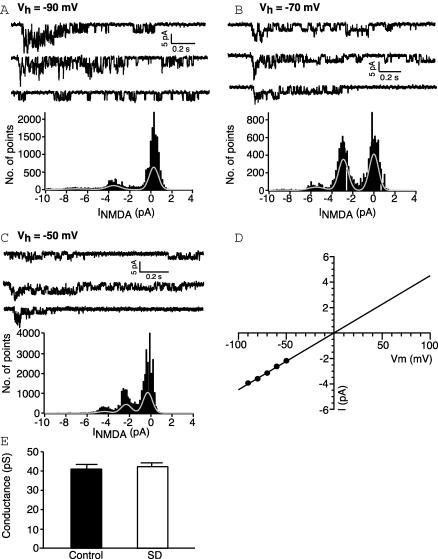
Although there have been no reports of activity regulating NMDA single channel conductance, these channels are reported to have several subconductance levels (Cull-Candy & Usowicz, 1987; Jahr & Stevens, 1987). A shift to a subconductance could result in a macroscopic current that is significantly attenuated. In many patches single NMDA receptor currents could be distinguished using a low concentration (10 μm for 10 ms) of glutamate. Figure 5 shows examples of single channel activity with corresponding all points histogram at a range of holding potentials. Single-channel I–V relationships for the main conductance state were approximately linear for both groups in the absence of magnesium. The reversal potential was close to 0 mV for both groups. Subconductances were sometimes visible but were not predominant and were not included in the analysis. Linear functions fitted to the I–V curves revealed a mean elementary conductance of 40.3 pS for control (n= 7) and 41.7 pS for NMDA receptors from sleep deprived rats (n= 6; P= 0.49, t test) (Fig. 5). These values are not significantly different from each other and are consistent with previous values obtained for NMDA receptors from CA1 neurones (Spruston et al. 1995; Gibb & Colquhoun, 1992). Therefore sleep deprivation does not appear to cause any alteration in the single channel conductance of NMDA receptors.
Figure 5. The single channel conductance is unchanged after sleep deprivation.
A–C, top, individual patch responses to 10 μm glutamate showing single channel activity at holding potentials of −90, −70 and −50 mV, respectively. Bottom, histogram of points for 5 such sweeps shown above. Each peak was fitted with a Gaussian distribution. D, I–V relationship for single channels in the same patch. Each point represents the mean for measurements made from 2 to 5 sweeps. The single-channel conductance calculated from the slope of a straight line fitted to the points was 44 pS. E, bar chart showing that the mean single channel conductance of NMDA receptors is unaffected by SD.
Probability that a channel bound by glutamate will open
There have been some reports describing the regulation of the gating properties of NMDA receptors. For example, a constitutively active form of PKC enhances NMDA currents in hippocampal neurones through an increase in the open probability of the channel (Xiong et al. 1998). Also, the neuromodulator adenosine decreases the open probability of the NMDA receptor in turtle brain (Buck & Bickler, 1998). Interestingly adenosine is involved in sleep–wake regulation and accumulates in the brain during the waking period.
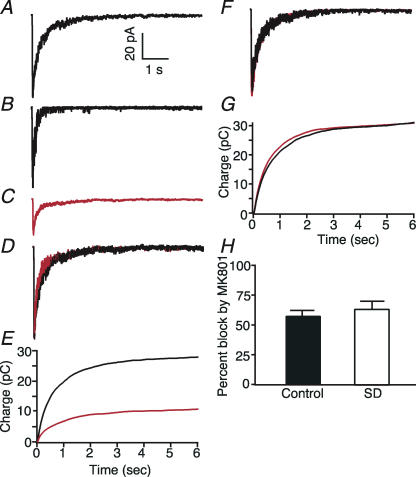
In order to investigate whether there could be any change in the opening probability of the receptor, we took advantage of the open channel blocking property of the anticonvulsant MK-801 (Huettner & Bean, 1988). In these experiments, after collecting 8–10 test traces in saturating concentrations of glutamate (1 mm) (Fig. 6A), both the control and glutamate solutions were switched to solutions containing 20 μm MK-801. A single 10 ms pulse of 1 mm glutamate was applied under these conditions after which the MK-801 was removed. This single application resulted in a current with a markedly faster decay time course (Fig. 6B). Subsequent glutamate applications resulted in currents that were stable and had normal time courses but were reduced in amplitude (Fig. 6C and D). As MK-801 can only block open channels, the percentage block produced can give us an indication of the proportion of receptors that open during one 10 ms application of glutamate. The concentration of MK-801 used was supramaximal so that any channels that opened should have been blocked by MK-801. Furthermore, the attenuation in amplitude was not caused by the MK-801 alone as patches that were exposed to MK-801 without any application of glutamate did not show any reduction in amplitude (Fig. 6E and F). In control patches the percentage decrease in charge transfer was 57.3 ± 4.8% (n= 11) and this represents the probability that a channel bound by glutamate will open before glutamate unbinds. We did not see any effect of sleep deprivation on NMDAR channel open probability. In patches from SD rats the percentage decrease in charge transfer was 63.7 ± 6.3% (n= 6) (P= 0.41, t test). These observed values were similar to previously reported values attained using the same method (Jahr, 1992).
