Abstract
A subset of integrin α subunits contain an I domain, which is important for ligand binding. We have deleted the I domain from the β2 integrin lymphocyte function-asssociated antigen-1 (LFA-1) and expressed the resulting non–I domain-containing integrin (ΔI-LFA-1) in an LFA-1-deficient T cell line. ΔI-LFA-1 showed no recognition of LFA-1 ligands, confirming the essential role of the I domain in ligand binding. Except for I domain monoclonal antibodies (mAbs), ΔI-LFA-1 was recognized by a panel of anti-LFA-1 mAbs similarly to wild-type LFA-1. However, ΔI-LFA-1 had enhanced expression of seven mAb epitopes that are associated with β2 integrin activation, suggesting that it exhibited an “active” conformation. In keeping with this characteristic, ΔI-LFA-1 induced constitutive activation of α4β1 and α5β1, suggesting intracellular signaling to these integrins. This “cross-talk” was not due to an effect on β1 integrin affinity. However, the enhanced activity was susceptible to inhibition by cytochalasin D, indicating a role for the cytoskeleton, and also correlated with clustering of β1 integrins. Thus, removal of the I domain from LFA-1 created an integrin with the hallmarks of a constitutively active receptor mediating signals into the cell. These findings suggest a key role for the I domain in controlling integrin activity.
INTRODUCTION
The integrin lymphocyte function-associated antigen-1 (LFA-1) (αL/β2, CD11a/CD18) is a leukocyte-specific receptor that mediates cell–cell interactions in the immune system (reviewed by Stewart and Hogg, 1996; Gahmberg, 1997). The ligands for LFA-1 are three members of the Ig superfamily of proteins, intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3. The extracellular portions of the α and β subunits of integrins consist of several types of domains. The N termini of the α subunits contain seven homologous repeats of ∼60 amino acids, which have been predicted to fold into a β-propeller domain (Springer, 1997). A subset of nine integrins incorporates an additional, autonomously folding domain of ∼200 amino acids, which is inserted between β-sheets 2 and 3 of the putative β-propeller and is termed the I (inserted) domain. The I domain is present in LFA-1 and the other β2 integrins Mac-1, p150,95, and αdβ2, as well as in α1β1, α2β1, α10β1, α11β1, and αEβ7 (Camper et al., 1998; Dickeson and Santoro, 1998; Velling et al., 1999). The crystal structures of the I domains of LFA-1, Mac-1, and α2β1 have been solved and show a dinucleotide-binding fold (reviewed by Loftus and Liddington, 1997; Humphries and Newham, 1998). An unusual Mg2+/Mn2+ binding site, termed the metal ion-dependent adhesion site, is located on the “top” of the domain, opposite the face that connects the I domain to the putative β-propeller domain. There is a conserved domain at the N terminus of the β subunit, which is predicted to adopt a fold similar to the α subunit I domain.
For I domain-containing integrins there is abundant evidence that this I domain contains the major ligand binding site. Recombinant I domains bind ligand not only with the same specificity as the parental integrin but, in most cases, also in the same cation-dependent manner (reviewed by Dickeson and Santoro, 1998). The importance of the I domain in ligand binding is further underscored by the fact that mutations within the I domain that affect cation coordination by the metal ion-dependent adhesion site motif abolish ligand binding in the context of the intact integrin. However, for the two I domain-containing integrins, LFA-1 and α2β1, there is also evidence that sequences in the α subunit outside the I domain contribute to ligand binding (Stanley et al., 1994; Dickeson et al., 1997), and mutagenesis shows that the conserved region of the β2 subunit is also important for ligand binding (Goodman and Bajt, 1996).
LFA-1 interaction with the ICAMs, like many other integrin–ligand interactions, is not constitutive but requires a signaling-induced activation event causing a transient increase in the ability of the integrin to bind ligand. There is some evidence to suggest a role for phosphoinositide 3-kinase (Shimizu and Hunt, 1996) and Ras/MAP kinase activation in LFA-1 adhesion (O'Rourke et al., 1998). Inactive integrin is maintained in the membrane by association of the membrane proximal sequences of the α and β cytoplasmic tails (Hughes et al., 1996). As a result of intracellular signal transduction, it is hypothesized that cytoplasmic adaptor proteins cause an altered arrangement or “unhinging” of the α and β cytoplasmic regions, and an active integrin ensues. Integrin activation has been correlated both with higher affinity forms of the receptors, which have undergone a conformational change, and with clusters of laterally associated integrins brought about by cytoskeletal alteration (Stewart and Hogg, 1996). In vivo, a mixture of both forms probably exists. Whether several types of signals are translated across the membrane or whether bidirectional signals give rise to the final active integrin population is presently unclear. These transiently expressed active forms of integrin are thought to be in equilibrium with nonactive forms. A second phase begins when active integrin engages ligand and signals back into the cell. The “outside-in” signals transduced by the fibronectin binding integrin α5β1 have been well investigated (Miyamoto et al., 1995), but little is known about the signaling capability of LFA-1.
The high-affinity LFA-1 is characterized by more efficient binding of soluble ICAM-1 and also expression of an epitope recognized by monoclonal antibody (mAb) 24 (Dransfield and Hogg, 1989; Dransfield et al., 1992; Stewart and Hogg, 1996). Certain mAbs that bind to the αL subunit, such as NKI-L16 (Keizer et al., 1988; van Kooyk et al., 1991) or the β2 subunit such as KIM-127 or KIM-185 (Robinson et al., 1992; Andrew et al., 1993), can also activate LFA-1. The nature of the change in conformation which an integrin such as LFA-1 undergoes to give rise to high-affinity integrin is poorly understood. We have recently found that one requirement for conversion of LFA-1 to the high-affinity form is interdomain movement of the I domain (McDowall et al., 1998). These findings suggested that the I domain, in addition to providing a ligand binding site, also has a role in activation of the integrin.
Approximately two-thirds of integrins do not have a I domain in their α subunit and the autonomously folding I domains are thought to have been inserted into the proteins during evolution. We therefore hypothesized that removal of the I domain from an integrin should retain expression of heterodimeric integrin and allow investigation of I domain functions that are independent of ligand binding. In this study, for the first time, we have removed the I domain from LFA-1 and have examined how the absence of this domain affects the structure, ligand binding capacity, and other functions of this leukocyte integrin. Although LFA-1 without the I domain can no longer bind its ligands, it has the characteristics of a constitutively active integrin. As an example of its ability to signal into the cell, we show that this I domain-minus LFA-1 is active as a mediator of integrin “cross-talk” causing the activation of β1 integrins on the same cell.
MATERIALS AND METHODS
Reagents
Restriction and modification enzymes were purchased from Boehringer Mannheim (Mannheim, Germany) or New England Biolabs (Hitchin, United Kingdom). The isolation of ICAM-1Fc, produced as a chimeric protein containing the five extracellular domains of human ICAM-1 fused to a human immunoglobulin G1 (IgG1) Fc sequence has been described before (Stanley and Hogg, 1998). Vascular cell adhesion molecule-1 (VCAM-1) Fc, produced as a chimeric protein consisting of the two N-terminal domains of human VCAM-1 fused to a human IgG1 sequence, was a gift from both R. Lobb (Biogen, Cambridge, MA) and M. Robinson (Celltech Chiroscience, Slough, United Kingdom). Fibronectin (0.1% solution from human plasma) was purchased from Sigma (Poole, United Kingdom).
