Abstract
Cholinergic signalling is critically involved in learning and memory processes in the hippocampus, but the postsynaptic impact of cholinergic modulation on morphologically defined subtypes of hippocampal interneurones remains unclear. We investigated the influence of muscarinic receptor (mAChR) activation on stratum oriens interneurones using whole-cell patch clamp recordings from hippocampal slices in vitro. Upon somatic depolarization, mAChR activation consistently enhanced firing frequency and produced large, sustained afterdepolarizations (ADPs) of stratum oriens–lacunosum moleculare (O-LM) interneurones. In contrast, stratum oriens cell types with axon arborization patterns different from O-LM cells not only lacked large muscarinic ADPs but also appeared to exhibit distinct responses to mAChR activation. The ADP in O-LM cells, mediated by M1/M3 receptors, was associated with inhibition of an M current, inhibition of a slow calcium-activated potassium current, and activation of a calcium-dependent non-selective cationic current (ICAT). An examination of ionic conductances generated by firing revealed that calcium entry through ICAT controls the emergence of the mAChR-mediated ADP. Our results indicate that cholinergic specializations are present within anatomically distinct subpopulations of hippocampal interneurones, suggesting that there may be organizing principles to cholinergic control of GABA release in the hippocampus.
Synchronous firing of neuronal assemblies mediates the flow of information in the brain (Lisman & Idiart, 1995; Raghavachari et al. 2001; Buzsaki, 2002; Kirk & Mackay, 2003). Rhythmic activity in the hippocampus at theta (5–12 Hz) frequencies correlates with exploration, voluntary movement, and REM sleep in mammals (Jouvet, 1969; Vanderwolf, 1969; Vanderwolf et al. 1977; Bischof & Boulanger, 2003; Cantero et al. 2003). During the theta cycle, interneurone subpopulations exhibit profound differences in the timing of their action potential (AP) discharge, yet the timing is relatively uniform within an interneurone subtype (Borhegyi et al. 2004; Klausberger et al. 2004; Somogyi & Klausberger, 2005). These unique and precise emergent network properties presumably arise from cellular, synaptic and morphological differences between hippocampal interneurone cell types.
Recent evidence suggests that theta rhythmicity is in part controlled by extrahippocampal afferents originating from the medial septum–diagonal band of Broca area (MS-DBB) (Buzsaki, 2002; Cobb & Davies, 2005). The MS-DBB innervates the hippocampus via GABAergic afferents (Freund & Antal, 1988), which target solely hippocampal interneurones, and cholinergic afferents (Dutar et al. 1995), which target both pyramidal cells and interneurones (Frotscher & Leranth, 1985). Cholinergic modulation appears to be critical in normal hippocampal processing, as suggested by the degradation in location-specific firing of hippocampal place cells upon muscarinic blockade (Ikonen et al. 2002; Brazhnik et al. 2003, 2004). Moreover, ablation of cholinergic neurones of the medial septum profoundly reduces the power of hippocampal theta oscillations (Lee et al. 1994), suggesting that the magnitude of theta oscillations can be controlled through cholinergic engagement of hippocampal pyramidal cell and interneurone targets.
Although the excitatory consequences of mAChR activation of pyramidal cells are well understood (Halliwell, 1990; Cobb & Davies, 2005), previous studies have shown that mAChR activation of individual interneurones in the hippocampus yields differential effects on resting membrane potential both across and within hippocampal layers (Parra et al. 1998; McQuiston & Madison, 1999a). In stratum oriens alone, interneurones exhibit a wide range of responses to cholinergic agonists; while some interneurones depolarize, others hyperpolarize, exhibit a biphasic response, or do not respond (McQuiston & Madison, 1999a). Furthermore, there appear to be as many as eight subclasses of stratum oriens interneurones, differing broadly in their axonal arborization pattern (Maccaferri et al. 2000; Maccaferri, 2005), physiological properties (Maccaferri et al. 2000; Gloveli et al. 2005), and neurochemical content (Somogyi & Klausberger, 2005). While initial investigations found no systematic relationship between mAChR effects on resting membrane potential and the anatomical phenotypes of interneurones across layers (Parra et al. 1998; McQuiston & Madison, 1999a), immunocytochemical findings suggested cell type-specific localization of mAChRs in different subtypes of interneurones (Levey et al. 1995; Hajos et al. 1998). Thus, whether physiological effects of mAChR activation differ between anatomically identified interneurone types remains unclear.
In this study, we show for the first time that a morphologically identifiable class of stratum oriens interneurone, the stratum oriens–lacunosum moleculare (O-LM) interneurone, exhibits a stereotypical phenotype associated with muscarinic receptor activation that is distinct from other stratum oriens interneurone cell types. mAChR activation robustly triggers an M1/M3-mediated ADP in O-LM interneurones, which is associated with the inhibition of several potassium (K+) conductances and the activation of ICAT. Our results indicate that muscarinic signalling is specialized within anatomically defined hippocampal interneurone cell types, suggesting cholinergic control of hippocampal rhythmicity and information processing may be orchestrated on a cell-type specific basis.
Methods
Hippocampal slice preparation
All experiments were conducted in accordance with animal protocols approved by the NIH. Fourteen- to twenty-one-day-old (16–18 day mode) 129J1 (50%) × CF1 (50%) mice were anaesthetized by isoflurane volatile inhalation and decapitated. The brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 75 sucrose, 7 MgCl2, 0.5 CaCl2 saturated with 95% O2–5% CO2, pH 7.4. Transverse hippocampal slices (300 μm) were cut on a VT1000S (Leica Microsystems, Bannockburn, IL, USA) or Vibratome 3000 Deluxe (Vibratome, St Louis, MO, USA) and placed in warm (36°C), continuously oxygenated ACSF for at least 30 min before use.
Electrophysiology
Slices were placed in a submerged chamber and imaged under 60× on a Zeiss FS1 upright microscope (Infrapatch; Luigs and Neumann, Ratingen, Germany). Solution was superfused at a rate of 1–2 ml min−1 under carbogen pressure. The extracellular solution contained (mm): 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 glucose, 2 MgCl2, 2 CaCl2, saturated with 95% O2–5% CO2. The extracellular solution also contained dl-APV (100 μm, 6,7-dinitro-quinoxaline-2,3-dione (DNQX) (25 μm), and gabazine (5 μm) to block NMDA, AMPA/kainate, and GABAA receptors, respectively (Sigma-Aldrich, St. Louis, MO, USA). mAChR antagonists (Tocris Bioscience, Avonmouth, UK) were added both to normal and muscarine-containing solutions. Recording pipettes of resistance 2.5–4.5 MΩ were pulled with a vertical puller (Narishige, East Meadow, NY, USA) and filled with (mm): 135 potassium gluconate, 10 Hepes, 0.1 EGTA, 2 Na2ATP, 0.3 Na2GTP, 2 MgCl2, 20 KCl. Recordings were performed with an Axoclamp 200B or Multiclamp 700A amplifier (Molecular Devices Corp., Union City, CA, USA) from visually identified interneurones with fusiform cell bodies and horizontal dendrites near the stratum oriens–alveus border. Bridge balance was employed throughout current clamp experiments. For ‘switch clamp’ experiments, mode switching between current clamp and voltage clamp was performed via a digital output into the mode port of the Multiclamp 700A, and manually toggling the external mode switch on and off on alternating episodes. Recordings were low pass filtered at 3 kHz (Bessel filter, Frequency Devices, Haverhill, MA, USA) and digitized at 10–20 kHz (Digidata 1320A and pCLAMP 9.2 Software, Molecular Devices Corp.). All recordings were performed at room temperature (∼22°C).