Figure 6. Glutamate activates NMDA receptors from control and sleep deprived rats with equal probability.
A, average NMDA response evoked by a 10 ms application of 1 mm glutamate to an outside out patch in the presence of 10 μm glycine and 5 μm NBQX. B, single response of the same patch as in A to a 10 ms exposure to 1 mm glutamate in the continuous presence of 20 μm MK-801. C, average response of the same patch to glutamate after washout of MK-801. D, superimposition of averaged currents recorded before (black) and after (red) the single exposure to glutamate in the presence of MK-801. The averaged current in C has been normalized to the peak amplitude of the response in A. E, currents in A (black) and C (red) have been integrated in time. F, superimposition of averaged responses of another patch to 1 mm glutamate (10 ms) before (black) and after (red) exposure to 20 μm MK-801 in the absence of glutamate. G, integration of currents in F in time. H, bar chart showing that the percentage block incurred by a 10 ms exposure to glutamate in the presence of MK-801 is similar for control and sleep deprived rats.
The data above show that the major channel properties of NMDA receptors are not affected by sleep deprivation. We see no effect on glutamate affinity, the dose–response relationship and single channel conductance of the receptors. Therefore the reduction in the whole cell recorded NMDA/AMPA ratio and change in NMDA current amplitude apparent in patches from sleep deprived rats must represent a loss in receptor number. If this is indeed the case then it is possible that the impairment in LTP may simply be the result of insufficient calcium influx during the stimulus. In other words, the problem may lie in the induction rather than the expression of LTP.
Cross-linking assay
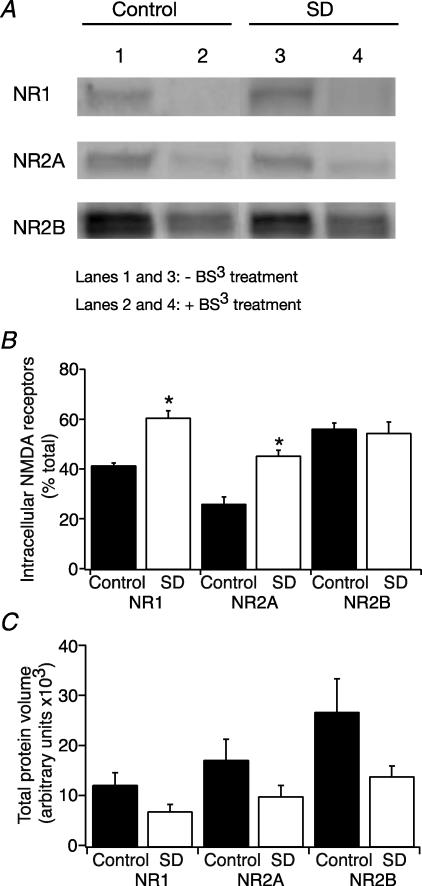
In order to determine whether NMDARs are preferentially being retained in the cytoplasm after sleep deprivation we preformed a cross-linking assay. All of the surface proteins were initially crosslinked with BS3. Total proteins were then separated by SDS-PAGE, transferred to a membrane and probed with antibodies against the different NMDAR subunits. Cross-linked proteins are considerably heavier and so remain at the top of the gel allowing for differentiation between intracellular and extracellular proteins. The intracellular protein as a percentage of total protein (measured when there was no cross-linking) was then compared between control and sleep deprived animals. Figure 7 shows that both the NR1 and NR2A subunits are more likely to be found in the intracellular fraction after sleep deprivation (NR1 intracellular fraction = 41.6 ± 0.78 (n= 5), and 61.1 ± 2.29% of total (n= 7), for control and SD, respectively (P < 0.05, t test); NR2A intracellular fraction = 26.2 ± 2.5 (n= 6), and 45.8 ± 1.68% of total (n= 7), for control and SD, respectively (P < 0.05, t test)). Although there appeared to be a trend towards lower total NMDAR subunit protein levels following SD, this was not statistically significant (Fig. 7). This suggests that the increased intracellular component is caused by a relocation of extracellular receptors rather that additional synthesis of subunits. The NR2B subunit does not appear to be affected (intracellular fraction = 56.42 ± 2.1 (n= 5) and 54.97 ± 3.87% of total (n= 4), for control and SD, respectively). This may be because of the limited role the NR2B subunit plays in NMDAR-mediated synaptic transmission in the adult hippocampus.