Monoclonal Antibodies
TS1/18 (CD18; β2), TS2/4 (CD11a; αL), TS1/22 (CD11a; αL), and P5D2 (CD29; β1) (all from American Type Culture Collection, Manassas, VA), and 24 (CD11; anti-αL, αM, αX), 38 (CD11a; αL), and 7.2R (CD49d; α4) were purified from tissue culture supernatant by protein A-Sepharose chromatography by the Imperial Cancer Research Fund Research Production Antibody Service. The following mAbs were generously provided: S6F1 (CD11a; αL; C. Morimoto, Dana Faber Cancer Institute, Boston, MA); 10D and 2.6E (CD11a; αL; D. Andrew, Amgen, Boulder, CO); and HP1/2 (CD49d; α4; R. Lobb, as above). CD18 (β2) mAbs were obtained as follows: KIM 170, KIM 182, KIM 215, and 6.5E (M. Robinson, as above); GRF1 (F. Garrido, Hospital Universitario Virgen de las Nieves, Granada, Spain); CLB54 (R. van Lier, University of Amsterdam, Amsterdam, The Netherlands); H52 and MHM23 (S.K.A. Law, Oxford University, Oxford, United Kingdom); and 60.3 (Bristol-Meyers Squibb, Seattle, WA). The following activating mAbs were generously provided: NKI-L16 (CD11a; αL; Keizer et al., 1988; van Kooyk et al., 1991; Y. van Kooyk, University Hospital Nijmegen, St. Radboud, Nijmegen, The Netherlands); KIM 127 and KIM 185 (CD18; β2; Robinson et al., 1992; Andrew et al., 1993; M. Robinson, as above); MEM 48 (CD18; β2; Binnerts et al., 1994; V. Horejsi, Academy of Sciences of the Czech Republic, Prague, Czech Republic); and 240Q (R. Jasman and D. Staunton, ICOS, Washington, DC). CBR LFA1/2 (CD18; β2; Petruzzelli et al., 1995) was obtained from Leukocyte Typing Workshop V (Boston, MA). The β1 integrin activation reporter mAbs HUTS-21 (Luque et al., 1996) and 15/7 (Yednock et al., 1995) were kindly provided by C. Cabanas (Universidad Complutense, Madrid, Spain) and T. Yednock (Elan Pharmaceuticals, San Francisco, CA), respectively. All other mAbs, CBR LFA-1/1, CBR LFA1/3, CBR LFA1/7, AZN-L20, AZN-L21, ICII, were obtained from Leukocyte Typing Workshops V (Boston, MA) and VI (Kobe, Japan). G25.2 (CD11a; αL) was purchased from Becton Dickinson (Oxford, United Kingdom), and SAM-1 (CD49e; α5) was from Eurogenetics (Hampton, United Kingdom).
cDNA Construct
To construct the I domain-deleted LFA-1 α subunit (ΔI-LFA-1), two fragments encoding the N-terminal region through to G128 (fragment A) and S319 through to G441 (fragment B) were generated by PCR amplification from a full-length cDNA clone, which had been subcloned into the pZErO-1 vector (Invitrogen, Leek, The Netherlands) (pZ-LFA-1). The necessary changes in the DNA sequence were designed such that the original amino acid sequence was retained. The 3′ primer for fragment A and the 5′ primer for fragment B contained extensions to add in-frame restriction sites for HindIII. The primers were as follows (with restriction enzyme sites given in bold type): fragment A 5′ (hybridizing in vector sequence): 5′-TCAAGCTATGCATCAAGCTT-3′; fragment A 3′: 5′-AGGTCTAAGCTTCCCTTG-3′; fragment B 5′: 5′-GGACCTGACAAGCTTCAA-3′; and fragment B 3′: 5′-CTTGGTCCACGTCGAC-3′. Fragment A (cut with NsiI and HindIII) and fragment B (cut with HindIII and SalI) were subcloned together into pZ-LFA-1 (cut with NsiI and SalI) after the corresponding wild-type (wt) fragment was removed. DNA sequencing was carried out using an automated sequencer (PE Biosystems, Warrington, United Kingdom). The cDNA encoding ΔI-LFA-1 was finally subcloned into the expression vector pcDNA3.1/Zeo (Invitrogen).
Cell Lines and Cell Culture
The human T lymphoma cell line clone J-β2.7, derived from Jurkat cells by mutagenesis (Weber et al., 1997), was a gift from L. Klickstein (Brigham and Women's Hospital, Boston, MA). Cells were maintained in RPMI 1640 medium supplemented with 10% FCS (Life Technologies, Paisley, United Kingdom) (complete medium). J-β2.7 transfectants were maintained in complete medium supplemented with 250 μg/ml Zeocin (Invitrogen).
cDNA Transfection and Generation of Stable Cell Lines
J-β2.7 cells (8 × 106 per transfection) in log phase growth were washed, resuspended in 0.7 ml RPMI 1640 medium, and mixed with 25 μg of wt LFA-1 or ΔI-LFA-1 DNA. Electroporation was carried out at 320 V and 960 μF. After 48 h of culture in complete medium, the medium was supplemented with 250 μg/ml Zeocin (Invitrogen). Cells expressing ΔI-LFA-1 were enriched for the highest expressing population by sterile cell sorting on a FACS Vantage cell sorter (Becton Dickinson, Oxford, United Kingdom) using anti-LFA-1 mAb G25.2. From this population clones were obtained by sterile sorting of single cells. Cells expressing wt LFA-1 were cloned by limiting dilution.
Flow Cytometric Analysis
Cells (2 × 105) were incubated with primary mAb in 100 μl of PBS and 0.2% BSA for 20–30 min on ice. Purified mAbs were used at 10 μg/ml; ascites were used at a 1:100 dilution. Incubation with mAb NKI-L16 was in HEPES buffer (20 mM HEPES, 140 mM NaCl, 2 mg/ml glucose) plus 1 mM Ca2+. For mAb 24 detection, cells were incubated with mAb 24 at 37°C in complete medium. Incubation with mAbs 15/7 and HUTS-21 was at room temperature in HEPES buffer with or without the specified concentrations of MnCl2. After the incubation with primary mAb, cells were washed three times with PBS and BSA and incubated with FITC-conjugated goat anti-mouse IgG (Sigma) for 30 min on ice. After three washes as above, the cells were resuspended in cold PBS and BSA and analyzed on a FACScan flow cytometer (Becton Dickinson).
Cell Adhesion to ICAM-1Fc
Immulon 3 96-well plates (Dynatech Technologies, Chantilly, VA) were coated overnight at 4°C with goat anti-human IgG (Fc specific; Sigma) at 20 μg/ml. ICAM-1Fc was added at 10 μg/ml in PBS for 2 h at 37°C. Nonspecific sites were then blocked with 2.5% BSA in PBS for 1 h, and the plates were washed in HEPES buffer. Cells were labeled with 2.5 μM 2′,7′-bis (carboxyethyl)-5(6′)-carboxyfluorescein acetomethyl ester (Calbiochem, Nottingham, United Kingdom) in HEPES buffer for 30 min at 37°C and then washed. Fifty microliters of cells at 3 × 106/ml were added to the ICAM-1Fc-coated plates in the presence of 50 μl of the appropriate adhesion-inducing stimuli. Phorbol 12,13-dibutyrate (PdBu; final concentration, 100 nM) and mAbs were diluted in RPMI (10 μg/ml final concentration for mAb 24, 5 μg/ml final concentrations for mAbs KIM 127, KIM 185, and NKI-L16). Mn2+ (1 mM final concentration) was diluted in HEPES buffer; Mg2+ (up to 5 mM final concentration) was diluted in HEPES buffer containing EGTA (1 mM final concentration). Plates were incubated on ice for 15 min, followed by a 30-min incubation at 37°C. Nonadherent cells were washed off by two washes in warmed HEPES buffer containing 1 mM Mg2+ and Ca2+. Adhesion was quantified by a fluorescence plate reader (Fluoro-scan II; Labsystems, Basingstoke, United Kingdom).
Fibronectin- or VCAM-1Fc-coated Bead Binding Assays
Fibronectin- and VCAM-1-coated bead binding assays were adapted from the method of Porter and Hogg (1997). Three-micrometer latex beads (Sigma) were coated with 5 μg/ml fibronectin or 1 μg/ml VCAM-1, blocked in 1% BSA in PBS, washed, and resuspended in complete medium. Multiwell Lab-Tek chamber slides (Nunc, Naperville, IL) were coated overnight at 4°C with rabbit anti-mouse Ig (Dako, Ely, United Kingdom) at 35 μg/ml. mAb UCHT2 (CD5) was added at 10 μg/ml in PBS for 3 h at room temperature. Wells were blocked with 1% BSA in PBS for 1 h and then washed in complete medium. Cells (200 μl of 6 × 105/ml, in complete medium) were added in the presence or absence of 100 μl of mAbs or PdBu (4× final concentration in complete medium) and allowed to settle for 30 min on ice. mAb 24 was used at a final concentration of 5 μg/ml; mAb NKI-L16 at 0.5 μg/ml; PdBu at 100 nM; cytochalasin D at 5 μM; and blocking mAbs at 10 μg/ml. Ligand-coated beads were added at a 100:1 beads:cell ratio in 100 μl. After a 15-min incubation on ice, the Lab-Tek slides were incubated for 90 min at 37°C. Unbound beads and cells were removed by four washes in warm RPMI. Cells were fixed with 1% formaldehyde in PBS for 20 min at room temperature, and then stained with hematoxylin. Beads and cells were counted per high-power field (40× oil immersion objective; Carl Zeiss, Thornwood, NY). The number of beads per 100 cells was determined as the mean of five high-power fields ± SD.