Biocytin (0.2%) was added for post hoc morphological identification of each recorded cell. Only cells positively identified as interneurones with somata located in stratum alveus/oriens were included in the study. Cells were visualized using 3,3′-diaminobenzidine as a chromogen as previously described (Toth & McBain, 1998) or cells were imaged with a Leica TCS RS confocal microscope with a 20× objective, using avidin-conjugated Alexa Fluor 488 from Molecular Probes at 1: 1000 dilution (original stock 2 mg ml−1; final concentration was 2 μg ml−1). Confocal images of cells were assembled in Photoshop 7.0 by overlaying adjacent 2D confocal projections.
Unless otherwise stated, data are in the form of the mean ±s.e.m.
Results
Large muscarinic ADPs are hallmark features of CA1 O-LM interneurones
We began our experiments by determining how mAChR activation changed the firing properties of stratum oriens interneurones and whether such changes were linked to cell type (Parra et al. 1998; McQuiston & Madison, 1999a). After an initial electrophysiological characterization (i.e. Fig. 1B), we monitored the effect of muscarinic receptor (mAChR) activation on the firing properties of stratum oriens interneurones elicited by delivering a 90 pA 1 s-duration current step every 15 s during bath application of either 10 μm muscarine (44 cells) or 100–300 μm acetylcholine (ACh; 20 cells). Cells were maintained at −60 mV throughout the experiment to isolate the depolarization-independent effects of mAChR activation on firing frequency and to avoid potential voltage-dependent effects on the afterhyperpolarization (Gu et al. 2005). After each recording, slices were fixed to determine the post hoc morphological identity of the cells.
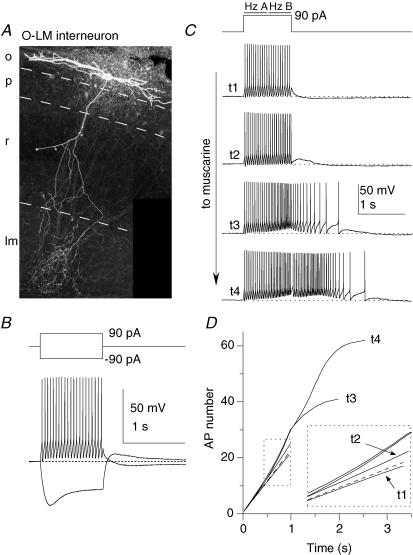
Figure 1. mAChR-induced ADPs are hallmark features of CA1 O-LM interneurones.
A, O-LM interneurone recovered after whole cell recording and processed with fluorescently labelled avidin against intracellularly introduced biocytin. B, current clamp response of the O-LM interneurone in A. C–D, second-long 90 pA current injections from −60 mV were delivered every 15 s upon wash-in of 10 μm muscarine. C, representative traces at progressive time points during wash-in of muscarine, illustrating elimination of the AHP and gain of a large ADP. Hz A and Hz B refer to the average action potential firing frequency during the first and second 500 ms of the current injection, respectively. D, plot of AP number versus time for traces t1–t4 in C. The slope of the dashed line indicates the average instantaneous AP frequency of the first 3 action potentials in t1; note that the t1 line is sublinear to the dashed line, denoting accommodation, while t2–t4 lines are supralinear, denoting acceleration in AP frequency.
One of the most common cell types we encountered in stratum oriens was the so-called ‘O-LM’ interneurone (Fig. 1A; see Maccaferri & Lacaille, 2003; Maccaferri, 2005 for reviews), so named for their horizontally orientated soma and dendrites located in stratum oriens (O) and an axon which projects to the lacunosum moleculare (LM) layer (McBain et al. 1994). In LM, the axon densely ramifies, where it exerts GABAergic inhibitory control over the distal dendrites of the CA1 pyramidal cells (Sik et al. 1995; Maccaferri et al. 2000). Under whole cell current clamp conditions these O-LM cells had relatively high input resistance (496 ± 25 MΩ) and slow membrane time constant (71.1 ± 5.8 ms), and could be maintained at −60 mV with the introduction of a small amount of negative bias current through the somatic recording pipette (−8.0 ± 4.0 pA, n= 11). Upon introduction of a 90 pA current injection, O-LM cells fired continuous, high frequency action potentials that underwent a modest spike frequency adaptation (Fig. 1B and C, t1). Upon termination of firing, O-LM cells often generated a long-lasting, slow afterhyperpolarization (AHP; Fig. 1B). In response to a −90 pA hyperpolarizing current injection, O-LM cells exhibited a conspicuous sag back towards the baseline potential, consistent with the presence of the hyperpolarization-activated current Ih (Fig. 1B). Upon repolarization, 10/15 O-LM cells exhibited rebound spikes (Fig. 2E). These intrinsic membrane properties are similar to that previously described for O-LM interneurones in rat hippocampal slices (Maccaferri & McBain, 1996; Lien et al. 2002; Gloveli et al. 2005).
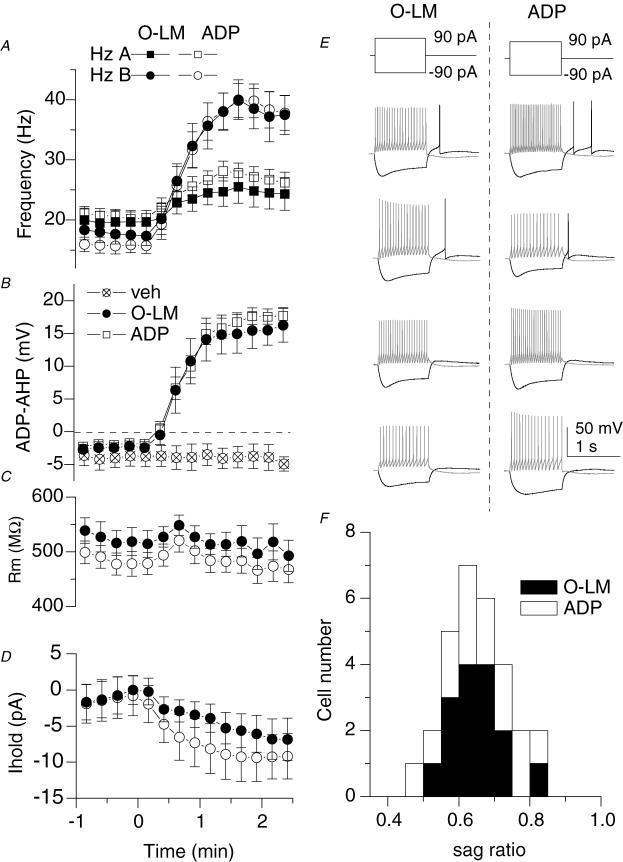
Figure 2. ADP-containing neurones and O-LM cells are highly overlapping cell populations.