Figure 7. Crosslinking assay showing that a higher proportion of NR1 and NR2A subunits of NMDA receptors are found intracellularly after sleep deprivation.
A, effect of sleep deprivation on surface NMDAR expression. Surface expression is determined by labelling extracellular proteins with a membrane-permeable crosslinking protein BS3. The crosslinking proteins are unable to enter the gel so only the intracellular protein is seen in Western blot. B, blots were analysed and quantified by densitometry. Bars represent the mean values and s.e.m. for intracellular protein expressed as a percentage of total protein. *P < 0.05 SD compared with control. C, the relative amount of protein isolated by Western blot was obtained by scanning each band. Protein volume (arbitrary unit) was measured by ImageQuant software. The results are expressed in arbitrary units and represent means ±s.e.m.
Effect of glycine on LTP induction
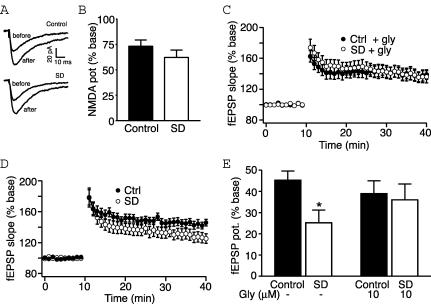
We would predict that if the impairment of LTP lies in the induction process through reduced NMDAR number, any measure that enhances the functioning of these receptors could rescue LTP. We have used the amino acid glycine for this effect. Glycine is a required coagonist at the NMDA receptor. It acts to increase the open probability of the NMDAR channels and produces an overall increase in the whole cell recorded current (Johnson & Ascher, 1987). We initially confirmed this effect on the whole cell current. In somatic voltage clamp recordings, NMDA currents were isolated in the presence of 5 μm NBQX and in the absence of external magnesium. The addition of 10 μm glycine increased the Schaffer collateral NMDA response by 73.3 ± 6.1% (n= 8). NMDA currents were similarly increased in slices from sleep deprived rats (62.3 ± 7.1%, n= 6; Fig. 8A and B; P= 0.47, t test). This is consistent with previous reports demonstrating that glycine is not present at saturating concentrations under these conditions (Wilcox et al. 1996).
Figure 8. Glycine rescues sleep deprivation-induced LTP impairment.
A, representative NMDA whole cell currents with and without 10 μm glycine from control (above) and sleep deprived rats (below). B, bar graph showing that glycine potentiation of NMDA currents is unaffected by sleep deprivation. C, graph showing addition of 10 μm glycine enhances LTP at Schaffer collateral synapses in slices from sleep deprived rats. D, graph reproduced with permission from McDermott et al. (2003) showing impairment of LTP after sleep deprivation. (©2003 by the Society for Neuroscience.) Graphs from C and D represent grouped data showing normalized fEPSP slope. LTP induction occurred at the 10 min time point. E, bar graph summarizing potentiation (mean ±s.e.m.) of fEPSP slope at 30 min post-tetanus for all treatment conditions. Control and SD data in the absence of glycine are from McDermott et al. (2003). *P < 0.05.
We then tested the impact of 10 μm glycine on LTP induction in field recordings. In the presence of glycine, 2 × 100 Hz stimuli (1 s, 30 s interval) resulted in a 39 ± 6.2% increase in the field response in slices from control rats (n= 14, Fig. 8C and E). This does not reflect any enhancement over LTP in the absence of glycine (McDermott et al. 2003; Fig. 8D and E), suggesting that we have reached the ceiling for potentiation using this induction protocol. However, LTP induction in slices from SD rats, which we have previously found to be significantly inhibited (McDermott et al. 2003; Fig. 8D and E), was similar to controls in the presence of glycine (36 ± 7.8%, n= 12; Fig. 8C and E; P= 0.26, t test). These results are consistent with our hypothesis that after sleep deprivation there is insufficient NMDAR activation for LTP induction.
Together these data point to a reduced density of NMDA receptors in CA1 pyramidal cells of sleep deprived rats. Our data indicate that measures to enhance NMDA receptor function rescue LTP and thus may serve to counteract SD-induced memory impairments.
