Abstract
Patients with panic disorder are at increased cardiac risk. While the mechanisms responsible remain unknown, activation of the sympathetic nervous system may be implicated. Using isotope dilution methodology, investigations of whole-body and regional sympathetic nervous activity have failed to show any differences between patients with panic disorder and healthy subjects. Using direct recording of single unit efferent sympathetic vasoconstrictor nerve activity by microneurography we examined sympathetic nervous function in patients with panic disorder more precisely than previously reported. The activity of multiunit and single unit vasoconstrictor sympathetic nerves was recorded at rest at the level of the peroneal nerve in 10 patients diagnosed with panic disorder and in nine matched healthy volunteers. Multiunit sympathetic activity was not different between the two groups (26 ± 3 bursts min−1 in patients with panic disorder and 28 ± 3 bursts min−1 in controls). The firing frequency of single unit vasoconstrictor neurones was also similar between the two groups (0.38 ± 0.09 versus 0.22 ± 0.03 Hz). However, the probability of firing during a sympathetic burst was higher in patients with panic disorder compared with healthy controls (45 ± 5%versus 32 ± 3%, P < 0.05). When only the neural bursts during which the vasoconstrictor neurone was active were considered, we found that in patients with panic disorder the neurones tended to fire more often in a ‘multiple spike’ pattern than in the controls (i.e. the probability of the neurone firing twice was 25 ± 3% in patients with panic disorder compared with 14 ± 3% in controls). Quantification from single vasoconstrictor unit recording provides evidence of a disturbed sympathetic firing pattern in patients with panic disorder.
Panic disorder is a distressing and disabling condition with patients experiencing recurrent episodes of unexpected intense anxiety of sudden onset. The anxiety peaks within 10 min and is associated with multiple somatic symptoms such as, palpitations, hyperventilation, chest discomfort, sweating, nausea and dizziness. Patients often fear that they have heart disease, and also often have persistent fear of having another attack or the consequences of such an attack. Indeed, earlier reports documented an abnormally high mortality rate due to cardiovascular and cerebrovascular events (Coryell et al. 1982; Weissman et al. 1990), and more recent studies indicate that there is increased risk of sudden death in patients with anxiety disorders (Kawachi et al. 1994a, b). The mechanism responsible is not known, but may involve activation or dysregulation of the sympathetic nervous system.
A relatively selective stimulation of the sympathetic nerves to the heart occurs during experimental mental stress (Esler et al. 1995). Using isotope dilution methodology for the assessment of the rate of spillover of noradrenaline to plasma we were unable to demonstrate any difference in resting whole body or cardiac sympathetic activity in patients with panic disorder compared with healthy control subjects (Wilkinson et al. 1998). Direct recording of the electrical activity of multiunit vasoconstrictor sympathetic nerves using the technique of microneurography has also indicated that resting sympathetic activity is normal in patients with panic disorder (Wilkinson et al. 1998; Lambert et al. 2002). During a panic attack, the pattern of multiunit nerve activity is characterized by an increase in burst amplitude rather than a change in firing frequency, which is unexpected given that a pronounced cardiac sympathetic activation was also evident. (Wilkinson et al. 1998). This pattern of nerve firing is different from the usual response associated with sympathetic nervous activation (Lambert & Schlaich, 2004). While measuring the rate of firing of multiunit vasoconstrictor muscle sympathetic nerve activity (MSNA) has provided important information in many aspects of cardiovascular medicine, the technique is limited in that multiunit recordings are unable to distinguish the pattern of firing or the number of the vasoconstrictor fibres associated with the burst. In order to resolve these drawbacks Macefield et al. (1994) refined the technique of clinical microneurography to enable the determination of the firing characteristics of single sympathetic vasoconstrictor units. This technique has recently been applied to the examination of sympathetic nerve activity in a variety of important clinical conditions including heart failure, obstructive sleep apnoea and hypertension (Greenwood et al. 1999; Macefield et al. 1999a; Elam et al. 2002). In these conditions examination of the sympathetic discharge pattern was found to favour the occurrence of multiple spikes during a neural burst. We hypothesized that a similar pattern of nerve firing would be evident in patients with panic disorder, a condition where, although the rate of nerve firing is normal, the regulation of sympathetic nerve firing seems altered (Wilkinson et al. 1998; Lambert et al. 2002). In the present study, in order to examine the firing properties of sympathetic vasoconstrictor fibres more thoroughly in patients with panic disorder we examined the resting firing characteristics of single unit sympathetic vasoconstrictor nerves.