A, plot of the AP frequency of the first 500 ms (Hz A; squares) versus the second 500 ms (Hz B; circles) during wash-in indicates a loss of accommodation. Open symbols represent ADP-containing cells (n= 31), a subset of which was anatomically identified as O-LM interneurones (n= 12; filled symbols). B, plot of the average membrane potential in a time window for the slow AHP (a 200–300 ms time window 100 ms after offset of current injection) during wash-in of muscarine. Crossed open symbols indicate the average of 3 cells in which no agonist (veh) was applied. C and D, plots of input resistance (C) and holding current (D) upon application of muscarine for 12 ADP-containing cells (○), including 8 O-LM cells (•). Input resistance was monitored by the peak deflection to 1 s, −20 pA current step occurring 10 s after each depolarizing current injection. Holding current was manually applied to maintain the voltage at −60 mV and monitored through a separate analog input channel. E, voltage responses to ± 90 pA, 1 s current injections from −60 mV for 4 O-LM cells and 4 ADP-containing unidentified cells. F, histograms of sag ratios for 29 ADP-containing cells (open bars), 15 of which were O-LM cells (filled bars). Sag ratio was calculated by the average voltage deflection in a 10 ms window at the end of a 1 s, −90 pA step divided by the peak voltage deflection during the current step.
O-LM cells underwent a profound, consistent transformation in firing properties upon mAChR activation (Figs 1C and D and 2A). In control conditions, the firing frequency decreased over the course of the depolarizing current injection, indicating a modest degree of spike frequency adaptation (Fig. 1C, t1; compare t1 and dashed line, denoting linearity, in Fig. 1D; Lien et al. 2002). However, in the presence of muscarine, the action potential frequency profoundly increased, persisting even after the depolarizing current injection had ended (Fig. 1C, t3–t4). As plotted in Fig. 2A, the firing frequency during the second 500 ms (Hz B in Fig. 1C) surpassed the firing frequency during the first 500 ms (Hz A in Fig. 1C), indicating that mAChR activation induces a switch in firing frequency from accommodating to accelerating during the depolarizing current injection (see also sublinear to supralinear slopes in Fig. 1D, t2–t4). Concomitant with the mAChR-induced change in firing properties, the AHP was abolished and replaced by a prominent afterdepolarization (ADP, Figs 1C and 2B). The large, mAChR-induced increase in AP frequency and emergence of an ADP was seen in all anatomically identified O-LM cells (Fig. 2A and B, filled symbols), indicating that mAChR activation had a highly consistent and stereotypical effect on this cell population. Despite these profound changes in firing properties, the input resistance (Rm), as measured by the peak voltage deflection to a −20 pA step delivered 10 s after depolarizing current injections, did not significantly change (Fig. 2C; 527 ± 24 versus 505 ± 25 MΩ; n= 8, P= 0.12). However, O-LM cells consistently depolarized in the presence of muscarine, as indicated by an increase in bias current needed to maintain the cells at −60 mV (Fig. 2D; −1.6 ± 2.5 versus−5.3 ± 2.6 pA, P= 0.011, n= 8).
mAChR activation also induced an ADP in 19 stratum oriens interneurones that had horizontally orientated dendrites in which severing or incomplete staining of the axon precluded the unambiguous identification of the cell type. However, the electrophysiological properties of these cells bore a striking resemblance to the identified subpopulation of O-LM cells, with input resistances (496 ± 25 versus 593 ± 41 MΩ, P= 0.10), membrane time constants (71.1 ± 5.8 versus 63.3 ± 3.0 ms, P= 0.20), and initial holding currents (from −60 mV; −8.0 ± 4.0 versus−10.4 ± 2.6 pA, P= 0.45) not significantly different from that of identified O-LM cells. Moreover, the intrinsic firing properties of these ADP-containing cells were similar (Fig. 2E), as was the degree of sag in response to hyperpolarization (Fig. 2F). When combined with the identified cells, the mAChR-induced increase in action potential frequency and time course of development of the ADP, as well as its magnitude, was virtually identical to that of anatomically confirmed O-LM interneurones (Fig. 2A–D, open circles). These findings strongly suggest that O-LM cells and ADP-containing interneurones are highly overlapping cell populations.
Other stratum oriens interneurone subtypes exhibit mAChR responses distinct from O-LM cells
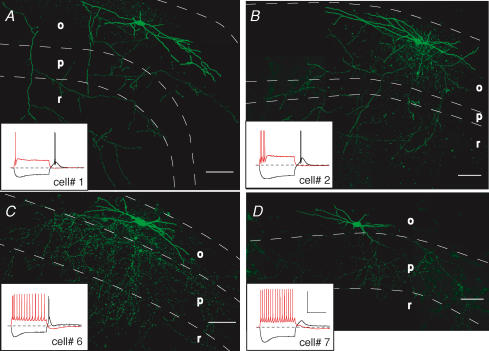
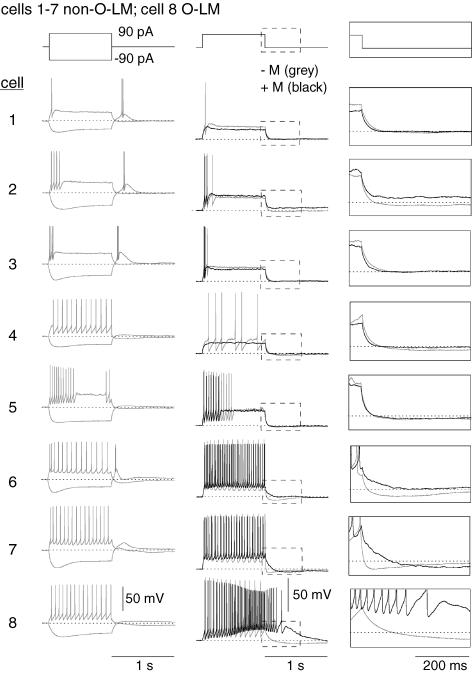
A subset of nine cells (7 cells using muscarine; 2 cells using ACh) exhibited morphological (Fig. 3) and electrophysiological (Fig. 4) characteristics different fom those of O-LM cells. These cells exhibited lower initial input resistance (311 ± 43 MΩ, P= 0.001) and shorter membrane time constants (44.2 ± 6.2 ms, P= 0.007, n= 7) than O-LM interneurones (unpaired t tests). While the precise morphological identity of these cells was difficult to unambiguously ascertain, the pattern of axonal arborizations for these cells could be sufficiently narrowed to exclude O-LM cells. For example, two cells exhibited diffuse arborizations within stratum radiatum, suggestive of a trilaminar cell (Fig. 3A and B; Sik et al. 1995; Gloveli et al. 2005). These cells exhibited strong spike frequency accommodation and broad rebound spikes (Fig. 3A and B, insets). In three cells that exhibited these morphological and electrophysiological characteristics, mAChR activation was accompanied by a reduction in input resistance and shunting of firing (Fig. 4A, cells 1–3). In a second class of stratum oriens interneurone, axon arborizations were seen primarily in stratum oriens and pyramidale (Fig. 3C and D), suggestive of basket, chandelier, or axo-axonic cell types (Macafferi, 2005). These cells differed subtly from O-LM cells in the rate of spike frequency accommodation and in the prominence of the hyperpolarization-induced sag (Fig. 3C and D, insets). mAChR activation caused an increase in firing frequency and an ADP much smaller in magnitude and duration (Fig. 4A, cells 6 and 7) than seen in O-LM cells (compare with Fig. 4A, cell no. 8).
Figure 3. Stratum oriens interneurones with axonal arborizations and electrophysiological characteristics different from those of O-LM cells.