Soluble VCAM-1Fc Binding Assay
Binding of soluble VCAM-1Fc was adapted from the method of Jakubowsky et al. (1995). Aliquots of 2 × 105 cells were incubated with VCAM-1Fc in HEPES buffer plus the indicated concentrations of MnCl2 and 0.02% NaN3 for 30 min at room temperature. Cells were then washed twice in the incubation buffers containing the same MnCl2 concentrations and incubated with FITC-conjugated goat anti-human IgG (Fc specific; Sigma) for 30 min on ice (in HEPES buffer plus 0.2% BSA). After three washes, cells were fixed in 2% formaldehyde and PBS. VCAM-1Fc binding was analyzed by a FACScan flow cytometer (Becton Dickinson) to give mean fluorescence intensity units.
Confocal Microscopy
Aliquots of 1 × 106 cells were incubated with mAb 7.2R or SAM-1 in RPMI 1640 medium for 30 min on ice and then washed three times in PBS. To prevent antibody-induced clusters, cells were fixed in 1% paraformaldehyde and PBS for 30 min on ice before a second incubation with Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR) for 30 min on ice. After three washes, cells were attached to poly-l-lysine-coated 13-mm round glass coverslips, fixed in 3% formaldehyde and PBS, and mounted onto slides in Mowiol (Calbiochem) dissolved in the antifade solution Citifluor (UKC Chemical Laboratory, Canterbury, United Kingdom). Fluorescence was analyzed using a Zeiss LSM 510 confocal laser scanning microscope equipped with a 63×, numerical aperture 1.4 objective, with an argon laser (wavelength, 488 nm). Cell surface distribution was evaluated by taking horizontal optical sections at 0.35-μm vertical steps throughout the whole height of representative cells. Images of optical sections (512 × 512 pixels) were digitally recorded, and their projections were generated using the LSM 510 program. The resulting images were processed using Adobe (Mountain View, CA) Photoshop software.
RESULTS
Expression of I Domain-deleted LFA-1 in Jurkat-β2.7 Cells
To study the function of LFA-1 minus the I domain, an αL subunit cDNA construct was generated by deleting DNA sequences predicted to encode the I domain of LFA-1 according to the model for the homologous β2 integrin Mac-1 (Springer, 1997; Figure 1A; see MATERIALS AND METHODS for details). The boundary of the domain was chosen such that the predicted disulfide bond arrangement for intact LFA-1 was not altered (i.e., the conserved C125 residue, which is predicted to form a disulfide bond with C94, was retained). The I domain-deleted protein, termed ΔI-LFA-1, lacked the sequence N129-T318 of the full-length LFA-1 α subunit but did not contain any additional sequences. cDNAs for ΔI-LFA-1 and wt LFA-1 were stably transfected into Jurkat-β2.7 cells, which are deficient for the endogenous LFA-1 α subunit but retain a functional β2 subunit (Weber et al., 1997). This β2 subunit is only transported to the cell surface upon heterodimerization with transfected αL. For both wt LFA-1- and ΔI-LFA-1-expressing cells, several clones were selected that exhibited comparable levels of surface expression as detected by immunoprecipitation (our unpublished results) and flow cytometry (see following). At least two independent clones were used for each experiment.
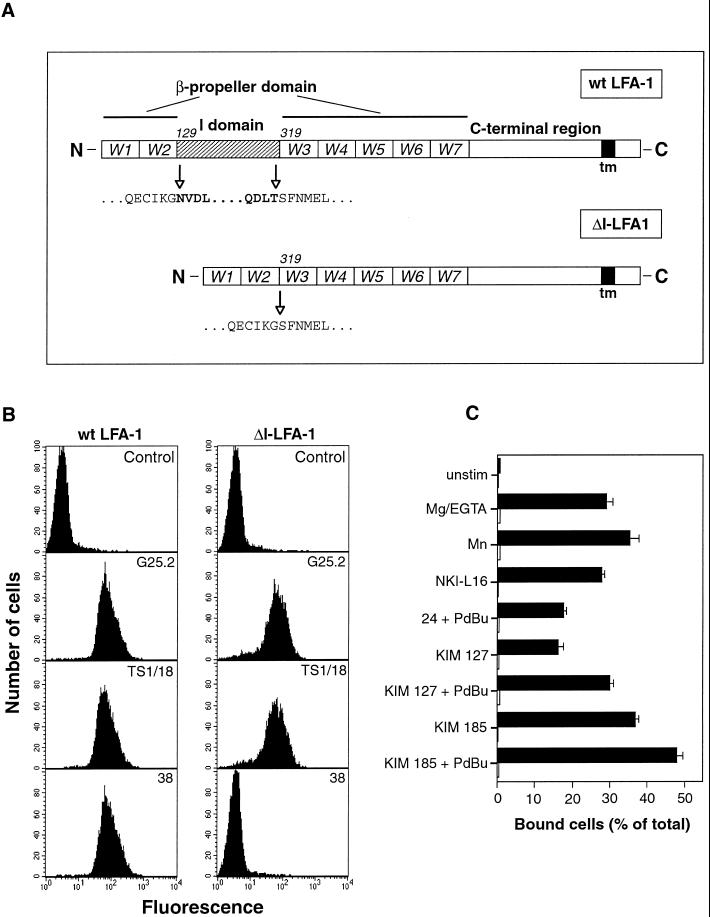
Figure 1.
Deletion of the I domain from LFA-1 and its effect on mAb epitopes and binding to ligand ICAM-1. (A) Schematic diagram of wt LFA-1 and ΔI-LFA-1 α subunits. W1–7 represent the individual β-sheets of the predicted β-propeller domain. The I domain of LFA-1 is inserted in the loop that connects β-sheets W2 and W3. Numbers (129 and 319) are positions of amino acid residues at the beginning of the I domain and of W3 of the β-propeller domain, respectively. In ΔI-LFA-1 the deletion encompasses residues N129-T318, thereby joining residue G128 to S319. tm, transmembrane domain. (B) Expression of epitopes recognized by mAbs G25.2 (anti-LFA-1 αL, epitope outside I domain), TS1/18 (anti-β2), and 38 (anti-LFA-1 αL, I domain-specific) on selected clones of J-β2.7 cells stably transfected with cDNAs encoding wt LFA-1 or ΔI-LFA-1. Cells were stained with the relevant mAbs followed by FITC-conjugated goat anti-mouse IgG and analysis by flow cytometry. As a negative control, the primary mAb was omitted. Data are representative of at least 10 determinations. (C) Adhesion of J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1 to ligand ICAM-1. Cells were allowed to bind to plastic immobilized ICAM-1 with or without stimulation for 30 min at 37°C before washing and quantification of bound cells. Stimuli were 3 mM Mg2+/1 mM EGTA, 1 mM Mn2+, and activating mAbs NKI-L16, KIM 127, KIM 185, and mAb 24. PdBu was used at 100 nM. Black bars, wt LFA-1-expressing cells; open bars, ΔI-LFA-1-expressing cells. One experiment representative of four is shown.
Expression of mAb Epitopes by ΔI-LFA-1 and wt LFA-1
We analyzed cell surface expression of LFA-1 α and β subunit epitopes on representative clones of wt LFA-1- and ΔI-LFA-1-expressing cells (Figure 1B). Both wt LFA-1- and ΔI-LFA-1-expressing cells showed very similar fluorescence levels of the non–I domain-specific αL mAb G25.2 as well as the β2-specific mAb TS1/18, indicating that the transfected α subunits and the endogenous β2 subunit were transported to the cell surface to the same extent in both cell lines. As expected, in contrast to wt LFA-1-expressing cells, ΔI-LFA-1-expressing cells did not react with the I domain-specific mAb 38. The reactivity of a panel of mAbs against the LFA-1 αL and β2 subunits was assessed (Table 1). ΔI-LFA-1 reacted with all of the tested αL subunit mAbs that map outside the I domain and, as expected, did not react with any I domain-specific mAbs including the mAb CBR LFA-1/1 whose epitope overlaps the I and β-propeller domains (Huang and Springer, 1995). Epitopes for all the tested β2 subunit-specific mAbs were present on ΔI-LFA-1 and were expressed to the same level as on wt LFA-1 (except activating mAbs, see below). Taken together, these results show that ΔI-LFA-1 is expressed on the cell surface, forms heterodimers with endogenous β2 subunit, and is folded for correct mAb recognition by a wide range of different anti-LFA-1 mAbs.