Methods
Participants
Data were obtained in 10 patients with panic disorder (3 males and 7 females, ages 24–61 years; mean 40 ± 4 years) and 9 healthy volunteers (3 males and 6 females, ages 21–64; mean 40 ± 5 years). All subjects were recruited by local advertisement and were examined by both the participating psychiatrist and a clinical psychologist in order to determine that they fulfilled the DSM-IV criteria for panic disorder (American Psychiatric Association, 1994) and had no other significant psychiatric comorbidity. Patients with panic disorder had no preexisting cardiac disease, diabetes or treated hypertension and had not taken any medications in the 2 weeks prior to testing. Healthy subjects had no history of chronic physical or mental illness and were not taking medication. Women of both groups were required to have a negative pregnancy test on the day of the study. All patients were asked to refrain from caffeinated beverages and tobacco smoking for 12 h before the study. The research protocol conformed to the relevant guidelines of the National Health and Medical Research Council of Australia and were approved by the Alfred Hospital Human Research Ethics Committee. All participants gave written informed consent for their participation.
Experimental protocol
On the experimental day Spielberger's State-Trait Anxiety Inventory (STAI) was used to assess the anxiety proneness (trait anxiety) and the situational anxiety (state anxiety) of each participant (Spielberger, 1988). This was administered immediately prior to the commencement of the study. The STAI consists of two separate 20-item questionnaires. The first required the respondent to assess how they ‘generally feel’ (trait anxiety) while the second questionnaire required subjects to report the intensity of their feelings of anxiety ‘right now, at this moment’ (state anxiety). Participants rated their degree of agreement with the 20 items on each questionnaire using a four-point Likert-type scale. Raw scores thus potentially range from a minimum of 20 to a maximum of 80 for each scale. Low scores indicate low anxiety proneness on the ‘trait’ scale, and relative calm on the ‘state’ scale. The construction and validity of Spielberger's inventory has previously been established (Spielberger, 1988). All patients had their diagnosis confirmed by a clinical interview with a psychiatrist as well as by a validated diagnostic interview the Mini International Neuropsychiatric Inventory (Sheehan et al. 1998).
All experiments were performed with participants in the supine position. Blood pressure was measured using radial arterial tonometry (CBM 7000, Colin Corp., Komak, Japan), heart rate was determined using a three-lead ECG recording and respiration rate was assessed using a strain-gauge transducer attached to a strap around the chest.
Muscle sympathetic nerve activity (MSNA)
Multiunit postganglionic sympathetic activity was recorded using microneurography in a muscle fascicle of the peroneal nerve at the fibular head. The common peroneal nerve was located by palpation and electrical stimulation via a surface probe. A tungsten microelectrode (FHC, Bowdoinham, ME, USA) was then inserted percutaneously and adjusted until satisfactory spontaneous MSNA was observed in accordance with previously described criteria (Sundlof & Wallin, 1977; Thompson et al. 1994). Resting measurements of multiunit postganglionic sympathetic activity were performed over a 15-min period during quiet breathing. The microelectrode was then manipulated until large unitary discharges (i.e. single-unit MSNA) appeared out of the multiunit sympathetic bursts. Resting measurements for single unit sympathetic activity were made for approximately 3 min.
Data acquisition and analysis
The nerve signal was amplified (× 50 000), filtered (bandpass, 700–2000 Hz) and integrated with a time constant of 0.1 s to produce a mean voltage neurogram. The signal was digitized with a sampling frequency of 1000 Hz during multiunit MSNA recording and of 20 000 Hz during single-unit MSNA recording (PowerLab recording system, model ML785/8SP, ADInstruments, Sydney, Australia).
Sympathetic bursts from the multiunit sympathetic activity recordings were counted manually over a period of 5–10 min. MSNA was expressed as burst frequency (bursts min−1). The amplitude of the largest burst during the analysed period of the recording was defined as 100 and all other bursts were expressed as the percentage of the largest one. The total power of MSNA was calculated by multiplying the mean burst amplitude per minute per burst rate and expressed as units per minute. The median burst amplitude was defined as the value at which 50% of the burst amplitudes were larger and 50% were smaller.
