Abstract
By birth, the regulatory neural network responsible for respiratory control is capable of generating robust rhythm-driving ventilation that can adjust to homeostatic needs. The advent of in vitro models isolated from prenatal rodents has significantly advanced our understanding of these processes. In this topical review, we examine the development of medullary respiratory rhythm-generating centres and phrenic motoneurone–diaphragm properties during the prenatal period.
Most mammalian brain structures do not become fully functional until they undergo significant postnatal development. While the neuronal and muscular components of the respiratory system mature postnatally, they must be developmentally advanced and functional by birth to generate a rhythm that allows for gas exchange in a highly compliant chest wall and to integrate swallowing and other behaviours with breathing. Neonatal intensive care units regularly deal with premature infants with inadequate prenatal maturation of the respiratory apparatus. Their maladies typically result from deficiencies of central respiratory rhythmogenesis or activation of respiratory musculature. Understanding the pathogenesis of breathing problems in the newborn and development of effective pharmacological treatment will greatly benefit from knowledge of in utero development of neural respiratory control.
The instrumentation of fetal sheep for physiological measurements allowed for the landmark studies demonstrating that mammals generate episodic fetal breathing movements (FBMs) that are modulated with changes in EEG and metabolic states (Dawes et al. 1970; Jansen & Chernick, 1991). Current understanding of neural circuits underlying FBMs and the development of these circuits to work at birth has resulted predominantly from the subsequent development of in vitro rodent models (Suzue, 1984; Smith et al. 1991). Specifically, rhythmically active brainstem–spinal cord or medullary slice preparations of rodents are amenable to intracellular recording, dynamic imaging and manipulation of specific neuronal groups by focal lesioning or pharmacological manipulation. In this review we will emphasize data from rodent models and discuss prenatal maturation of respiratory neural control.
Fetal respiratory activity in rodent preparations
Respiratory rhythmogenesis first emerges in the rat at embryonic day (E) 16.5–17 (Fig. 1). This observation was initially based on in vitro electrophysiological recordings of the isolated rat fetal brainstem–spinal cord (Greer et al. 1992; DiPasquale et al. 1992), and has been confirmed in ultrasound recordings of rat FBMs in utero from anaesthetized dams (Kobayashi et al. 2001) and direct electrophysiological recordings from the pre-Bötzinger complex (pre-BötC) in fetal medullary slices (Pagliardini et al. 2003). Fitting with the shorter gestation period of the murine model, the first indication of inspiratory discharge in utero and in vitro starts at E15 in the fetal mouse (Viemari et al. 2003; Thoby-Brisson et al. 2005).
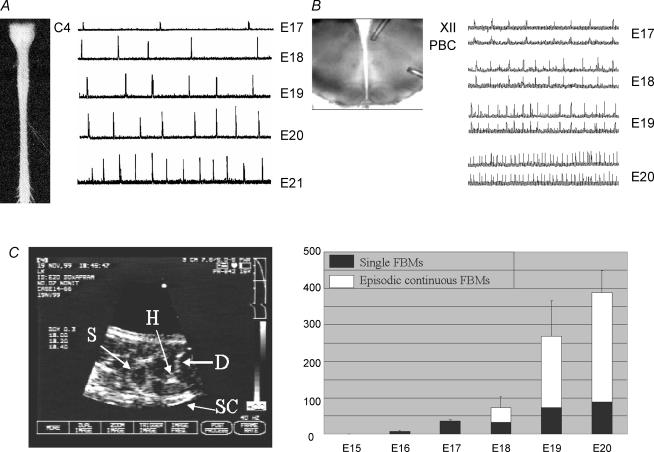
Figure 1. In vitro and in vivo recordings of fetal respiratory activity in rat.