Examples of stratum oriens interneurones that innervate stratum radiatum diffusely (A and B) or innervate stratum oriens and pyramidale (C and D). Scale bar: 100 μm. Insets, voltage responses to 1 s, +90 pA (red traces) or −90 pA (black traces) current injections from −60 mV. Scale bar: 50 mV, 500 ms. Cell layers o, p and r refer to stratum oriens, pyramidale and radiatum, respectively.
Figure 4. Other stratum oriens interneurone subtypes exhibit mAChR responses distinct from O-LM cells.
For 7 morphologically identified non-O-LM cells (cells 1–7), voltage responses to 1 s, ± 90 pA somatic current injections from −60 mV (grey traces). Also illustrated for each cell is an overlay of the voltage response to a 90 pA somatic current injection upon mAChR activation (black) compared to control conditions (grey). Panels on the right indicated magnified views of the voltage responses upon termination of the current injection. Note that the O-LM cell (cell no. 8) is the only cell that elicits a large muscarinic ADP.
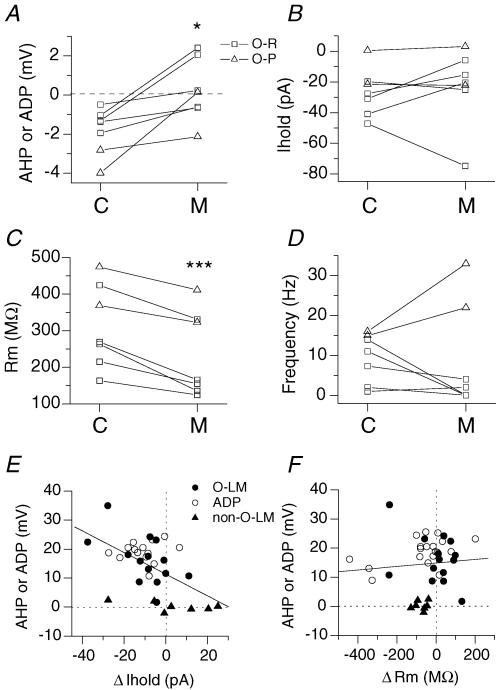
As a population, responses of non-O-LM cells to mAChR activation appeared to be distinct from that of O-LM cells in many ways (Fig. 5). First, although mAChR activation reduced the magnitude of the AHP (Fig. 5A; from −1.8 ± 0.5 mV to 0.2 ± 0.6 mV, P= 0.01, n= 7), no cell produced a large ADP characteristic of O-LM interneurones. Secondly, mAChR activation in O-LM cells was accompanied by depolarization, as indicated by an increase in inward current required to maintain them at −60 mV (Fig. 2D). Moreover, the magnitude of this inward current correlated with the magnitude of the muscarinic ADP (Fig. 5E; P= 8.7 × 10−4, n= 35). In contrast, in 4 of 7 non-O-LM cells, positive bias current was required to maintain the cells at −60 mV (Fig. 5B and E), indicating that mAChR activation often hyperpolarized these cells. Thirdly, O-LM cells showed no net change in input resistance (Figs 2C and 5F), whereas non-O-LM cells exhibited a consistent reduction in input resistance (from 311 ± 43 MΩ to 235 ± 44 MΩ, P= 9 × 10−4, n= 7, Fig. 5C and F) and membrane time constant (from 44.2 ± 6.2 ms to 28.6 ± 6.7 ms, P= 0.007, n= 7). Finally, all O-LM cells exhibited an AChR-induced increase in firing frequency (Fig. 2A) whereas only 2 of 7 non-O-LM cells showed an increase in firing frequency (Fig. 5F). In summary, non-O-LM cells consisted of several morphologically distinct cell types that exhibited considerable heterogeneity in responses to mAChR activation. However, these responses were distinct from O-LM interneurones, particularly in the capacity of these neurones to generate a large muscarinic ADP.
Figure 5. Electrophysiological differences between non-O-LM and O-LM cell populations.
A–D, for 7 non-O-LM cells, plot of the magnitude of the AHP or ADP (average voltage amplitude in a 200–300 window 100 ms after the termination of the current injection) (A), holding current at −60 mV (B), input resistance (C), and firing frequency in the first 500 ms (D), in control (C) and in the presence of muscarine (M). For A–D, cells in which the axon ramified primarily in stratum radiatum (O-R) are indicated by squares; cells in which the axon ramified primarily in stratum pyramidale (O-P) are indicated by triangles. Note that O-R and O-P cells can be differentiated on the basis of mAChR effects of firing frequency. E, plot of the mAChR-induced AHP or ADP versus mAChR change in holding current at −60 mV for total of 35 cells, including 14 O-LM cells, 14 ADP-containing cells, and 7 non-O-LM cells. F, plot of magnitude of the AHP or ADP versus change in Rm for the same cells as in E.
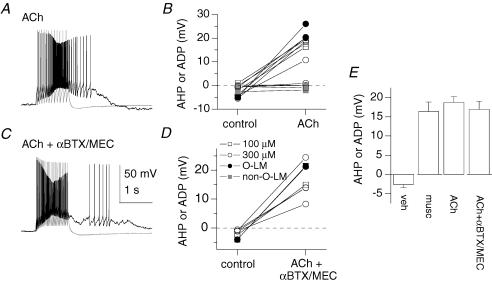
In 13 stratum oriens interneurones, we also tested the effect of the endogenous mAChR agonist acetylcholine (Fig. 6A and B). In eight neurones an ADP was induced using either 100 μm or 300 μm ACh (Fig. 6B; from −2.6 ± 0.9 mV to 18.7 ± 1.5 mV, P= 7 × 10−6, n= 8). These ADP-containing cells showed electrophysiological properties consistent with those of O-LM cells (Figs 1B and 2E), two of which were morphologically identified as O-LM cells (Fig. 6B, filled circles). In addition, five cells did not induce an ADP in response to 100 μm or 300 μm ACh. These cells exhibited firing properties different from that of O-LM cells, two of which were confirmed to be non-O-LM cells (Fig. 6B, grey squares). These results are consistent with the cell-type specific mAChR effects observed using muscarine.
Figure 6. ACh-induced responses in stratum oriens interneurones.
A, voltage responses to a 1 s, 90 pA current step from −60 mV before (grey) and after bath application of ACh (black). B, average voltage in a 200–300 ms window 100 ms after the offset of a +90 pA current injection in control conditions or in the presence of 100 μm (squares) or 300 μm (circles) acetylcholine for a population of 13 cells, including 2 O-LM cells (filled circles) and 2 non-O-LM cells (grey squares). C and D, same as A and B, but experiments were performed in the continued presence of nAChR antagonists α-BTX and MEC. E, summary graph of corresponding population data. Cell numbers (with anatomically identified O-LM cells in parentheses) are: vehicle, n= 3 (0); muscarine, n= 12 (12); ACh, n= 8 (2); ACh in the presence of nAChR antagonists (ACh +αBTX/MEC), n= 7 (2).
To address whether nicotinic acetylcholine receptors (nAChRs) contributed to the ACh-induced ADP, we applied α-bungarotoxin (α-BTX; 100 nm) and mecamylamine (MEC; 10 μm), which block α7 and non-α7 nAChRs, respectively, to control and ACh-containing solutions in seven neurones. mACh-induced ADPs were seen in all seven cells, two of which were anatomically confirmed to be from O-LM cells (Fig. 6C and D). The amplitude of the ACh-mediated ADP occurring in the presence of nAChR blockers (16.9 ± 2.1 mV) was not significantly different from that seen in ACh alone (Fig. 6E; 18.7 ± 1.5 mV, n= 8, P= 0.50, unpaired t test), indicating that emergence of the ADP is due to ACh acting exclusively at mAChRs.