Discussion
Summary
In this study we have characterized the NMDA receptor properties in dendrites of CA1 pyramidal neurones from control and sleep deprived rats and have identified an alteration, which may be responsible for the SD induced impairment of LTP. The NMDA/AMPA ratio at Schaffer collateral synapses is reduced after 72 h sleep deprivation. This effect is not due to any alteration in AMPA receptor function as AMPA mEPSC parameters were identical between control and sleep deprived rats. Using rapid application of glutamate to outside out patches from the distal dendrites of CA1 neurones we saw that the amplitude of NMDA currents was significantly reduced in sleep deprived animals at a level that is comparable to the reduced NMDA/AMPA ratio. The glutamate affinity, single channel conductance and probability of opening of NMDA receptors were not altered. However, there was a significant increase in the intracellular proportion of the NR1 and NR2A subunits. Total protein levels were unchanged, indicating that there is a reduced surface expression of NMDARs in sleep deprived animals and that this underlies the reduced NMDA/AMPA ratio. That NR1 and -2A subtypes were affected by SD, while NR2B was not, may simply reflect the relative functional importance of the different receptor subtypes. The increased expression of NR2A in adult rat hippocampus supports this idea (Watanabe et al. 1992; Monyer et al. 1994; Portera-Cailliau et al. 1996).
There is overwhelming evidence indicating that NMDA receptors are mediators of LTP induction and spatial memory in area CA1 of the hippocampus. Numerous studies blocking NMDAR activity or knocking out genes for selected subunits have been carried out and have led to reduced LTP induction and impaired spatial memory (Coan et al. 1987; Bashir et al. 1990; Sakimura et al. 1995; McHugh et al. 1996; Tsien et al. 1996). Furthermore, overexpression of the NR2B subunit of the NMDA receptor leads to an enhancement of learning and memory in mice (Tang et al. 1999). Therefore, we believe these alterations in NMDA receptors may underlie SD-induced impairments in long-term potentiation and memory.
Movement of receptors out of the synapse
Although it has long been thought that NMDA receptors are stationary at the synapse, there is now significant evidence demonstrating that this is not the case. Although they are not as dynamic as AMPAR receptors, they do show constitutive and regulated movements into and out of the synapse. NMDA receptors not only display lateral mobility within the membrane (Rao & Craig, 1997; Tovar & Westbrook, 2002), they can also be rapidly internalized in a clathrin dependent manner (Roche et al. 2001; Snyder et al. 2001).
Changes in the surface expression of NMDA receptors appear to be regulated by activity. For example, long-term exposure to NMDAR antagonists results in increased clustering of NMDARs at the synapse (Rao & Craig, 1997). Functionally, NMDAR currents are increased by activity blockade and decreased by enhanced activity (Watt et al. 2000). Snyder et al. (2001) reported significant reductions in synaptic NMDA receptors after chemically induced mGluR-dependent LTD. This LTD-induced loss of synaptic receptors was initially due to migration of NMDARs within the membrane followed by internalization. Conversely, increased surface expression of NMDA receptors has been described after the induction of LTP (Grosshans et al. 2001).
Some of the molecular elements controlling NMDAR trafficking have been identified. For example, the activation of protein kinase C, a molecule, which has been implicated in the induction of LTP, results in the exocytosis of new NMDAR channel molecules at the cell surface (Lan et al. 2001). Other factors which are believed to play a role include members of the membrane-associated guanylate kinase (MAGUK) family. When overexpressed, PSD-95 enhances the surface expression of NMDA receptors, both through reduced internalization and increased channel insertion (Roche et al. 2001; Lin et al. 2004). PSD-93 also promotes cell surface clustering of NMDAR subunits (Kim et al. 1996) and its deletion results in impaired NMDAR-mediated postsynaptic function in spinal dorsal horn neurones (Tao et al. 2003). The MAGUKs may themselves be subject to activity-dependent regulation (Ehlers, 2003) and as such may serve as mediators for activity-related changes.
The attenuation of the NMDA current by SD without affect on opening probability, glutamate affinity or single channel conductance indicates that there is an overall reduction in the number of functional NMDA receptors at the synapse. Activity in the hippocampus is responsive to the behavioural state of the animal and it is clear that sleep deprivation disrupts the normal behavioural state of the animal. This no doubt could lead to an altered state of activity in the hippocampus. Such changes in activity over an extended period of time could feasibly lead to the changes in NMDAR surface expression reported here.