A, rectified and integrated suction electrode recordings of C4 ventral root activity from prenatal brainstem–spinal cord preparations. B, rectified and integrated suction electrode recordings made from the pre-BötC (PBC) and XII motoneurone pool of prenatal medullary slice preparations. Rhythmic respiratory discharge commenced at E17 and the frequency and amplitude of inspiratory bursting increased in an age-dependent manner in both types of in vitro preparations. C, ultrasound image of fetal rat used to measure the incidence of fetal breathing movements. Right panel shows graph depicting the age-dependent increase in FBMs from E16–E20. FBMs occurred as isolated single movements or as episodes of clustered movements lasting 40–180 s. Abbreviations: H, heart; D, diaphragm; S, stomach; SC, spinal cord.
Episodic respiratory activity, a characteristic feature of fetal breathing in mammals including rats (Jansen & Chernick, 1991; Kobayashi et al. 2001), is not seen in vitro. This presumably reflects the lack of intact supramedullary structures that impart the episodic nature of FBMs (Blanco, 1994), and their suppression during hypoxia (Johnston & Gluckman, 1989; Ackland et al. 1997). Regardless of pattern, however, two features common to fetal breathing in vitro and in utero are that respiratory network activity is depressed and that this depression decreases, or excitation increases, as the fetus develops. In rats, between E17 and E20, the rate of FBMs increases ∼10-fold in utero (Kobayashi et al. 2001) and the frequency of inspiratory bursting increases ∼5-fold in vitro (Greer et al. 1996; Onimaru & Homma, 2002; Pagliardini et al. 2003; Ballanyi, 2004). The amplitude and duration of respiratory bursts also increase with gestational age, while variability in both frequency and amplitude decreases (Greer et al. 1992; DiPasquale et al. 1996; Hilaire & Duron, 1999).
Ontogeny of the pre-Bötzinger complex
Sequential serial sectioning from the rostral and caudal aspects of the isolated brainstem–spinal cord neonatal rat preparation developed by Suzue (1984) revealed that a restricted area of the medulla containing the pre-BötC (Fig. 2A) was necessary and sufficient for generating respiratory rhythm in vitro (Smith et al. 1991; reviewed in Rekling & Feldman, 1998; Feldman et al. 2003). A central role of the pre-BötC for inspiratory rhythmogenesis in perinates and adults is widely accepted although there remains considerable debate about the cellular mechanisms (Richter & Spyer, 2001; Feldman et al. 2003; Ezure, 2004; Duffin, 2004; Ramirez et al. 2004; Del Negro et al. 2005). Whether the pre-BötC cooperates with a second, more rostral system in the region of the parafacial respiratory group (pFRG) is currently also under investigation (Janczewski et al. 2002; Onimaru & Homma, 2003; Mellen et al. 2003). Data from imaging, electrophysiological and pharmacological studies suggest that neurones within the pFRG are intrinsically rhythmogenic and control expiratory musculature (Onimaru & Homma, 2002, 2003; Janczewski et al. 2002).
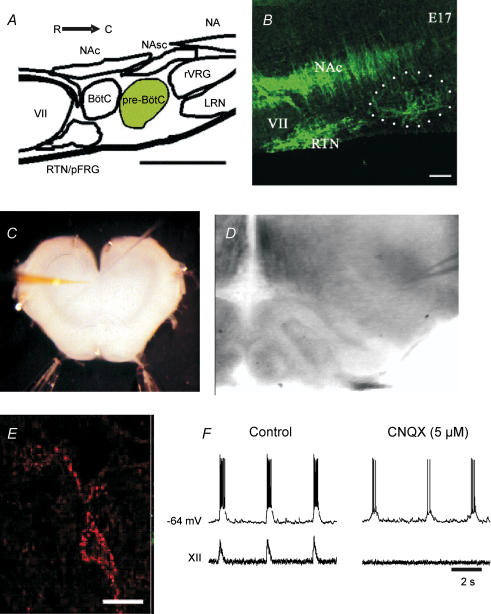
Figure 2. Identification and targeting of pre-BötC neurones within the ventrolateral medulla of perinatal rats.