ADPs in O-LM cells are mediated by M1/M3 receptors
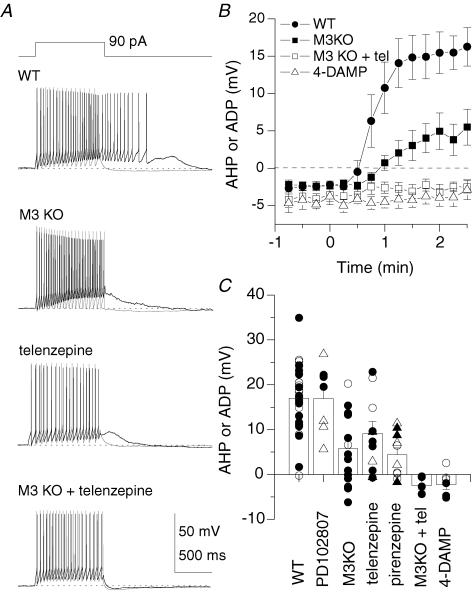
To determine which mAChR subtypes mediate the ADP in O-LM interneurones, we monitored the development of the mAChR-induced ADP in slices from mAChR subtype knockout (KO) mice and/or in the continued presence of mAChR antagonists (Fig. 7). In 13 O-LM cells from M3 KO mice, a mAChR-mediated ADP developed (4.6 ± 2.1 mV) but was reduced in amplitude compared to WT O-LM cells (Fig. 7A and B; P= 0.001). Similarly, in eight O-LM cells, the mAChR-mediated ADP developed in the presence of the M1 antagonist telenzepine (100 nm; 6.9 ± 2.7 mV) but at reduced amplitude compared to WT O-LM cells (Fig. 7A–C; P= 0.02). Consistent with the reduction in both M3 KO mice and in the presence of an M1 receptor antagonist, the mAChR-mediated ADP was abolished by application of telenzepine in M3 KO mice (Fig. 7A–C; −2.5 ± 0.9 mV, P= 7 × 10−6, n= 5 O-LM cells), confirming that the ADP was generated by M1 and M3 mAChRs. In three O-LM cells, the ADP was also eliminated in the presence of the broad spectrum mAChR antagonist 4-DAMP (Fig. 7B and C; −3.9 ± 1.0 mV, P= 4 × 10−6). As summarized in Fig. 7C, we also tested the M4 antagonist PD102807 (100 nm) and M1 antagonist pirenzepine (400 nm) in slices from both WT (circles) and M2 KO mice (triangles). These data also support the conclusion that the mAChR-induced ADP is mediated primarily by M1/M3 mAChRs.
Figure 7. The mAChR-induced ADP in O-LM cells is mediated by M1/M3 mAChRs.
Experiments were conducted similar to Fig. 1C–E, but in slices from mAChR knockout (KO) mice and/or in the continued presence of mAChR antagonists present in both control and muscarine-containing solutions. A, representative traces from identified O-LM cells in WT animals, in a slice from a M3 KO mouse, in the presence of the M1 antagonist telenzepine, and in the presence of telenzepine in slices from a M3 KO mouse. B, plot of emergence of the ADP in WT (n= 12), in slices from M3 KO mice (n= 13), in the presence of telenzepine in M3 KO mice (n= 5), and from WT mice in the presence of 4-DAMP (n= 3), in anatomically identified O-LM cells. C, summary plot of amplitude of the AHP or ADP in the presence of mAChR antagonists and/or in mAChR KO mice. Identified O-LM cells are indicated as filled symbols. Circles denote WT; triangles denote M2 KO mice.
The mAChR-induced ADP is associated with the inhibition of the M-current, a Ca2+-activated potassium current, and activation of ICAT
What ionic conductances are targets of mAChR modulation in O-LM cells? We first investigated the M-current (IM), a K+ conductance named historically for its susceptibility to mAChR modulation (Brown & Adams, 1980). In CA1 pyramidal cells, IM has been shown not only to be linked to a subthreshold resonance at theta frequencies (Hu et al. 2002) but also to control the emergence of an ADP (Yue & Yaari, 2004). Horizontally orientated stratum oriens interneurones, which presumably include O-LM interneurones, exhibit a similar theta frequency preference (Pike et al. 2000), but it is unclear whether IM may be present in these cells. While KCNQ2 channels have been detected in both parvalbumin-positive and -negative stratum oriens interneurones (Cooper et al. 2001), no study has yet examined whether IM is present in these cells.
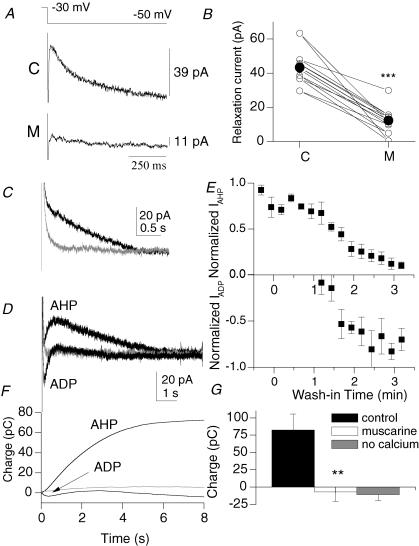
In a subset of experiments in which a muscarinic ADP was detected under current clamp conditions (Fig. 1C), we examined IM under voltage clamp conditions (Fig. 8A and B) before and after wash-in of muscarine. The M-current can be distinguished from other outward potassium currents by its slow relaxation kinetics upon stepping from −30 to −50 mV (i.e. Hadley et al. 2003). We fitted the time course of this current with a single exponential (τ= 224 ± 24 ms, n= 7) and used the amplitude as an index for the magnitude of the M-current. In control conditions in an example cell (C), this amplitude was 39 pA (Fig. 8A, top panel). In the presence of muscarine (M), the amplitude was reduced to 11 pA (Fig. 8A, bottom panel). Averaging across seven cells, the M-current was reduced from 43.4 ± 4.0 pA to 12.4 ± 3.5 pA (P= 0.001) in ADP-containing cells (Fig. 8B). The M-current was also reduced by the KCNQ channel antagonist linopirdine (10 μm; from 26.8 ± 5.2 pA to 8.9 ± 2.6 pA, P= 0.001, n= 7), confirming the presence of IM (data not shown; see also Fig. 9A–E). Thus, the M-current is consistently inhibited by mAChR activation in cells that generate a mAChR-induced ADP.
Figure 8. mAChR activation is associated with the inhibition of IM and IAHP, and the appearance of ICAT.
A, a −20 mV step from a holding potential of −30 mV induces a relaxation of IM in control (upper trace), which is attenuated in the presence of 10 μm muscarine (lower trace). Each trace is the average of 5 traces. Single exponential fits are shown in grey. B, cells associated with a mAChR-induced ADP exhibit a strong inhibition of IM by muscarine (***P < 0.001). Means are indicated by the filled circles. C, outward tail currents (measured at −50 mV) activated by a 600 ms step to 0 mV, delivered from a holding potential of −50 mV once every 15 s (black). The slow IAHP is inhibited in Ca2+-free solutions (grey). D, overlaid traces from control (black, labelled ‘AHP’) and in the presence of muscarine (black, labelled ‘ADP’) indicate the loss of IAHP and the appearance of ICAT (n= 5). ICAT is abolished in the presence of Ca2+-free solutions (grey). E, the time course of the inhibition of IAHP and appearance of ICAT during wash-in of muscarine. Normalization was based from a 10 ms window around peak outward or peak inward currents. F, cumulative charge upon integration of traces in E. G, muscarine significantly reduces the charge in a 6–8 s time window after the offset of the 50 mV depolarizing pulse (P < 0.01, n= 9).