For the analysis of single unit recordings, we applied similar methods as those developed by Macefield et al. (1994), which use very stringent criteria for the acceptance of a recording as being truly unitary. The morphology of every candidate spike was carefully analysed over a period of approximately 2 min. All spikes of similar amplitude (after allowing for the variation induced by the background noise) were selected and their shape was analysed using computer software developed in the laboratory that allowed the superimposition of all spikes to confirm that the signals originated from a single nerve fibre. Only the units satisfying these criteria were included in the analysis. For each unit the following parameters were determined.
Probability of firing per heartbeat = the percentage of heart beats during which one or more spikes occurred.
Probability of firing per burst = the percentage of bursts during which one or more spikes occurred.
Incidence of multiple within burst firing = the percentage of neural bursts where at least one spike occurred in association with another number of spikes (1, 2, 3 and 4 or more).
Firing rate = total number of spikes/duration of recording (s).
Statistical analysis
Values are reported as the mean ±s.e.m. Variables between the two groups of participants were compared using a two-tailed unrelated samples t test or by using the Mann-Witney rank sum test when appropriate. P-values below 0.05 were considered to indicate statistical significance.
Results
Characteristics of the participants and multiunit postganglionic sympathetic activity
There was no difference between patients with panic disorder and healthy volunteers with respect to blood pressure and heart and respiration rates (Table 1). The pattern of breathing, as assessed from examination of amplitude and continuity of breathing, was also not different between groups. Patients with panic disorder experienced significantly higher degrees of anxiety than the healthy volunteers (anxiety trait: 45 ± 4 versus 29 ± 3, P < 0.05). The frequency of panic attacks ranged from 2 to 34 per month, and the duration of the illness from 4 months to 8 years.
Table 1.
Demographic data and resting characteristics of the healthy subjects and patients with panic disorder (PD)
| Healthy | Patients with panic disorder | P-value (t test) | |
|---|---|---|---|
| No of subjects | 9 | 10 | — |
| Sex | 6F, 3M | 7F, 3M | — |
| Age (year) | 40 ± 5 | 40 ± 4 | 0.852 |
| BMI (kg m−2) | 27 ± 2 | 27 ± 1 | 0.740 |
| Duration of the illness (years) | NA | 3.1 ± 0.8 | — |
| Number of panic attack (per month) | NA | 9.2 ± 3.1 | — |
| Anxiety state | 35 ± 4 | 39 ± 4 | 0.483 |
| Anxiety trait | 33 ± 3 | 45 ± 4 | 0.032 |
| SBP (mmHg) | 129 ± 5 | 127 ± 4 | 0.768 |
| DBP (mmHg) | 66 ± 2 | 71 ± 4 | 0.391 |
| HR (bpm) | 61 ± 2 | 65 ± 2 | 0.196 |
| Respiration (breaths min−1) | 16 ± 2 | 16 ± 2 | 1.00 |
| Multiunit sympathetic activity | |||
| Bursts min−1 | 28 ± 3 | 26 ± 3 | 0.616 |
| Units min−1 | 1389 ± 151 | 1269 ± 187 | 0.628 |
| Bursts amplitude (median) | 47 ± 2 | 43 ± 2 | 0.210 |
BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; NA, not applicable.
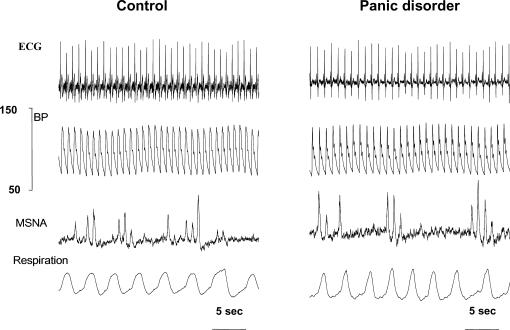
Multiunit postganglionic sympathetic activity was similar in the two groups of patients when burst frequency and amplitude were considered (Table 1). An example of blood pressure (BP), ECG, MSNA and respiration recordings in one patient with panic disorder and in one healthy subject is shown in Fig. 1.
Figure 1. Original recordings of ECG, blood pressure (BP), multiunit muscle sympathetic nerve activity (MSNA) and respiration from a control subject (left) and a patient with panic disorder (right).