A, an illustration in sagittal view of the primary structures in the ventrolateral medulla along the rostral (R)–caudal (C) axis. B, immunolabelling for NK1R (green) in E17 sagittal section of the ventrolateral medulla. The dotted circle demarcates the approximate area of the pre-BötC (calibration bar = 100 µm). C, photomicrograph of medullary slice preparation used for whole-cell recordings. D, IR-DIC image of the ventrolateral medulla with recording electrode positioned in the vicinity of the pre-BötC. E, neurone within the pre-BötC that has been fluorescently tagged via the internalization of TMR-conjugated SubP. F, whole-cell recording of membrane potential from a Tetramethylrhodamine (TMR)-SubP-positive neurone and simultaneous recording of integrated XII nerve inspiratory activity before and during block of rhythmic activity by bath application of CNQX. The neurone continues to burst after application of CNQX, consistent with it having pacemaker properties. Abbreviations: VII, facial nucleus; NAc, nucleus ambiguus, pars compacta; NAsc, nucleus ambiguus, semicompact NA, nucleus ambiguus; RTN, retrotrapezoid nucleus; pFRG, parafacial respiratory group; BötC, Bötzinger complex; pre-BötC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group; LRN, lateral reticular nucleus.
Molecular markers can delineate the critical population of pre-BötC neurones as pioneered by Feldman and colleagues for immunolabelling for neurokinin-1 receptors (NK1R; Gray et al. 1999). Subsequent studies supported the idea that there is a population of small fusiform, glutamatergic neurones in the pre-BötC expressing NK1R that have characteristics consistent with their involvement in rhythmogenesis (Pilowsky & Feldman, 2001; Wang et al. 2001; Guyenet et al. 2002; Stornetta et al. 2003). Further evidence came from experiments in adult rats demonstrating that bilateral destruction of NK1R-expressing neurones within the pre-BötC by substance P (SubP) conjugated to saporin results in ataxic breathing during wakefulness (Gray et al. 2001).
The NK1R expression data provided the basis for a study of the ontogeny of the pre-BötC in the rat (Pagliardini et al. 2003). A combination of immunohistochemical labelling and neuronal birth-dating with bromodeoxyuridine (BrdU) indicated that the NK1R-positive, pre-BötC neurones are born within the ventricular zone on E12 and E13, approximately 2 days later than adjacent NK1R-positive neurones within the semicompact division of the nucleus ambiguus. The neurones reach the region of the pre-BötC between E16.5 and E18 (Fig. 2B), which coincides with the time that respiratory-related neural discharge is first detected electrophysiologically. Thoby-Brisson et al. (2005) used a combination of NK1R labelling, electrophysiological recordings and calcium imaging to demonstrate a similar developmental profile of pre-BötC anatomy and functional development in mice. Less is known about the prenatal development of the pFRG. However, imaging of respiratory network activity in rat with voltage-sensitive dyes suggests that rhythmic activity of the pFRG commences at E18, approximately 1 day later than in the rat pre-BötC (Onimaru & Homma, 2005).
While the anatomical aspects of pre-BötC prenatal development have been amenable to study, important questions regarding the ontogeny of neuronal properties and synaptic connectivity within the pre-BötC have been difficult to address. DiPasquale et al. (1996) demonstrated that the firing frequency and stability of medullary respiratory neuronal activity increases markedly from E18 to birth (reviewed in Hilaire & Duron, 1999). However, there have been no reports during the prenatal period of intracellular or whole-cell analyses of identified NK1R-expressing pre-BötC neurones. The fact that the pre-BötC contains a functionally heterogeneous pool of neurones has been an impediment to progress. However, a recent technical advancement that identifies NK1R-expressing neurones for whole-cell patch-clamp recording, based on their internalization of fluorescently conjugated SubP, should greatly facilitate the analysis of these key pre-BötC neurones (Fig. 2B–F; Pagliardini et al. 2005a). Further, both the activity and structure of populations of pre-BötC neurones can be assessed online with two-photon calcium imaging prior to whole-cell recording (Ruangkittisakul & Ballanyi, 2006). Thus, it should now be possible to more clearly examine the spatiotemporal pattern of expression of multiple voltage- and ligand-gated ion channels in the different populations of respiratory neurones.