Table 1.
Reactivity of anti LFA-1 mAbs to J-β2.7 cells and J-β2.7 cells stably transfected with cDNAs encoding wt LFA-1 or ΔI-LFA-1
| mAb | Specificity | J-β2.7 | wt LFA-1 | ΔI-LFA-1 |
|---|---|---|---|---|
| S6F1 | αL non-I dom | − | + | + |
| TS2/4 | αL non-I dom | − | + | + |
| CBR LFA-1/3 | αL non-I dom | − | + | + |
| AZN-L20 | αL non-I dom | − | + | + |
| AZN-L21 | αL non-I dom | − | + | + |
| G25.2 | αL non-I dom | − | + | + |
| CBR LFA-1/1 | αL I dom/non-I dom | − | + | − |
| 38 | αL I dom | − | + | − |
| 2.6E | αL I dom | − | + | − |
| 10D | αL I dom | − | + | − |
| TS1/22 | αL I dom | − | + | − |
| TS1/18 | β2 | − | + | + |
| H52 | β2 | − | + | + |
| 60.3 | β2 | − | + | + |
| MHM23 | β2 | − | + | + |
| CLB54 | β2 | − | + | + |
| GRF1 | β2 | − | + | + |
| ICII | β2 | − | + | + |
| 6.5E | β2 | − | + | + |
| KIM 170 | β2 | − | + | + |
| KIM 215 | β2 | − | + | + |
| CBR LFA-1/7 | β2 | − | + | + |
Cells were stained with the indicated mAbs for 20 min on ice, washed, stained with FITC-conjugated goat anti-mouse IgG and analyzed by flow cytometry. αL non-I dom, epitope mapped to αL outside the I domain; αL I dom/non-I dom, epitope mapped to region overlapping I domain and β-propeller domain; αL I dom, epitope mapped on αL I domain. −, staining not greater than that seen with secondary mAb alone (see Fig. 1B). +, positive staining, usually similar to that seen in Fig 1B. Staining of ΔI-LFA-1-expressing cells always to same extent as that seen on wt LFA-1-expressing cells relative to G25.2 expression. Data are representative of at least three determinations on at least two different clones.
The I Domain of LFA-1 Is Necessary for Adhesion to ICAM-1
To test whether ΔI-LFA-1 showed detectable ligand binding activity, adhesion assays using the LFA-1 ligands ICAM-1 (Figure 1C) and ICAM-3 (our unpublished results) were performed. Adhesion-inducing agents covered a range of stimuli activating the integrin from the outside (i.e., divalent cations Mn2+ or Mg2+/EGTA, activating mAbs KIM 127 [Robinson et al., 1992], KIM 185 [Andrew et al., 1993], or NKI-L16 [van Kooyk et al., 1991]), or a combination of activating mAbs and the phorbol ester PdBu, which activates LFA-1 by triggering signal transduction pathways from within the cell. Although wt LFA-1 adhered to both ligands under all the conditions tested, ΔI-LFA-1 did not adhere at all to ICAM-1 or ICAM-3. A more sensitive adhesion assay, which uses buoyancy rather than washing to remove nonadherent cells (Goodwin and Pauli, 1995), also failed to detect any adhesion of ΔI-LFA-1-transfected cells to ICAM-1 (our unpublished results). As a third approach, ICAM-1-coated latex beads were added to cells together with LFA-1-activating stimuli, but ΔI-LFA-1-transfected cells failed to show any specific interactions with ICAM-1-coated beads, even after incubation times as long as 24 h (our unpublished results). wt LFA-1-expressing cells were strongly positive in both of these assays, which detect weak adherence reactions. Taken together, these results are consistent with the interpretation that the I domain of LFA-1 contains the major ligand binding site and is essential for the binding reaction of LFA-1 to ICAM-1.
ΔI-LFA-1 Expresses Higher Levels of Activation Epitopes than wt LFA-1
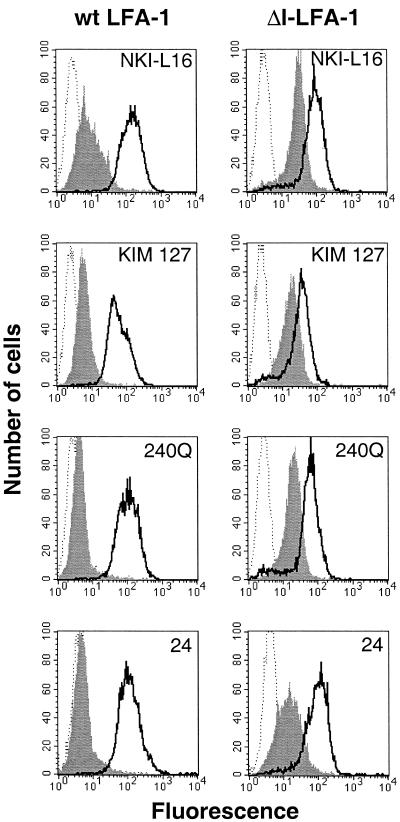
Certain activating mAbs (with epitopes outside the I domain) can promote LFA-1 ligand binding activity from the outside of the cell, and this is thought to involve conformational changes in the integrin, which are either induced or stabilized by these mAbs (Stewart and Hogg, 1996). It was of interest to investigate whether removal of the I domain from LFA-1 altered the expression of epitopes detected by several activating anti-β2 mAbs and the activating anti-αL mAb NKI-L16. Compared with wt LFA-1-expressing cells, ΔI-LFA-1-expressing cells showed approximately five times higher fluorescence levels of the αL mAb NKI-L16 and the β2 mAb KIM 127 and six to eight times higher fluorescence levels of the β2 mAb 240Q (Figure 2). All three of these mAb epitopes were expressed at low levels on wt LFA-1-expressing cells. Expression of three other activating β2 mAbs, KIM 185, MEM48, and CBR LFA-1/2, was also enhanced on ΔI-LFA-1-expressing cells compared with wt LFA-1-expressing cells (our unpublished results). The mAb 24 epitope, which can be induced by divalent cations Mg2+ or Mn2+, reflects a conformational change in LFA-1 characteristic of a higher-affinity receptor and is considered to act as an activation reporter (Dransfield and Hogg, 1989; Dransfield et al., 1992; Stewart and Hogg, 1996). This epitope was not expressed by the wt LFA-1-expressing cells, but, as for the activation mAbs, there was enhanced expression on the ΔI-LFA-1-expressing cells (Figure 2). Therefore, certain epitopes, all of which are associated with LFA-1 activation, are more highly expressed when the I domain is removed.
Figure 2.
Expression of epitopes recognized by activating mAbs NKI-L16 (anti-αL), KIM 127 (anti-β2), 240Q (anti-β2), and 24 (anti-αL activation reporter) on J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1. Cells were stained on ice with the relevant mAbs followed by FITC-conjugated goat anti-mouse IgG and analysis by flow cytometry. Dotted lines, negative control; thick lines, mAb G25.2; filled histograms, activating mAbs. Data for each set of mAbs are from experiments conducted in parallel, and one experiment representative of three is shown.