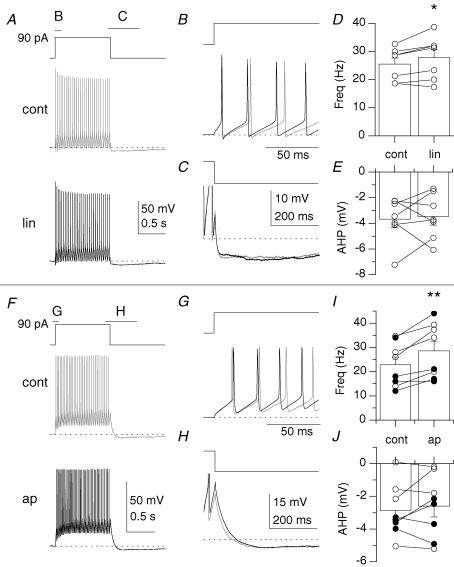
Figure 9. KCNQ- and SK2-mediated K+ channels do not control the emergence of the ADP.
A, voltage response to the introduction of a 90 pA current step from −60 mV in control (cont; grey) and in the presence of linopirdine (lin; black). B and C illustrate overlaid regions in A at expanded time scales. D and E, plots of action potential frequency in the first 500 ms (D) or average membrane potential in a 200–300 ms time window 100 ms after offset of current injection (E) for 7 stratum oriens interneurones. F, voltage response to the introduction of a 90 pA current step from −60 mV in control (cont) and in the presence of apamin (ap). G and H illustrate regions in F at expanded time scales. I and J, plots of action potential frequency in the first 500 ms (I) or average membrane potential in a 200–300 ms time window 100 ms after offset of current injection (J) for 8 stratum oriens interneurones. Filled circles indicate anatomically identified O-LM cells.
We next determined whether the slow Ca2+-activated K+ conductance (IAHP) was modulated by muscarine in stratum oriens interneurones. IAHP, associated with mAChR-induced ADPs in pyramidal cells (Cole & Nicoll, 1984; Madison et al. 1987), contributes to spike frequency adaptation and the slow AHP following sustained depolarization. Although stratum oriens interneurone subpopulations also exhibit spike frequency accommodation to some extent (i.e. Fig. 1D), IAHP has so far been studied only in interneurones of the stratum lacunosum-moleculare (Aoki & Baraban, 2000). Upon stepping back from 0 mV to to 50 mV in voltage clamp mode, this outward conductance decayed slowly (mean duration: 6.7 ± 0.6 s, n= 9) and was blocked by removing extracellular Ca2+ (Fig. 8C and G), confirming the presence of this conductance in these cells. Upon wash-in of muscarine, this outward current gradually diminished (Fig. 8D and E, upper panel). Interestingly, this outward current was gradually replaced by a transient inward current (Fig. 8D and E, lower panel; n= 5). The time course of loss of IAHP and gain of the inward current was similar to the time course of loss and gain of the AHP and ADP observed in current clamp mode (Fig. 2B). The emergent inward current was also blocked by removal of extracellular Ca2+ (Fig. 8D, grey trace), also indicating that this conductance was Ca2+ dependent. In integrating the trace over the course of 8 s (Fig. 8F), the mean charge associated with the outward conductance was 82.3 ± 23.2 pC and was abolished in the presence of muscarine (to −6.9 ± 13.7 pC; P= 0.004, n= 9), which is not statistically different from that observed in the Ca2+-free condition (to −10.8 ± 8.5 pC; P= 0.63; Fig. 8G). mAChR activation had no consistent effect on other ionic conductances, such as Ih, the A-type K+ conductance, and the K+ leak conductance (data not shown). Therefore, mAChR activation is associated with inhibition of IM and IAHP, and activation of ICAT.
Calcium entry through ICAT controls the emergence of the muscarinic ADP in O-LM cells
To what extent does each of these conductances impact the firing properties and control the emergence of the ADP in O-LM interneurones? We examined firing properties and the emergence of the ADP under different pharmacological and ionic conditions (Figs 9 and 10).
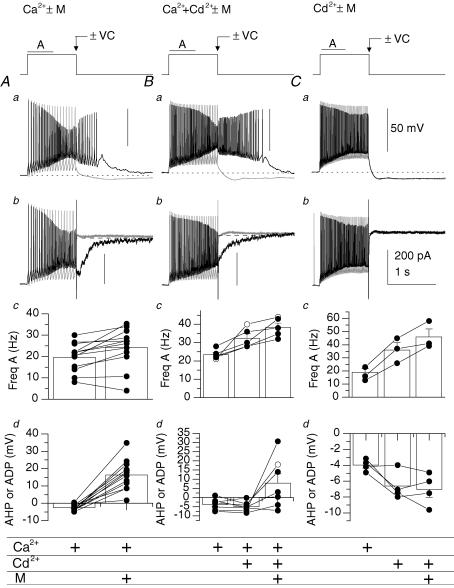
Figure 10. Ca2+ entry through ICAT controls the emergence of the mAChR-induced ADP.
A–C, firing properties in the absence (−M; grey) or presence (+M; black) of muscarine examined with 2 mm CaCl2 present (n= 12) (A), 2 mm CaCl2+ 0.2 mm CdCl2 present (n= 7) (B), or with 0.2 CdCl2 alone (n= 4) (C). To reveal conductances activated as a consequence of firing, the amplifier was switched from current clamp (−VC; Aa–Ca) to voltage clamp mode (+VC; Ab–Cb) at the end of the 1 s current pulse. Panels c and d, summary of average changes in firing in the first 500 ms of the current pulse (Hz A) (Ac–Cc) or amplitude (Ad–Cd) of the AHP or ADP. Two cells in Bc and one cell in Cc were excluded because the action potential frequency could not be reliably measured because the level of depolarization induced Na+ channel inactivation in these conditions. Anatomically identified O-LM cells are indicated by filled circles.
M-current has been shown to control the emergence of an ADP in pyramidal cells (Yue & Yaari, 2004), and we first asked whether it can produce a similar effect in stratum oriens interneurones (Fig. 9A–E). Linopirdine, the KCNQ channel antagonist, produced a modest increase in frequency (Fig. 9A–B and D) from 25.5 ± 2.2 Hz to 27.8 ± 2.8 Hz, P= 0.03), but had little effect on the magnitude of the AHP (Fig. 9A, C and E; from −3.7 ± 0.3 mV to −3.5 ± 0.7 mV, P= 0.73, n= 7). The SK2-channel blocker apamin also increased action potential frequency (Fig. 9F, G and I; from 22.8 ± 3.1 Hz to 28.6 ± 4.0 Hz, P= 0.003) but had no effect on AHP magnitude (Fig. 9F, H and J; from −2.9 ± 0.6 mV to −2.6 ± 0.7 mV, P= 0.45, n= 8). Thus, while inhibition of SK2- or KCNQ-mediated channels may potentially account for the increase in action potential frequency seen in muscarine (Fig. 2A, Hz A; Fig. 10Ac), neither potassium conductance controlled the emergence of an ADP.