Neurochemical control of fetal respiratory rhythm
A fundamental issue pertaining to the ontogeny of respiratory rhythmogenesis is whether fetal networks oscillate at a slower rate than neonatal networks because: (i) of age-dependent differences in the neurones and/or the network underlying rhythmogenesis; (ii) fetal network activity is suppressed by endogenous inhibitory modulators; or (iii) modulatory systems that provide excitatory drive to the respiratory networks are not well developed. Certainly the increase in respiratory frequency and stability that accompanies development in utero will, in part, reflect network maturation. However, it also appears to reflect the latter two options. Fetal rhythm-generating centres can oscillate at frequencies comparable to those of the neonate if agonists of excitatory modulators or antagonists of inhibitory modulators are administered. For example, in utero, the frequency of FBMs in rats is increased by administration of the respiratory stimulants doxapram and aminophylline (Kobayashi et al. 2001). Application of the excitatory neuromodulators serotonin (5-HT), thyrotropin-releasing hormone (TRH) and SubP in vitro also markedly increases the frequency of prenatal respiratory rhythm (Greer et al. 1996; Pagliardini et al. 2003; Ballanyi, 2004).
The primary excitatory phasic drive that maintains the oscillatory state in the pre-BötC of neonates arises from activation of non-NMDA glutamatergic receptors (Greer et al. 1991; Funk et al. 1993). This has also been demonstrated for prenatal in vitro preparations (Thoby-Brisson et al. 2005). Further conditioning is provided by a diverse group of neuromodulators, including those acting via cyclic nucleotides, including noradrenaline, opioids, prostaglandins and SubP (Moss & Inman, 1989; Richter et al. 1997; Ballanyi et al. 1997, 1999). The medullary raphe nuclei are an important source of neuromodulatory input regulating respiratory rhythmogenesis (Bonham, 1995). The three major neurotransmitters released from the raphe complex, 5-HT, TRH and SubP, all have excitatory actions on respiratory rhythmogenesis from early fetal stages when rhythmic respiratory activity first appears (DiPasquale et al. 1994; Al-Zubaidy et al. 1996; Pagliardini et al. 2003). The neurotransmitters γ-aminobutyric acid (GABA) and glycine are the principal mediators of fast chloride-mediated inhibitory transmission in the mammalian central nervous system. They modulate mammalian respiratory rhythmogenesis and the patterning of motor output (Johnson et al. 1996; Shao & Feldman, 1997; Brockhaus & Ballanyi, 1998; Parkis et al. 1999; Ritter & Zhang, 2000). Changes in membrane potential in response to GABAA and glycine receptor ligands are determined by the chloride equilibrium potential which depends on a developmental decrease in the expression of the Na+–K+–2Cl− cotransporter (NKCC), which elevates intracellular [Cl−], and concomitant up-regulation of the K+–Cl− cotransporter (KCC2), which lowers intracellular [Cl−] (Payne et al. 2003). Data from in vitro preparations indicate that the transition from a depolarizing to a hyperpolarizing action via chloride-mediated conductances within the pre-BötC occurs prenatally (Brockhaus & Ballanyi, 1998; Ren et al. 2001).