Higher Ligand Binding Activity of α4β1 and α5β1 on ΔI-LFA-1-expressing Cells than on wt LFA-1-expressing Cells
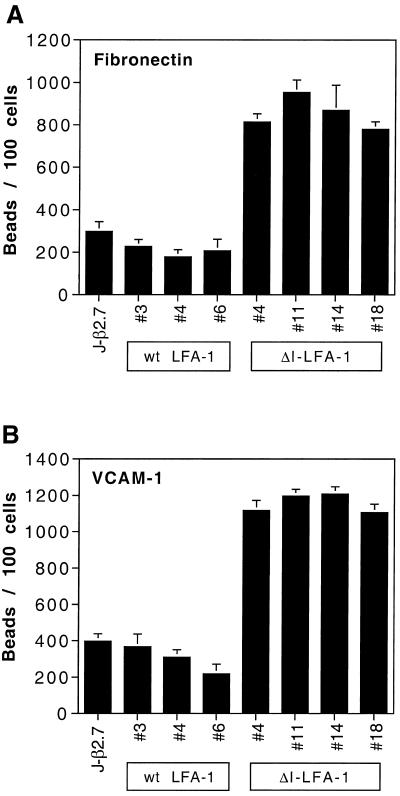
Although ΔI-LFA-1 was deficient in ligand binding, it exhibited enhanced expression levels of activation epitopes and the activation reporter epitope 24. These findings suggested that LFA-1 without its I domain was in an “active” conformation, which might be able to transmit signals into the cell. LFA-1 has been shown to regulate the ligand binding capacity of β1 integrins through intracellular signaling termed cross-talk (Porter and Hogg, 1997). To test the possibility that ΔI-LFA-1 was active in signal transduction, we therefore asked whether the presence of ΔI-LFA-1 on J-β2.7 cells influenced the basal ligand binding activity of β1 integrins in these cells. Fibronectin was used as a ligand that is recognized by both α4β1 and α5β1, and VCAM-1 was used as a ligand for α4β1 alone. Figure 3 shows that nontransfected J-β2.7 cells and three independent clones expressing wt LFA-1 each bound comparable numbers of fibronectin- or VCAM-1-coated beads per cell. By comparison, all four of the tested ΔI-LFA-1 clones exhibited significantly higher bead binding activity. Fibronectin and VCAM-1 binding on cells expressing wt LFA-1 or ΔI-LFA-1 was completely blocked by the blocking anti-β1 mAb P5D2 (see below; our unpublished results).
Figure 3.
Fibronectin-coated (A) and VCAM-1-coated (B) bead binding of J-β2.7 cells and different clones of J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1. Cells were adhered to plastic with an anti-CD5 mAb and incubated with fibronectin- or VCAM-1-coated beads for 90 min at 37°C before washing off unbound beads. Bound cells and beads were fixed in 1% formaldehyde. Quantification was carried out by counting cells and beads per high-power field. Data are represented as beads per 100 cells from the mean of five high-power fields ± SD. Data are representative of two experiments with identical results.
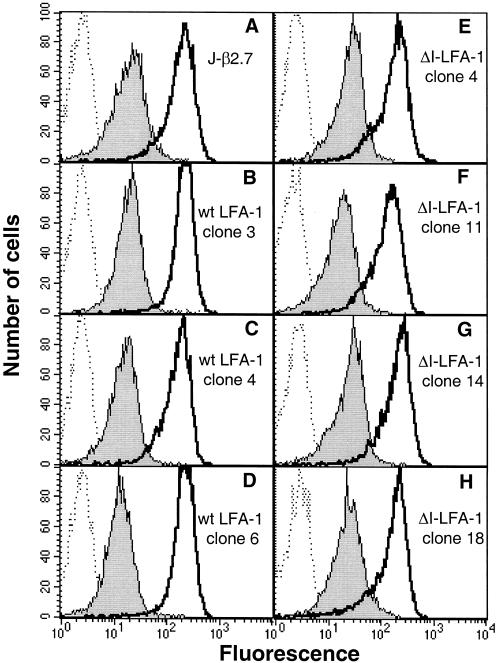
To analyze whether this increase in fibronectin and VCAM-1 binding activity might be explained by enhanced expression of α4β1 or α5β1 in clones expressing ΔI-LFA-1, the surface expression of these integrins was measured by flow cytometry. Similar surface expression levels of α4β1 and α5β1 were found on the parental J-β2.7 cells (Figure 4A) and on the clones expressing wt LFA-1 (Figure 4, B–D) as well as ΔI-LFA-1 (Figure 4, E–H). Therefore, the activity and not the surface expression of α4β1 and α5β1 was up-regulated in clones expressing ΔI-LFA-1.
Figure 4.
Expression of α4β1 and α5β1 on untransfected J-β2.7 cells and on different J-β2.7 clones expressing wt LFA-1 or ΔI-LFA-1. Cells were stained on ice with the mAbs SAM-1 (anti-α5) or HP1/2 (anti-α4) followed by FITC-conjugated goat anti-mouse IgG and analysis by flow cytometry. Dotted lines, negative control; filled histograms, mAb SAM-1; open histograms, mAb HP1/2.
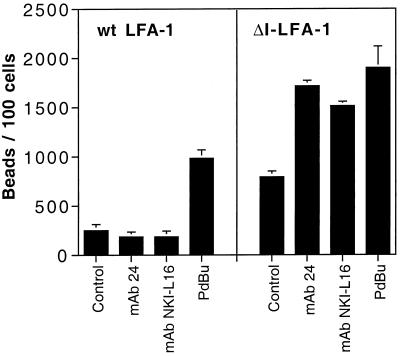
Activating ΔI-LFA-1 by mAbs Further Up-regulates the Function of β1 Integrins
To confirm that the enhanced β1 integrin activity of the cells expressing ΔI-LFA-1 was directly linked to the presence of ΔΙ-LFA-1 and not caused by some coincidental alteration in these transfectants, we analyzed whether direct targeting of ΔI-LFA-1 by anti-LFA-1 mAbs NKI-L16 or 24 would influence fibronectin binding (Figure 5). On cells expressing ΔI-LFA-1, stimulation with either of these mAbs led to a further increase in fibronectin binding above the constitutive level, which was comparable with stimulation of the cells with PdBu. Cells expressing wt LFA-1 were stimulated by PdBu to bind fibronectin, as expected, but mAbs 24 or NKI-L16 had no effect. All fibronectin binding was completely blocked by the β1-specific mAb P5D2 or by a combination of mAbs against α4 and α5 integrins (our unpublished results; see Figure 7). Therefore, stimulation of ΔI-LFA-1 with mAbs that either bind to or stabilize only active LFA-1 led to an increase in fibronectin binding mediated by α4β1 and/or α5β1. These results further established that ΔI-LFA-1 had a direct role in signaling into the cells.
Figure 5.
Fibronectin-coated bead binding of J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1 after treatment with stimulating mAbs. Cells were adhered to plastic with an anti-CD5 mAb and incubated with fibronectin-coated beads and the indicated stimuli for 90 min at 37°C before washing off unbound beads. Data are represented as beads per 100 cells from the mean of five high-power fields ± SD. Data are representative of five experiments.
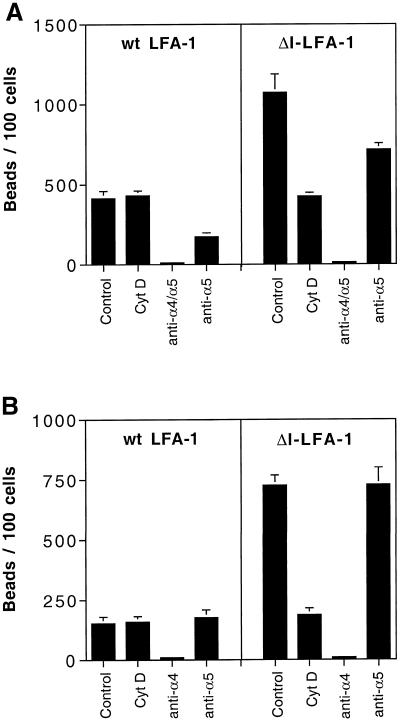
Figure 7.
Fibronectin-coated (A) and VCAM-1-coated (B) bead binding of J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1 after treatment with cytochalasin D or function-blocking mAbs. Cells were adhered to plastic with an anti-CD5 mAb and incubated with fibronectin- or VCAM-1-coated beads in the presence or absence of 5 μM cytochalasin D (Cyt D) or blocking mAbs for 90 min at 37°C before washing off unbound beads. Data are represented as beads per 100 cells from the mean of five high-power fields ± SD. Anti-α4-blocking mAb was HP1/2; anti-α5-blocking mAb was SAM-1. Data are representative of two experiments with identical results.