We next determined to what extent calcium-mediated conductances controlled the emergence of the ADP by reducing calcium entry by blocking voltage-gated calcium channels and/or reducing external calcium concentrations (Fig. 10). In addition, we also performed ‘switch clamp’ experiments in which the amplifier was abruptly switched from current clamp to voltage clamp mode at the end of the current pulse to reveal conductances activated as a consequence of firing. In control solutions (2 mm Ca2+), a depolarizing stimulus caused an accommodating train of high frequency APs followed by an AHP (Fig. 10Aa, grey trace) and a slow IAHP in voltage clamp mode (Fig. 10Ab, grey trace). In contrast, in the presence of muscarine (+M), an increase in firing frequency and accompanying ADP was observed (Fig. 10Aa, black trace). In switching to voltage clamp mode, ICAT was observed to underlie the ADP (Fig. 10Ab, black trace). In 12 O-LM cells, mAChR activation increased action potential frequency from 19.7 ± 1.9 Hz to 24.3 ± 2.7 Hz (P= 0.007, Fig. 10Ac) and transformed the AHP to an ADP (from −2.5 ± 0.5 mV to 16.4 ± 2.5 mV, P= 1 × 10−5; Fig. 10Ad). Inhibiting voltage-dependent calcium channels (VDCCs) and Ca2+-dependent IAHP with 0.2 mm Cd2+ (Fig. 10B) increased AP frequency from 23.5 ± 1.2 Hz to 32.4 ± 2.4 Hz (P= 0.03, n= 5) but did not remove the AHP (Fig. 10Ba, grey trace; Fig. 10Bd, −3.7 ± 1.1 versus−4.9 ± 1.0 mV, n= 7, P= 0.23). Moreover, preincubation with 0.2 mm Cd2+ did not prevent the generation of the mAChR-induced ADP (Fig. 10Ba, black trace; Fig. 10Bd, 7.8 ± 5.1 versus 16.4 ± 2.5 mV, n= 7, P= 0.17) and associated ICAT (Fig. 10Bb, black trace) indicating that Ca2+ entry through VDCCs is not required for ADP generation. However, incubation with Cd2+ only (omitting 2 mm CaCl2) fully prevented the mAChR-induced ADP (Fig. 10Ca, black trace; Fig. 10Cd, −6.6 mV versus−7.1 mV ± 1.0 mV, n= 4, P= 0.61) and ICAT (Fig. 10Cb, black trace), indicating that the emergence of the ADP is Ca2+ dependent yet does not depend on calcium entry through VDCCs. Thus, Ca2+ entry through ICAT appears to be a dominant factor in controlling the emergence of the ADP, with mAChR-induced inhibition of IM and IAHP playing secondary roles in increasing the firing frequency.
Discussion
Cholinergic specializations in stratum oriens interneurone cell types
There is increasing evidence that the diversity of features of hippocampal interneurones reflect specializations that converge to yield precise and unique roles for subclasses of interneurones in network and oscillatory activities (Klausberger et al. 2003; Pouille & Scanziani, 2004). Increasingly, both in vivo and in vitro evidence suggests that anatomically and neurochemically defined subsets of hippocampal interneurones can exhibit remarkably homogeneous electrophysiological and network properties (Pouille & Scanziani, 2004; Gloveli et al. 2005; Somogyi & Klausberger, 2005). However, our current understanding of whether such specializations extend to cholinergic neuromodulation has remained unclear. In two previous studies, mAChR-induced changes in resting membrane potential were compared in interneurones across all hippocampal layers (Parra et al. 1998; McQuiston & Madison, 1999a). No obvious relationship was found between the type of mAChR response and morphologically identified cell types. In both studies, very few examples were presented investigating the effect of mAChR activation within a morphologically defined interneurone phenotype. We took a narrower approach, instead concentrating on a particular subclass of interneurone within stratum oriens that had fairly homogeneous firing patterns and axonal arborizations, the O-LM cell. O-LM cells are also known to be homogeneous in neurochemical content: the vast majority of O-LM cells contain the peptide somatostatin (Maccaferri et al. 2000; Martina et al. 2000; Oliva et al. 2000). Here, we found that mAChR activation was indeed intimately linked to cell type; in O-LM cells, M1/M3 activation induced a homogeneous response profile that comprised the reliable activation of a repertoire of identified mAChR-sensitive conductances, leading to profound alterations in the cells' firing properties. In this and in previous investigations, cells that underwent depolarization were the most common response type in stratum oriens (Parra et al. 1998; McQuiston & Madison, 1999a, b). Depolarizing cells are most likely to be O-LM cells for several reasons. First, both O-LM cells and depolarizing cells are the frequently encountered cells in stratum oriens (McQuiston & Madison, 1999a; Maccaferri, 2005). Second, a significant correlation exists between mAChR-induced depolarization and the presence of the ADP (Figs 2B and D and 5E). Finally, O-LM and ADP- containing cells are highly overlapping cell populations that share many electrophysiological similarities (Fig. 2). Thus, it is likely that the majority of cells that depolarize in response to mAChR activation in stratum oriens are O-LM cells.
Do distinct cholinergic specializations also exist in other morphologically defined interneurone types within stratum oriens, and in other layers? Although non-O-LM cells were encountered more rarely within stratum oriens and contained a more diverse morphological and neurochemical cell population, several observations were noted. The distinct morphological phenotypes that were present not only had similar firing patterns but had mAChR-induced responses distinct from other morphologically identified phenotypes, including O-LM cells. For example, two cells had axons ramifying in stratum pyramidale, shared similar electrophysiological properties, and exhibited a mAChR-induced increase in firing frequency, yet lacked an ADP. Furthermore, three other cells showed more diffuse ramifications in stratum radiatum resembling that of a trilaminar cell. Remarkably, these cells also exhibited similar firing patterns in control conditions, yet all three decreased in firing in response to mAChR activation. Trilaminar cells may have a different compliment of mAChRs than O-LM cells; rather than M1/M3 receptors, as in O-LM cells, immunocytochemical evidence suggests that trilaminar cells are rich in M2 receptors (Hajos et al. 1998; Somogyi & Klausberger, 2005). Although our data set does not include all morphologically identified stratum oriens cell types, on the basis of our current findings, it appears likely that each morphologically identified interneurone subclass may respond to mAChR activation in a distinct and unique way. Future studies using other neurochemically, morphologically, and electrophysiologically homogeneous interneurone types will likely reveal the extent that this organizing principle extends to other cell populations.
Potential mechanisms underlying the muscarinic ADP in O-LM cells
In this study, we identified several ionic conductances that were modulated by mAChR activation. In accordance with an earlier study (McQuiston & Madison, 1999a), where mAChR activation was associated with inhibition of a K+ conductance, here, we distinguished two K+ conductances that were inhibited by muscarine (Fig. 6). Although both the Ca2+-dependent AHP and IM had clear effects on firing frequency, block of neither conductance led to an ADP (Fig. 9). However, we cannot conclusively rule out that some Ca2+-independent portion of the AHP may play a role in controlling the emergence of the ADP. Although we detected appreciable Ca2+ sensitivity of the AHP in our voltage clamp experiments (Fig. 8), interestingly, a significant AHP persists in the presence of Cd2+ and zero Ca2+ in current clamp mode (Fig. 10). Apamin- and Ca2+-insensitive AHPs have been observed in interneurones previously (Zhang & McBain, 1995; McQuiston & Madison, 1999b), though not explicitly raised as a point of discussion in these papers.