Prenatal maturation of respiratory motoneurones and muscle
The phrenic and hypoglossal motoneurone groups have been studied extensively in the newborn and adult period (reviewed in Berger et al. 1996; Rekling et al. 2000; Cameron & Nunez-Abades, 2000). However, exploration of prenatal development has primarily been limited to phrenic motoneurone (PMN) and diaphragm muscle properties. By E17, when PMNs are first recruited for the generation of FBMs, the motoneurones have migrated to their position in the ventral horn, extended intramuscular branches throughout the full extent of the developing diaphragm, received synaptic input from spinal afferents, undergone the major period of naturally occurring cell death and expressed the necessary ionic conductances for the generation of sodium-dependent action potentials (Harris & McCaig, 1984; Allan & Greer, 1997a, b; Martin-Caraballo & Greer, 1999). While PMNs do not receive inspiratory drive prior to E17, they are recruited prior to E17 as part of a robust, regular rhythmic motor pattern that is generated along the full extent of the developing spinal cord and medulla (Greer et al. 1992; Ren & Greer, 2003). Spontaneous embryonic rhythmogenesis, quite distinct from FBMs, is postulated to play a key role in regulating the events involved in the early development of neuronal circuits and establishing motoneuronal phenotype (reviewed in Ben-Ari, 2001).
While PMNs are functional at E17 and can induce diaphragmatic contractions, there are several key characteristics of the PMN–diaphragm unit that mature prior to birth. These include the following. (1) Morphologically, the distinct rostrocaudal bundling of PMN dendrites, which may facilitate the synchronized activation of PMNs by descending synaptic drive, occurs during the 48–72 h following the onset of descending inspiratory drive (Allan & Greer, 1997b). (2) At the inception of inspiratory drive, PMNs have relatively depolarized resting membrane potentials and very high input impedance (Martin-Caraballo & Greer, 1999). Both of these characteristics increase the propensity for reaching firing threshold despite the rather weak inspiratory drive currents at this point in development (DiPasquale et al. 1996). (3) PMN firing rates increase 2-fold during the last few days in utero with the expression of new ion channels, most notably Ca2+-activated K+ conductances (Martin-Caraballo & Greer, 2000, 2001). (4) The speed of twitch contraction and the range and absolute amount of force generated by diaphragm muscle fibres change in concert with PMN properties (Martin-Caraballo et al. 2000). Thus, prior to birth, PMN and diaphragm properties ensure gross movement of the ribcage despite relatively weak synaptic drive. By birth, the motoneurone and muscle properties allow for the generation of more substantial ribcage movements that can be graded to meet the varied demands of breathing ex utero. Our current working hypothesis states that the rapid maturation of PMN–diaphragm properties is in part due to phenotypic changes induced by synaptically mediated events associated with inspiratory drive transmission (e.g. Ca2+ influx, neurotrophic factor release).
Summary and future perspectives
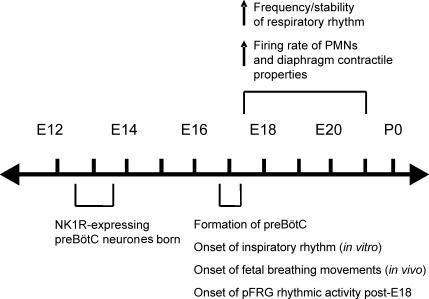
Rodent in vitro models are providing significant advances in our understanding of respiratory neural control during the prenatal period. There is now solid foundational data regarding the anatomical development of respiratory neuronal populations, onset of rhythmic respiratory drive, the actions of neuromodulators that control respiratory frequency and functional development of the phrenic nerve and diaphragm musculature (Fig. 3). The advent of experimental approaches to examine putative rhythmogenic neurones within the pre-BötC should facilitate the understanding of their basic properties and developmental processes. Further, experimental approaches based on advances in developmental genetics are emerging. Large-scale screening of the spatiotemporal distribution of transcription factors in the developing brainstem should reveal additional pre-BötC neuronal markers (Gray et al. 2004). Genetic manipulations will provide the opportunity to inactivate, eliminate or alter specific neurones to dissect and understand central rhythm and pattern generating networks at the molecular, cellular, network and physiological levels (Kiehn & Kullander, 2004; Goulding & Pfaff, 2005). An increasing number of genetic mutations in mouse models are associated with hypoventilation, which has been linked to abnormalities of the central control of respiratory rhythmogenesis (reviewed in Blanchi & Sieweke, 2005). Data from these models will provide insight into the transcriptional control mechanisms underlying respiratory network development and breathing disorders in the perinate, including apnoeas of prematurity, congenital central hypoventilation syndrome and Prader Willi syndrome (Ren et al. 2003; Pagliardini et al. 2005b; Blanchi & Sieweke, 2005). Finally, there is increasing awareness that an inhospitable in utero environment has long-term consequences on physiological function in adulthood (reviewed in Barker, 2004). The respiratory control system, including normoxic ventilatory parameters and responses to hypoxia, is clearly influenced by perturbations during the newborn period (reviewed in Mitchell & Johnson, 2003; Gozal, 2004) and there is emerging evidence for long-term plasticity in response to intermittent prenatal hypoxia (Gozal et al. 2003) that warrants further investigation.