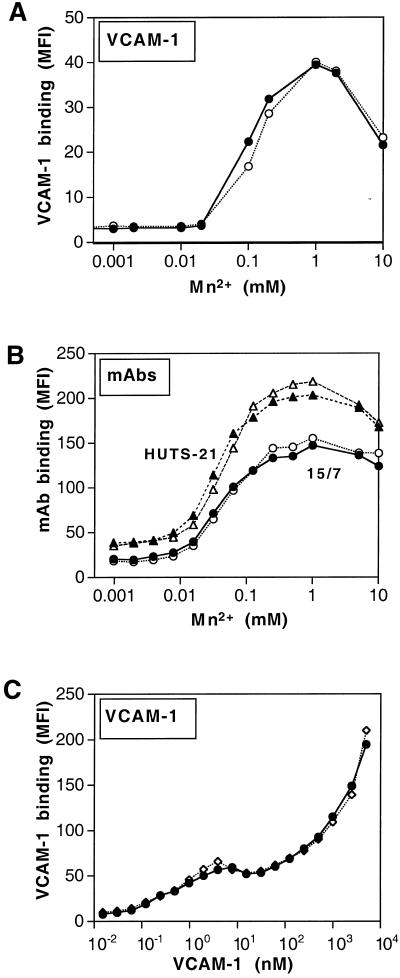
ΔI-LFA-1 Does Not Cause an Increase in α4β1 Integrin Affinity
We next wanted to characterize the enhanced activated state of the β1 integrins on cells expressing ΔI-LFA-1. As the ability to bind soluble ligand is a measure for integrin affinity, we first investigated the state of soluble VCAM-1 binding by α4β1 on ΔI-LFA-1- and wt LFA-1-expressing cells. Over a range of Mn2+ concentrations no differences were observed between the two types of cells in their ability to bind VCAM-1 (held constant at 10 nM) (Figure 6A). Again, no significant differences in VCAM-1 binding between the two types of cells were observed when the VCAM-1 concentration was varied and the Mn2+ concentration was held constant at 1 mM (Figure 6C). The titration curves for both cell lines show bivalent VCAM-1 binding between 1 and 10 nM followed by monovalent binding to the level of 5 μM (Jakubowsky et al., 1995; Lobb et al., 1995; Pujades et al., 1997).
Figure 6.
(A and C) Soluble VCAM-1 binding; (B) expression of β1 integrin activation epitopes HUTS-21 and 15/7 by J-β2.7 cells expressing wt LFA-1 or ΔI-LFA-1. VCAM-1 binding was determined as a function of Mn2+ concentration in the presence of 10 nM VCAM-1Fc (A) or as a function of ligand concentration in the presence of 1 mM MnCl2 (C). Cells were incubated with VCAM-1Fc for 30 min at room temperature followed by incubation with FITC-conjugated goat anti-human Fc IgG and analysis by flow cytometry. (B) Cells were incubated with the anti-β1 mAbs HUTS-21 or 15/7 at room temperature followed by FITC-conjugated goat anti-mouse IgG and analysis by flow cytometry. Filled symbols, wt LFA-1-expressing cells; open symbols, ΔI-LFA-1-expressing cells. Results are expressed as mean fluorescence intensities (MFI), and data are representative of three experiments.
To examine whether ΔI-LFA-1 affects the conformation of the β1 integrins, we used the mAbs HUTS-21 (Luque et al., 1996) and 15/7 (Yednock et al., 1995), the epitopes for which are induced by Mn2+. Epitope expression of these mAbs is also a measure of β1 integrin affinity. There was a direct correlation between Mn2+ concentration and expression of the epitopes, as expected, but no difference in epitope expression between the ΔI-LFA-1- and wt LFA-1-expressing cells (Figure 6B). It is of interest that the β1 epitope curves mirrored the VCAM-1 binding curves after titration of Mn2+ (Figure 6A). Taken together, these results indicate that there was no increase in β1 integrin affinity or change in conformation as detected by mAbs HUTS-21 and 15/7 on cells expressing ΔI-LFA-1 compared with wt LFA-1-expressing cells.
The Increased Activity of β1 Integrins in ΔI-LFA-1-expressing Cells Is Dependent on an Intact Cytoskeleton
To gain some insight into the nature of the signals transduced by ΔI-LFA-1-expressing cells, we analyzed the effect of the cytoskeleton-disrupting drug cytochalasin D (Figure 7). On wt LFA-1-expressing cells, cytochalasin D had no effect on fibronectin or VCAM-1 binding. However, on ΔI-LFA-1-expressing cells, cytochalasin D inhibited both fibronectin and VCAM-1 binding to the same basal levels exhibited by wt LFA-1-expressing cells. The specificity of the β1 integrin-mediated adhesion is shown by complete blocking of fibronectin binding of both cell lines by a combination of mAbs against α4 and α5 integrins and partial blocking by the anti-α5 blocking mAb SAM-1 alone. VCAM-1 binding was completely blocked by the anti-α4 blocking mAb HP1/2 and not affected by the anti-α5 mAb SAM-1. The results with cytochalasin D imply a role for the cytoskeleton itself or processes dependent on the cytoskeleton in the signaling, which gives rise to enhanced β1 integrin-mediated function in ΔI-LFA-1 expressing cells.
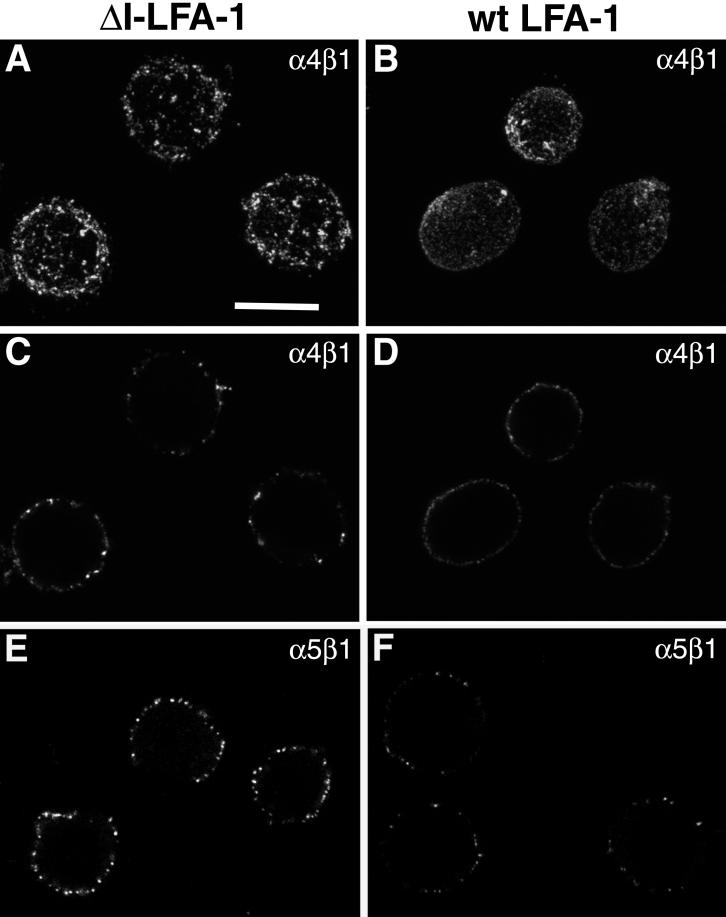
ΔI-LFA-1 Causes an Increase in β1 Integrin Clustering
A characteristic feature of activated integrin that is dependent on the cytoskeleton is integrin clustering. We therefore assessed the state of integrin clustering on wt and ΔI-LFA-1-expressing cells using confocal laser microscopy. As illustrated in Figure 8, on cells expressing ΔI-LFA-1, α4β1 was found in large clusters on the cell surface. In contrast, on wt-LFA-1-expressing cells, α4β1 was more diffusely distributed (Figure 8, A and C vs. B and D). Staining for α5β1 on cells expressing ΔI-LFA-1 showed a significant increase in signal strength compared with cells expressing wt LFA-1, indicating that α5β1 is also more clustered on ΔI-LFA-1 expressing cells (Figure 8, E and F). Pretreatment of ΔI-LFA-1-expressing cells with 5 μM cytochalasin D reduced clustering of α4β1 and α5β1 to levels observed on wt LFA-1-expressing cells, whereas cytochalasin D had no effect on the distribution of these β1 integrins on wt LFA-1-expressing cells (our unpublished results). Therefore, ΔI-LFA-1 appears to signal through the cytoskeleton to cause constitutive β1 integrin clustering.
Figure 8.
Distribution of α4β1 and α5β1 on J-β2.7 cells expressing ΔI-LFA-1 or wt LFA-1 as determined by confocal microscopy. Cells were stained on ice with the anti-α4 mAb 7.2R (A–D) or the anti-α5 mAb SAM-1 (E and F), fixed, and incubated with Alexa 488-conjugated goat anti-mouse IgG, followed by confocal microscopy. (A and B) Projections onto the x–y plane of all individual optical sections taken along the z-axis using maximum fluorescence values. (C–F) One optical section taken at midheight of the cells. Data are representative of four experiments (A–D) and five experiments (E and F). Bar, 10 μm.