One possible molecular candidate for mediating the Ca2+-independent component of the AHP is the Slack/Slick family of Na+-activated K+ channels. These channels have been shown to produce a prolonged Ca2+-independent AHP in response to repetitive firing in visual cortical neurones (Sanchez-Vives et al. 2000; Bhattacharjee & Kaczmarek, 2005), thus sharing some similarities to the Ca2+-independent AHP observed here (Fig. 10B and C). Moreover, Slick mRNA is present in CA1 stratum oriens interneurones (Bhattacharjee et al. 2005). Since Na+ entry is blocked with TTX in voltage clamp mode, the presence of Na+-activated K+ channels may also help explain the apparent higher Ca2+ sensitivity of the AHP observed in voltage clamp mode than in current clamp mode. In preliminary experiments, we were able to remove both Ca2+-dependent and -independent components of the AHP with bath application of 10 mm TEA, unmasking an inward current in control conditions. However, unlike the large mAChR-induced inward current, the inward current revealed with TEA was much smaller in magnitude (∼10 pA) and duration (< 300 ms), and was not accompanied by an increase in channel noise (Fig. 10Ab). Thus, although reduction of the AHP may contribute to a small extent towards revealing the ADP, calcium entry through ICAT must occur in order to generate the large inward current that accompanies the prolonged mAChR-induced ADP (Fig. 10).
What is the molecular identity of ICAT? Interestingly, mGluR activation also activates a large inward current in O-LM cells that is similar to ICAT in ionic permeability and is not dependent on Ca2+ entry through VDCCs (McBain et al. 1994). There is an unusually high mGluR1 concentration in O-LM cells (Baude et al. 1993), raising the possibility that ICAT may be linked to a TRP family of cationic conductances, as seen in cerebellar Purkinje cells (Kim et al. 2003). However, preliminary experiments using antagonists selective for some TRP channel subtypes (i.e. SKF96365, lanthanum) have failed to unequivocally identify the molecular identity of ICAT (J. J. Lawrence and C. J. McBain, unpublished observations). Nevertheless, M1/M3 receptors and mGluR1 receptors may be coupled to similar cellular machinery (Guerineau et al. 1997). The development of more specific pharmacological tools for discriminating TRP channel subunits may clarify the molecular identity of ICAT.
Functional implications of cholinergic signalling onto hippocampal interneurones
The MS-DBB, a major extrahippocampal source of cholinergic innervation, innervates both pyramidal cells and interneurones in the hippocampus (Cobb & Davies, 2005). Electrical stimulation of cholinergic afferents can generate large depolarizations in pyramidal cells (Madison et al. 1987; Morton & Davies, 1997). In a recent review, Cobb & Davies (2005) demonstrate that a long-lasting, atropine-sensitive ADP can also be synaptically evoked onto CA1 interneurones. Given the susceptibility of O-LM cells to cholinergic modulation and the participation of MS-DBB neurones in theta oscillations (Buzsaki, 2002), we propose that cholinergic afferents play an important role in engaging O-LM cells during theta oscillations. mAChR-induced excitation of O-LM cells would increase the inhibitory tone onto distal dendrites of pyramidal cells; robust GABA release from the dense axonal arborizations of O-LM cells may contribute to the large sink–source alternation between stratum pyramidale and stratum lacunosum moleculare (Kamondi et al. 1998; Buzsaki, 2002). However, we predict that mAChR activation of other interneuronal types, such as trilaminar interneurones, would lead to the disengagement of these neurones from the hippocampal network. Still other interneurone types, such as parvalbumin-positive basket cells, may be modulated in more complex ways. In addition to postsynaptic mAChR-mediated modulation, these cells may be modulated presynaptically via M2 receptors (Hajos et al. 1998; Freund, 2003). Thus, cholinergic afferents from the medial septum may fine-tune different interneurone subclasses through distinct cholinergic specializations, contributing to the emergence of a theta-permissive state.
Acknowledgments
J.M.S. and Z.M.G. are HHMI-NIH Research Scholars. This work was supported by a NICHD intramural funding to CMcB. We thank Dr Jurgen Wess (NIMH) for providing the M2- and M3-muscarinic receptor knockout mice, Brian Jeffries for immunofluorescence labelling, and Drs Maciej Lazarewicz and Mark Stopfer for reading an earlier version of the manuscript.
References
- Aoki T, Baraban SC. Properties of a calcium-activated K+ current on interneurons in the developing rat hippocampus. J Neurophysiol. 2000;83:3453–3461. doi: 10.1152/jn.2000.83.6.3453. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, von Hehn CA, Mei X, Kaczmarek LK. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J Comp Neurol. 2005;484:80–92. doi: 10.1002/cne.20462. [DOI] [PubMed] [Google Scholar]
- Bischof WF, Boulanger P. Spatial navigation in virtual reality environments: an EEG analysis. Cyberpsychol Behav. 2003;6:487–495. doi: 10.1089/109493103769710514. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Varga V, Szilagyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik E, Borgnis R, Muller RU, Fox SE. The effects on place cells of local scopolamine dialysis are mimicked by a mixture of two specific muscarinic antagonists. J Neurosci. 2004;24:9313–9323. doi: 10.1523/JNEUROSCI.1618-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik ES, Muller RU, Fox SE. Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J Neurosci. 2003;23:611–621. doi: 10.1523/JNEUROSCI.23-02-00611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol. 2005;562:81–88. doi: 10.1113/jphysiol.2004.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau NC, Bossu JL, Gahwiler BH, Gerber U. G-protein-mediated desensitization of metabotropic glutamatergic and muscarinic responses in CA3 cells in rat hippocampus. J Physiol. 1997;500:487–496. doi: 10.1113/jphysiol.1997.sp022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Halliwell JV. Physiological mechanisms of cholinergic action in the hippocampus. Prog Brain Res. 1990;84:255–272. doi: 10.1016/s0079-6123(08)60910-3. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex. 2003;39:993–1008. doi: 10.1016/s0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Maccaferri G. Stratum oriens horizontal interneuron diversity and hippocampal network dynamics. J Physiol. 2005;562:73–80. doi: 10.1113/jphysiol.2004.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. Interneuron diversity series: Hippocampal interneuron classifications – making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–571. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity has multiple effects on the resting membrane potentials of CA1 hippocampal interneurons. J Neurosci. 1999a;19:5693–5702. doi: 10.1523/JNEUROSCI.19-14-05693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity induces an afterdepolarization in a subpopulation of hippocampal CA1 interneurons. J Neurosci. 1999b;19:5703–5710. doi: 10.1523/JNEUROSCI.19-14-05703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Davies CH. Regulation of muscarinic acetylcholine receptor-mediated synaptic responses by adenosine receptors in the rat hippocampus. J Physiol. 1997;502:75–90. doi: 10.1111/j.1469-7793.1997.075bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus. Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Kramis R, Robinson TE. Hippocampal electrical activity during waking behaviour and sleep: analyses using centrally acting drugs. Ciba Found Symp. 1977;58:199–226. doi: 10.1002/9780470720394.ch10. [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995;488:661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]