Single unit postganglionic sympathetic activity
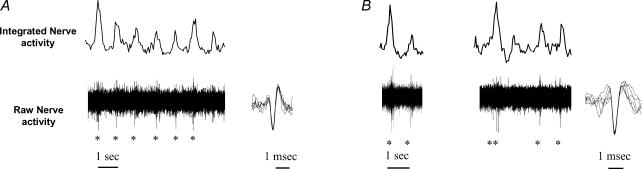
Activities from single unit vasoconstrictor fibres were obtained from 12 units of the 10 patients with panic disorder (2 recording sites were obtained in 2 patients) and from 11 units of the nine healthy volunteer (3 recording sites were obtained in 1 person). Figure 2 shows examples of single unit postganglionic sympathetic nerve recordings in two participants (one healthy volunteer and one patient with panic disorder).
Figure 2. Segments of recording from single muscle vasoconstrictor neurone in a control subject (A) and in a patient with panic disorder (B).
In recording A, one unit (identified with *) fires only once per sympathetic burst. In recording B, one unit fires either once or twice per sympathetic burst. Superimposed spikes on enlarged scale (right) show uniform spike morphology.
Firing rate and firing probability of single vasoconstrictor neurones
The firing rate of individual vasoconstrictor neurones was not significantly different between the two groups: 0.38 ± 0.09 Hz (range, 0.09–1.36 Hz) in patients with panic disorder and 0.22 ± 0.03 Hz (range, 0.08–0.43 Hz) in healthy volunteers (P= 0.091, Mann–Whitney Rank Sum Test). The firing probability per heartbeat (percentage of cardiac intervals in which the fibre is active) ranged from 7 to 55% in patients with panic disorder (mean 23 ± 4%), which was not different from that observed in healthy subjects (range 8–29%, mean 18 ± 2%). The firing probability per burst (percentage of bursts in which the fibre is active) was increased in patients with panic disorder (range 16–70%, mean 45 ± 5%) compared with healthy controls (range 13–50%, mean 32 ± 3, P < 0.05).
Firing pattern of single vasoconstrictor neurones during the neural bursts
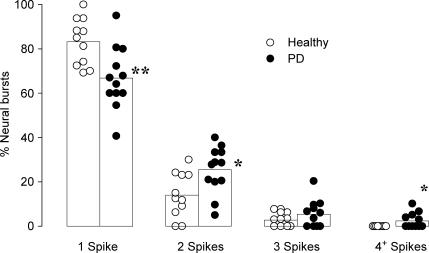
When only the neural bursts in which a unit fired were considered, a single spike occurred in 66.8 ± 4.1% of the bursts in the patients with panic disorder, which was less than that observed in healthy participants (83.2 ± 3.2%, P < 0.01, Fig. 3). This resulted in a shift towards multiple within-burst firing in panic disorder patients with double spikes occurring in 25.4 ± 3.0% of the neural bursts. This was significantly higher than that observed in healthy volunteers (14.0 ± 3.0%, P < 0.05). The occurrence of three or more spikes per burst was very low. In patients with panic disorder, we found that 7 units out of 12 occasionally generated three spikes (5.1 ± 1.9% of burst), which was no different from that seen in healthy volunteers (2.7 ± 0.9% of bursts). In patients with panic disorder, we found that five units occasionally generated four spikes or more, with the maximum number of spikes generated by one unit being seven (on one occasion only). On average, the occurrence of four or more spikes represented 2.4 ± 1.0% of neural bursts in patients with panic disorder, which was significantly different to that seen in healthy volunteers as no unit generated more than three spikes during a neural burst (P < 0.05).
Figure 3. Bar graphs with individual and pooled data from single vasoconstrictor fibres showing the percentage of neural bursts in which units fired one, two, three and four or more spikes.
Only neural bursts in which units were active were considered. *P < 0.01 PD (panic disorder) versus healthy subjects.
Discussion
The examination of single unit MSNA has been used to quantify central sympathetic discharge to the periphery and has advanced the understanding of the mechanisms underpinning the characteristic sympathoexcitation associated with conditions such as heart failure (Macefield & Wallin, 1999), obstructive sleep apnoea syndrome (Elam et al. 2002) and hypertension (Greenwood et al. 1999). Analogous to these reports, in this study we have demonstrated that while multiunit resting vasoconstrictor sympathetic nervous activity in patients with panic disorder appears to be normal, the discharge pattern of single units is significantly different from that observed in healthy subjects. Indeed, in patients with panic disorder with normal blood pressure and no underlying cardiovascular disease, the probability of a given vasoconstrictor fibre firing during a sympathetic burst is higher. Moreover, during a sympathetic burst in these patients the fibres tend to fire more often than in healthy people. It would seem that the apparent normality of multiunit MSNA measurement in patients with panic disorder belies the complexity of sympathetic regulation in this condition.