Figure 3.
Time line illustrating key events in the development of respiratory neuronal activity in fetal rats.
Acknowledgments
We thank Jack Feldman for his valuable comments on the manuscript. J.J.G. and K.B. are Scientists of the Alberta Heritage Foundation for Medical Research (AHFMR) and G.D.F. is a Senior Scholar of the AHFMR.
References
- Ackland GL, Noble R, Hanson MA. Red nucleus inhibits breathing during hypoxia in neonates. Respir Physiol. 1997;110:251–260. doi: 10.1016/s0034-5687(97)00090-x. [DOI] [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J Comp Neurol. 1997a;382:459–468. doi: 10.1002/(sici)1096-9861(19970616)382:4<459::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol. 1997b;381:469–479. doi: 10.1002/(sici)1096-9861(19970616)382:4<469::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol. 1997;504:127–134. doi: 10.1111/j.1469-7793.1997.127bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of well-being. Philos Trans R Soc Lond B Biol Sci. 2004;359:1359–1366. doi: 10.1098/rstb.2004.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81:1039–1048. doi: 10.1152/jappl.1996.81.3.1039. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Sieweke MH. Mutations of brainstem transcription factors and central respiratory disorders. Trends Mol Med. 2005;11:23–30. doi: 10.1016/j.molmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Blanco CE. Maturation of fetal breathing activity. Biol Neonate. 1994;65:182–188. doi: 10.1159/000244050. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Resp Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Nunez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons during postnatal development. Brain Res Bull. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and paradoxical sleep in the foetal lamb. J Physiol. 1970;210:47P–48P. [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPasquale E, Monteau R, Hilaire G. Endogenous serotonin modulates the fetal respiratory rhythm: an in vitro study in the rat. Brain Res Dev Brain Res. 1994;80:222–232. doi: 10.1016/0165-3806(94)90107-4. [DOI] [PubMed] [Google Scholar]
- DiPasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- DiPasquale E, Tell F, Monteau R, Hilaire G. Perinatal developmental changes in respiratory activity of medullary and spinal neurons: an in vitro study on fetal and newborn rats. Brain Res Dev Brain Res. 1996;91:121–130. doi: 10.1016/0165-3806(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Duffin J. Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp Physiol. 2004;89:517–529. doi: 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- Ezure K. Reflections on respiratory rhythm generation. Prog Brain Res. 2004;143:67–74. doi: 10.1016/S0079-6123(03)43007-0. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Gozal D. New concepts in abnormalities of respiratory control in children. Curr Opin Pediatr. 2004;16:305–308. doi: 10.1097/01.mop.0000127159.74908.27. [DOI] [PubMed] [Google Scholar]
- Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–1547. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;30:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires pre-Bötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the pre-Bötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Al-Zubaidy ZA, Carter JE. Thyrotropin releasing hormone (TRH) stimulates perinatal rat respiration in vitro. Am J Physiol. 1996;271:R1160–R1164. doi: 10.1152/ajpregu.1996.271.5.R1160. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JF. The role of excitatory amino acids in the generation and transmission of respiratory drive in the neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Generation of respiratory and locomotor patterns by an in vitro brainstem-spinal cord fetal rat preparation. J Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AJ, McCaig CD. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci. 1984;4:13–24. doi: 10.1523/JNEUROSCI.04-01-00013.