DISCUSSION
In this study, LFA-1 lacking the I domain (ΔI-LFA-1) was expressed in the αL-deficient Jurkat T cell line, J-β2.7, which allowed analysis of LFA-1-dependent functions in a lymphocyte background. The major findings of this study are 1) ΔI-LFA-1 is expressed as an αβ heterodimer on the cell surface, demonstrating that the I domain is not necessary for heterodimer formation; 2) the I domain of LFA-1 is essential for ligand binding, because ΔI-LFA-1 showed no detectable ligand binding activity to ICAM-1 or ICAM-3; 3) removal of the I domain leads to enhanced expression of activation epitopes as well as expression of the activation reporter epitope 24, which suggests that the I domain regulates conversion to the high-affinity conformation; 4) ΔI-LFA-1 signals constitutively into the cell, as illustrated by the activation of β1 integrins on the same cell through integrin cross-talk; the nature of the signals transmitted by ΔI-LFA-1 is dependent on an intact actin cytoskeleton; and 5) ΔI-LFA-1 does not signal an increase in affinity of the β1 integrins but does cause enhanced integrin clustering.
ΔI-LFA-1 was detected on the cell surface by a number of different anti-LFA-1 mAbs, indicating correct folding of the α and β subunits in the absence of the I domain. In fact, all tested mAb epitopes outside the I domain were expressed by ΔI-LFA-1 and wt LFA-1 to a similar extent. The specific expression of epitopes dependent on association of αL with β2 (e.g., TS2/4 and TS1/18; Dustin et al., 1992) indicates that ΔI-LFA-1 formed heterodimers with the endogenous β2 subunit on the cell surface. Therefore, correct folding of the β-propeller and C-terminal domains of the αL subunit and heterodimerization of αL with β2 are independent of the I domain. In agreement, another study showed that, in the context of intact LFA-1, folding of the β-propeller domain was independent of the I domain (Huang and Springer, 1997).
The I domain contains the major ligand binding site in LFA-1. However, because additional sites contributing to ligand binding are predicted in both the αL subunit (Stanley et al., 1994) and the β2 subunit (Goodman and Bajt, 1996; Goodman et al., 1998), it was possible that an I domain-deleted LFA-1 might bind ligand similarly to a non–I domain-containing integrin. The data in the present study clearly demonstrate that there is no residual ICAM-1 or ICAM-3 ligand binding capacity in ΔI-LFA-1. Therefore, the additional sites, although participants in ligand binding in intact LFA-1, are not sufficient to independently sustain ligand binding in the absence of the I domain. The I domain may cooperate with these other sites for stable interaction with ligand.
Although LFA-1 without an I domain has lost its capacity to bind ligand, a significant feature of ΔI-LFA-1 is the enhanced expression of mAb epitopes, which are associated with activation of LFA-1. For example, the Ca2+-dependent αL-specific NKI-L16 epitope is expressed by a subset of LFA-1 that is primed for activation (van Kooyk et al., 1994). In addition, the activation epitopes detected by KIM 127, KIM 185 (Ortlepp et al., 1995), MEM48, and 240Q (McDowall, unpublished data) are also expressed by subsets of total cellular LFA-1 on other leukocytes. The fact that these activation epitopes are expressed only at a low level by intact LFA-1, as in our study, suggests that these sites are masked but exposed upon activation. mAbs KIM 127, KIM 185, MEM48, and CBR LFA-1/2, have been mapped to the cysteine-rich region of the β2 subunit (Stephens et al., 1995; Huang et al., 1997). Thus, in addition to the α subunit, the conformation of the cysteine-rich region in the β2 subunit may be altered on ΔI-LFA-1 compared with wt LFA-1. Alternatively, removal of the I domain could lead to unmasking of the cysteine-rich region. This latter explanation is favored by the finding that KIM 127 recognizes the immature unassociated β2 subunit but not the mature β2 subunit of the αLβ2 heterodimer (Huang et al., 1997). It is of interest that ligand binding and integrin activation of α5β1 integrin has been linked to uncovering of the β1 cysteine-rich region (Tsuchida et al., 1998).
In the present study, wt LFA-1 showed no constitutive expression of the activation reporter mAb 24 epitope, whereas ΔI-LFA-1 cells expressed this epitope. Expression of the 24 epitope is a hallmark of higher-affinity LFA-1, a form of the receptor that is capable of binding soluble ligand (Stewart et al., 1996; Ganpule et al., 1997). In vivo this high-affinity LFA-1 conformation is not constitutively found on resting leukocytes, but increased epitope expression has been correlated with human T cell activation in secondary lymphoid tissues (Picker et al., 1993). Expression of this activation reporter epitope further confirmed the activated status of ΔI-LFA-1 compared with wt LFA-1.
Although ΔI-LFA-1 could no longer bind ligand, it was of interest to know whether the active conformation of ΔI-LFA-1 was correlated with signal transduction into the cell. The signaling capabilities of LFA-1 have usually been tested by analyzing LFA-1 functions as a costimulator in conjunction with other membrane receptors, which has made it difficult to resolve whether LFA-1 can signal independently. However, a signaling activity of LFA-1 that is dependent on LFA-1 alone is the ability to influence the activity of other integrins such as α4β1 and α5β1, termed cross-talk (Porter and Hogg, 1997). A characteristic of the ΔI-LFA-1-expressing cells is constitutively elevated fibronectin and VCAM-1 binding activity mediated by the β1 integrins α4β1/α5β1 (fibronectin binding) and α4β1 alone (VCAM-1 binding). Evidence that this was functionally related to the presence of ΔI-LFA-1, and not some coincidental activity, was shown by the additional enhanced β1 integrin activity of ΔI-LFA-1-expressing cells after exposure to the LFA-1-specific mAbs NKI-L16 and 24.
How integrins effect cross-talk to other integrins has not yet been defined in molecular terms. However, distinctive features ascribed to active integrins fall into two categories. In response to intracellular signals, integrins such as αIIbβ3 can alter their conformation and bind ligand with higher affinity (Hato et al., 1998). Alternatively, integrins cluster in response to intracellular signals and bind ligand with greater adhesive strength (Yauch et al., 1997; Stewart et al., 1998). We found no evidence for an affinity alteration of α4β1 on ΔI-LFA-1-expressing cells, as assessed by binding of soluble ligand VCAM-1 or expression of β1 subunit reactive HUTS-21 and 15/7 activation epitopes, which register the active conformation particularly of α4β1 (Bazzoni et al., 1998). However, the β1 integrins α4β1 and α5β1 were observed to be in a constitutively highly clustered state on ΔI-LFA-1-expressing cells. These results contrast with the dominant inhibition that αIIbβ3 had on the affinity of α5β1 (Diaz-Gonzalez et al., 1996).
The fact that cytochalasin D blocked the enhanced activity and the clustering of β1 integrins on ΔI-LFA-1-expressing cells implies that the cytoskeleton or processes dependent on the cytoskeleton are targets of ΔI-LFA-1-mediated signaling. These findings suggest that active LFA-1 might reorganize the cytoskeleton in a manner that instructs other integrins to link into it, a process that happens during cell migration (Felsenfeld et al., 1996). Another possibility to be considered is that clustering may occur after removal of cytoskeletal constraints by the signaling integrin.
In addition to our findings, another example of positive integrin cross-talk involves activation of α2β1 by the interaction of α5β1 with ligand (Pacifici et al., 1994). In contrast, other examples of inter-integrin communication can be termed “trans-dominant inhibition” because of the negative effect on target integrin function (Blystone et al., 1994; Blystone et al., 1995; Huhtala et al., 1995; Diaz-Gonzalez et al., 1996; Hodivala-Dilke et al., 1998). In fact, LFA-1-mediated cross-talk in primary T cells was detected as a negative effect on α4β1 function (Porter and Hogg, 1997). These conflicting results raise the issue as to why there is positive regulation of integrin function in some situations and, in others, negative regulation. It has been suggested that a prerequisite for negative regulation is high expression of the “dominating” integrin (Diaz-Gonzalez et al., 1996). The choice between positive or negative cross-talk may depend on the availability of adaptor proteins for cytoskeletal connections or components of critical signaling pathways. Potentially highly expressed integrins such as αIIβ3 transfected into Chinese hamster ovary cells (Diaz-Gonzalez et al., 1996), α3β1 on keratinocytes (Hodivala-Dilke et al., 1998), or LFA-1 on cultured primary T cells (Porter and Hogg, 1997) might sequester such essential adaptor or signaling molecules. However, in other situations such as described in this study, in which the activating integrin is expressed at relatively low levels, the adaptor–signaling protein(s) may be generated in excess amounts and available to other integrins on the same cell. Signaling enzymes that have been implicated in cross-talk are protein kinase C (Pacifici et al., 1994) and calmodulin-dependent kinase II (Blystone et al., 1999). Future work will be required to investigate whether these kinases or other signaling components are activated by LFA-1 to operate through the cytoskeleton to cause clustering of “target” integrins.