The firing frequency of individual sympathetic vasoconstrictor neurones in healthy people has been demonstrated to range from 0.24 to 0.47 Hz (Macefield et al. 1994; Greenwood et al. 1999). While we found that the firing frequency in panic disorder patients was normal, examination of the sympathetic discharge pattern was found to favour the occurrence of multiple spikes during a neural burst. Macefield & Elam (2003) have previously observed that postganglionic sympathetic axons have a tendency to fire only one spike because sympathetic bursts are too short to allow prolonged firing. These authors propose that the occurrence of two or more spikes may occur as a result of a single postganglionic neurone receiving convergent inputs from two or more preganglionic neurones and occasionally the postganglionic fibre receives several inputs almost simultaneously generating in turn multiple spikes (Macefield et al. 2002).
The occurrence of two or more spikes per burst generates high instantaneous discharge frequencies and may influence the effector organ response to sympathetically mediated adrenergic receptor activation (Nilsson et al. 1985; Kunimoto et al. 1992). Whether irregular patterns of stimulation are associated with increased neurotransmitter release in the human setting remains unknown. Similarly, whether the pattern of firing that we observe in muscle also occurs in the heart in these patients is not known.
It is important to note that our observation of an altered sympathetic nerve firing pattern in panic disorder patients occurred when subjects were at rest and not when they were experiencing a panic attack. The patients did not appear anxious throughout the study, as indicated by their relatively normal situational anxiety scores, and no patient experienced a panic attack during nerve recording. In an earlier study by our group, Wilkinson et al. (1998) performed multiunit recording of MSNA in four patients during a panic attack. Although there was only a modest increase in firing rate during the acute attack, sympathetic nerve burst amplitude was dramatically increased. An increase in burst amplitude in the absence of an alteration in burst frequency may reflect an elevation in the firing rate of individual vasoconstrictor neurones possibly by increasing the number of spikes generated during a sympathetic burst. Furthermore, the occurrence of the large sympathetic bursts during a panic attack was accompanied by marked increases in the release of noradrenaline and adrenaline from the heart, an increase in heart rate (Wilkinson et al. 1998) and an elevation in the release of the potent vasoconstrictor neuropeptide Y into the coronary sinus venous effluent (Esler et al. 2004). It is tempting to speculate that the irregular pattern of sympathetic nerve firing reported in the present study may be of particular relevance to the onset of panic attacks, as irregular discharges may promote a more vigorous cardiac response. Such an alteration in sympathetic vasoconstrictor nerve firing pattern may also underlie recent findings of an increased sympathetic reactivity in response to postural changes in patients with panic disorder (Coupland et al. 2003). This latter observation is consistent with our previous demonstration of an increased sympathetic response to spontaneous fluctuations in diastolic blood pressure in this patient group (Lambert et al. 2002).
Interestingly, the incidence of multiple within-burst firing of vasoconstrictor fibres in our patients with panic disorder is very similar to that observed both in healthy subjects during an acute increase in muscle sympathetic outflow induced by a sustained inspiratory capacity apnoea (Macefield & Wallin, 1999b) and in patients with obstructive sleep apnoea syndrome (Elam et al. 2002). This suggests that the pattern of multiple within-burst firing of vasoconstrictor fibres may be related to respiratory control inputs. This is of particular relevance in the setting of panic disorder as abnormalities in respiration may be an important feature in the development of the disorder (Wilhelm et al. 2001). Voluntary hyperventilation has been used to provoke panic attacks (Gorman et al. 1984), and dyspnoea (shortness of breath), together with palpitations and faintness, have been found to be the most commonly reported symptoms associated with panic disorder (McNally et al. 1995). A prolonged irregular breathing pattern is thought to generate a disturbed CO2 homeostasis, which in turn may activate the firing of the locus ceruleus and impact on the sympathetic nervous system (Brawman-Mintzer & Lydiard, 1997). It is known that the pattern of breathing exerts marked influences on the within-breath modulation of MSNA in humans (Seals et al. 1990). Patients with panic disorder may display greater irregularity in tidal volume and a higher rate of breathing pauses due to the occurrence of frequent sighs (Abelson et al. 2001). In the present study we found no evidence of an alteration in breathing rate or pattern in the panic disorder group. Moreover, although the probability of firing of vasoconstrictor fibres is lowest during late inspiration and early expiration (Macefield et al. 1994), the dominant determinants of respiratory modulation of MSNA are feedback from arterial baroreceptors and pulmonary stretch receptors (St Croix et al. 1999).