Loss of surface NMDA receptors could occur through lateral movement, reduced insertion or increased internalization of receptors. Tovar & Westbrook (2002) saw 40% recovery of synaptic currents in a matter of hours after complete irreversible block by MK-801. This was due to lateral movement of receptors into the synapse. However, we saw a reduction in both synaptic currents and excised patch currents. As our patches contain a proportion of extrasynaptic receptors, we would expect that patch currents would not be affected or would even be increased by SD if lateral mobility were increased. Furthermore, the crosslinking assay demonstrates that after SD the intracellular population of NR1 and NR2A receptor subunits is increased. There is no change in total protein levels, which suggests that the increased intracellular population is caused by a relocation of surface receptors. However, we cannot determine from our data whether this effect is produced through reduced exocytosis of new receptor molecules to the cell surface or increased internalization. It should be noted that glial receptor molecules are also included in the crosslinking assay. The effect of SD on the physiology of these receptors was not explored and so we do not know how they can affect the results.
In conclusion, we have revealed a sleep loss induced alteration at the Schaffer collateral synapse, which we believe is a key element in SD-induced LTP impairment. As synaptic plasticity at this synapse is thought to be critical for the formation of memories, this alteration may also be the basis of SD-induced memory deficits. The identification of an impairment in NMDAR-mediated synaptic transmission after sleep deprivation may represent a novel target for intervention studies.
Acknowledgments
This work was supported by DARPA grant N65236-01-1-5438.
References
- Andrásfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrásfalvy BK, Magee JC. Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones. J Physiol. 2004;559:543–554. doi: 10.1113/jphysiol.2004.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrásfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Tam B, Collingridge GL. Activation of the glycine site in the NMDA receptor is necessary for the induction of LTP. Neurosci Lett. 1990;108:261–266. doi: 10.1016/0304-3940(90)90651-o. [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Buck LT, Bickler PE. Adenosine and anoxia reduce N-methyl-D-aspartate receptor open probability in turtle cerebrocortex. J Exp Biol. 1998;201:289–297. doi: 10.1242/jeb.201.2.289. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2001;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Chen N, Ren J, Raymond LA, Murphy TH. Changes in agonist concentration dependence that are a function of duration of exposure suggest N-methyl-D-aspartate receptor nonsaturation during synaptic stimulation. Mol Pharm. 2001;59:212–219. doi: 10.1124/mol.59.2.212. [DOI] [PubMed] [Google Scholar]
- Coan EJ, Saywood W, Collingridge GL. MK-801 blocks NMDA receptor-mediated synaptic transmission and long term potentiation in rat hippocampal slices. Neurosci Lett. 1987;80:111–114. doi: 10.1016/0304-3940(87)90505-2. [DOI] [PubMed] [Google Scholar]
- Coenen AML, Van Luijtelaar ELJM. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav. 1985;35:501–504. doi: 10.1016/0031-9384(85)90130-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Usowicz MM. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987;325:525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signalling via the ubiquitin-proteasome system. Nature Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nature Neurosci. 2001;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirements for GluR1 and PDZ domain. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992;255:470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325:522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kim E, Cho K-O, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience and EEG dynamics. J Neurosci. 1999;19:4090–4104. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J-Y, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MVL, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MVL, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nature Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JC, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calcium on synaptic transmission. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P. REM sleep modifications following a morse code learning session in humans. Physiol Behav. 1989;46:639–642. doi: 10.1016/0031-9384(89)90344-2. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van Der Linden M, Smith C, Cleeremans A. Experience-dependent changes in cerebral activation during human REM sleep. Nature Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Maquet P, Passingham R, Frith C. Sleep related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci. 2003;23:1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev H, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of the four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationship for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience dependent phase reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. N-methyl-D-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous systems revealed by subunit specific antibodies. J Neurochem. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Ly CD, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nature Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Romcy-Pereiro R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampal-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking the NMDA receptor ɛ1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Lapp L. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol Behav. 1986;36:1053–1057. doi: 10.1016/0031-9384(86)90479-8. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W. Acquisition impairments following rapid eye movement sleep deprivation in rats. Physiol Behav. 1971;7:345–352. doi: 10.1016/0031-9384(71)90312-x. [DOI] [PubMed] [Google Scholar]
- Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tao Y-X, Rumbaugh G, Wang G-D, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci. 2003;23:6703–6712. doi: 10.1523/JNEUROSCI.23-17-06703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Wang J-H, Kelly PT. Postsynaptic injection of Ca2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron. 1995;15:443–452. doi: 10.1016/0896-6273(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MCW, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Xiong Z-G, Raouf R, Lu W-Y, Wang L-Y, Orser BA, Dudek EM, Browning MD, MacDonald JF. Regulation of N-methyl-D-aspartate receptor function by constitutively active protein kinase C. Mol Pharm. 1998;54:1055–1063. [PubMed] [Google Scholar]
- Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RBS. Sleep deprivation by the ‘flower pot’ technique and spatial refrence memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]