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Fetal breathing and development of control of breathing. J Appl Physiol. 1991;70:1431–1446. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gluckman PD. Lateral pontine lesions affect central chemosensitivity in unanesthetized fetal lambs. J Appl Physiol. 1989;67:1113–1118. doi: 10.1152/jappl.1989.67.3.1113. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–321. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lemke RP, Greer JJ. Development of fetal breathing movements in the rat. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- Martin-Caraballo M, Campagnaro PA, Gao Y, Greer JJ. Contractile properties of the rat diaphragm during the perinatal period. J Appl Physiol. 2000;88:573–580. doi: 10.1152/jappl.2000.88.2.573. [DOI] [PubMed] [Google Scholar]
- Martin-Caraballo M, Greer JJ. Electrophysiological properties of rat phrenic motoneurons during perinatal development. J Neurophysiol. 1999;81:1365–1378. doi: 10.1152/jn.1999.81.3.1365. [DOI] [PubMed] [Google Scholar]
- Martin-Caraballo M, Greer JJ. Development of potassium conductances in perinatal rat phrenic motoneurons. J Neurophysiol. 2000;83:3497–3508. doi: 10.1152/jn.2000.83.6.3497. [DOI] [PubMed] [Google Scholar]
- Martin-Caraballo M, Greer JJ. Voltage-sensitive calcium currents and their role in regulating phrenic motoneuron electrical excitability during the perinatal period. J Neurobiol. 2001;46:231–248. doi: 10.1002/1097-4695(200103)46:4<231::aid-neu1005>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Moss IR, Inman JG. Neurochemicals and respiratory control during development. J Appl Physiol. 1989;67:1–13. doi: 10.1152/jappl.1989.67.1.1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Development of the rat respiratory neuron network during the late fetal period. Neurosci Res. 2002;42:209–218. doi: 10.1016/s0168-0102(01)00322-4. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Developmental changes in the spatio-temporal pattern of respiratory neuron activity in the medulla of late fetal rat. Neuroscience. 2005;131:969–977. doi: 10.1016/j.neuroscience.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005a;25:2591–2596. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Greer JJ. Ontogeny of the pre-Botzinger complex in perinatal rats. J Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Wevrick W, Greer JJ. Developmental abnormalities of neuronal structure and function in mice lacking the Prader-Willi syndrome gene necdin. Am J Pathol. 2005b;167:175–191. doi: 10.1016/S0002-9440(10)62964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Feldman JL. Identifying neurons in the pre-Bötzinger complex that generate respiratory rhythm: visualizing the ghost in the machine. J Comp Neurol. 2001;434:125–127. doi: 10.1002/cne.1168. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Ann Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong X, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- Ren J, Lee S, Pagliardini S, Gerard M, Stewart CL, Greer JJ, Wevrick R. Absence of Ndn, encoding the Prader-Willi syndrome deleted gene necdin, results in congenital deficiency of central respiratory drive in neonatal mice. J Neurosci. 2003;23:1569–1573. doi: 10.1523/JNEUROSCI.23-05-01569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Zhang W, Greer JJ. Modulation of respiratory neural discharge by chloride-mediated conductances during the perinatal period. Soc Neurosci Abstr 633.3. 2001 doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld F. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Ballanyi K. Neuron-glia-imaging. In: Binder M, Hirsch M, Hirokawa N, Windhorst U, editors. In Encyclopedic Reference Of Neuroscience. Heidelberg, New York, Tokyo: Springer; 2006. (in press) [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bevengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]