Acknowledgments
This study was supported by a grant from the National Health and Medical Research Council (NHMRC) of Australia (ID 225126). Ms Tye Dawwod is supported by a Post-graduate research scholarship from the National Heart Foundation of Australia. Dr Gavin Lambert is supported by an NHMRC RD Wright Career Development Fellowship.
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001;49:588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Brawman-Mintzer O, Lydiard RB. Biological basis of generalized anxiety disorder. J Clin Psychiatry. 1997;58(Suppl. 3):16–25. discussion 26. [PubMed] [Google Scholar]
- Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch General Psychiatry. 1982;39:701–703. doi: 10.1001/archpsyc.1982.04290060051010. [DOI] [PubMed] [Google Scholar]
- Coupland NJ, Wilson SJ, Potokar JP, Bell C, Nutt DJ. Increased sympathetic response to standing in panic disorder. Psychiatry Res. 2003;118:69–79. doi: 10.1016/s0165-1781(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Elam M, McKenzie D, Macefield V. Mechanisms of sympathoexcitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol. 2002;93:297–303. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- Esler M, Alvarenga M, Lambert G, Kaye D, Hastings J, Jennings G, Morris M, Schwarz R, Richards J. Cardiac sympathetic nerve biology and brain monoamine turnover in panic disorder. Ann N Y Acad Sci. 2004;1018:505–514. doi: 10.1196/annals.1296.062. [DOI] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF. Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry. 1984;141:857–861. doi: 10.1176/ajp.141.7.857. [DOI] [PubMed] [Google Scholar]
- Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994a;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994b;90:2225–2229. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- Kunimoto M, Kirno K, Elam M, Karlsson T, Wallin BG. Neuro-effector characteristics of sweat glands in the human hand activated by irregular stimuli. Acta Physiol Scand. 1992;146:261–269. doi: 10.1111/j.1748-1716.1992.tb09415.x. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the ‘pressor reactor’ hypothesis of hypertension development. Am J Hypertens. 2004;17:863–868. doi: 10.1016/j.amjhyper.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Thompson J, Schlaich M, Laude D, Elghozi JL, Esler MD, Lambert GW. Sympathetic and cardiac baroreflex function in panic disorder. J Hypertens. 2002;20:2445–2451. doi: 10.1097/00004872-200212000-00025. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Elam M. Why do human postganglionic neurones primarily only fire once during a sympathetic burst. Acta Physiol Scand. 2003;177:247–253. doi: 10.1046/j.1365-201X.2003.01078.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Elam M, Wallin BG. Firing properties of single postganglionic sympathetic neurones recorded in awake human subjects. Auton Neurosci. 2002;95:146–159. doi: 10.1016/s1566-0702(01)00389-7. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation. 1999a;100:1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol. 1999b;516:293–301. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Hornig CD, Donnell CD. Clinical versus nonclinical panic: a test of suffocation false alarm theory. Behav Res Ther. 1995;33:127–131. doi: 10.1016/0005-7967(94)00037-k. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Ljung B, Sjoblom N, Wallin BG. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol Scand. 1985;123:303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Spielberger C. State-Trait Anger Expression Inventory: Professional Manual. Odessa: Psychological Assessment Resources; 1988. [Google Scholar]
- St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM, Jennings GL, Chin JP, Esler MD. Measurement of human sympathetic nervous responses to stressors by microneurography. J Auton Nerv Syst. 1994;49:277–281. doi: 10.1016/0165-1838(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Markowitz JS, Ouellette R, Greenwald S, Kahn JP. Panic disorder and cardiovascular/cerebrovascular problems: results from a community survey. Am J Psychiatry. 1990;147:1504–1508. doi: 10.1176/ajp.147.11.1504. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gevirtz R, Roth WT. Respiratory dysregulation in anxiety, functional cardiac, and pain disorders. Assessment, phenomenology, and treatment. Behav Modif. 2001;25:513–545. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- Wilkinson DJ, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch General Psychiatry. 1998;55:511–520. doi: 10.1001/archpsyc.55.6.511. [DOI] [PubMed] [Google Scholar]