In summary, LFA-1 expressed without its I domain does not bind its ICAM ligands, has the features of an activated integrin, and appears to signal constitutively back into the T cell. The altered conformation of ΔI-LFA-1 compared with wt LFA-1 suggests that a quarternary structural change has occurred in the integrin ectodomain, which could alter the configurations of the αL and β2 cytoplasmic domains, leading to a constitutively active signaling integrin. Alternatively, the absence of the I domain might alter the associations of LFA-1 with other membrane proteins. We have recently shown that the I domain participates in interdomain movement upon activation (McDowall et al., 1998), which could be a prerequisite for the subsequent activated conformation. Thus, as well as binding ligand, the I domain controls activation of LFA-1 extracellularly and complements the regulation of adhesiveness provided by the cytoplasmic sequences of both subunits (O'Toole et al., 1994; Lu and Springer, 1997). We show here that the activation of LFA-1 has a major effect on the activity of β1 integrins on the same T cell membrane. Thus at least some integrins appear not to operate in isolation but, as a consequence of their activation status, directly influence the activity of other classes of integrin on the same cell.
ACKNOWLEDGMENTS
We are extremely grateful to Lloyd Klickstein for the J-β2.7 cell line. We are also indebted for gifts of mAbs to Yvette van Kooyk, Martyn Robinson, Alex Law, and Rick Jasman and for supplies of VCAM-1Fc to Roy Lobb and Martyn Robinson. We thank our colleagues in the Leukocyte Adhesion Laboratory for their helpful comments on the manuscript. This work was supported by the Imperial Cancer Research Fund. B.L. is a recipient of a European Union Training and Mobility of Researchers fellowship.
Abbreviations used:
- ICAM
intercellular adhesion molecule
- IgG
immunoglobulin G
- LFA
lymphocyte function-associated antigen
- mAb
monoclonal antibody
- PdBu
phorbol 12,13-dibutyrate
- VCAM
vascular cell adhesion molecule
- wt
wild-type
REFERENCES
- Andrew D, Shock A, Ball E, Ortlepp S, Bell J, Robinson M. KIM 185, a monoclonal antibody to CD18 which induces a change in the conformation of CD18 and promotes both LFA-1- and CR3-dependent adhesion. Eur J Immunol. 1993;23:2217–2222. doi: 10.1002/eji.1830230925. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Ma L, Blue M-L, Hemler ME. Divalent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J Biol Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, van Kooyk Y, Simmons DL, Figdor CG. Distinct binding of T lymphocytes to ICAM-1, -2 or -3 upon activation of LFA-1. Eur J Immunol. 1994;24:2155–2160. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin αvβ3 differentially regulates adhesive and phagocytic function of the fibronectin receptor α5β1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Lindberg FP, LaFlamme SE, Brown EJ. Integrin β3 cytoplasmic tail is necessary and sufficient for regulation of α5β1 phagocytosis by αvβ3 and integrin-associated protein. J Cell Biol. 1995;130:745–754. doi: 10.1083/jcb.130.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Slater SE, Williams MP, Crow MT, Brown EJ. A molecular mechanism of integrin cross-talk: αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH. Trans-dominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–1961. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickeson SK, Santoro SA. Ligand recognition by the I domain-containing integrins. Cell Mol Life Sci. 1998;54:556–566. doi: 10.1007/s000180050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickeson SK, Walsh JJ, Santoro SA. Contributions of the I and EF hand domains to the divalent cation-dependent collagen binding activity of the α2β1 integrin. J Biol Chem. 1997;272:7661–7668. doi: 10.1074/jbc.272.12.7661. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunits. EMBO J. 1989;12:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Carpen O, Springer TA. Regulation of locomotion and cell-cell contact area by the LFA-1 and ICAM-1 adhesion receptors. J Immunol. 1992;148:2654–2663. [PubMed] [Google Scholar]
- Felsenfeld D, Choquet D, Sheetz M. Ligand binding regulates the directed movement of β1 integrins. Nature. 1996;383:438–440. doi: 10.1038/383438a0. [DOI] [PubMed] [Google Scholar]
- Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- Ganpule G, Knorr R, Miller JM, Carron CP, Dustin ML. Low affinty of cell surface lymphocyte function-associated antigen-1 (LFA-1) generates selectivity for cell-cell interactions. J Immunol. 1997;159:2685–2692. [PubMed] [Google Scholar]
- Goodman TG, Bajt ML. Identifying the putative metal ion-dependent adhesion site in the β2 (CD18) subunit required for αLβ2 and αMβ2 ligand interactions. J Biol Chem. 1996;271:23929–23736. doi: 10.1074/jbc.271.39.23729. [DOI] [PubMed] [Google Scholar]
- Goodman TG, DeGraaf ME, Fischer HD, Bajt ML. Expression of a structural domain of the β2 subunit essential for αMβ2 ligand recognition. J Leukocyte Biol. 1998;64:767–773. doi: 10.1002/jlb.64.6.767. [DOI] [PubMed] [Google Scholar]
- Goodwin AE, Pauli BU. A new adhesion assay using buoyancy to remove nonadherent cells. J Immunol Methods. 1995;187:213–219. doi: 10.1016/0022-1759(95)00187-6. [DOI] [PubMed] [Google Scholar]
- Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIbβ3. J Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, DiPersio CM, Kreidberg JA, Hynes RO. Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin function in mouse keratinocytes. J Cell Biol. 1998;142:1357–1369. doi: 10.1083/jcb.142.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Lu C, Springer TA. Folding of the conserved domain but not of flanking regions in the integrin β2 subunit requires association with the α subunit. Proc Natl Acad Sci USA. 1997;94:3156–3161. doi: 10.1073/pnas.94.7.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Springer TA. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1) J Biol Chem. 1995;270:19008–19016. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- Huang C, Springer TA. Folding of the β-propeller domain of the integrin αL subunit is independent if the I domain and dependent on the β2 subunit. Proc Natl Acad Sci USA. 1997;94:3162–3167. doi: 10.1073/pnas.94.7.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. J Biol Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by α5β1 and α4β1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Newham P. The structure of cell-adhesion molecules. Trends Cell Biol. 1998;8:78–83. [PubMed] [Google Scholar]
- Jakubowsky A, Rosa MD, Bixler S, Lobb R, Burkly LC. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct states of the very late antigen-4 (VLA-4) receptor. Cell Adhes Commun. 1995;3:131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- Keizer GD, Visser W, Vliem M, Figdor CG. A monoclonal antibody (NKI-L16) directed against a unique epitope on the α-chain of human leukocyte function-associated antigen-1 induces homotypic cell-cell interactions. J Immunol. 1988;140:1393–1400. [PubMed] [Google Scholar]
- Lobb RR, Antognetti G, Pepinsky RB, Burkly LC, Leone DR, Whitty A. A direct binding assay for the vascular cell adhesion molecule-1 (VCAM-1) interaction with α4 integrins. Cell Adhes Commun. 1995;3:385–397. doi: 10.3109/15419069509081293. [DOI] [PubMed] [Google Scholar]
- Loftus JC, Liddington RC. New insights into integrin-ligand interaction. J Clin Invest. 1997;99:2302–2306. doi: 10.1172/JCI119408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C-F, Springer TA. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated coformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common β1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- McDowall A, Leitinger B, Stanley P, Bates PA, Randi AM, Hogg N. The I domain of integrin leukocyte function-associated antigen-1 is involved in a conformational change leading to high affintiy binding to ligand intercellular adhesion molecule 1 (ICAM-1) J Biol Chem. 1998;273:27396–27403. doi: 10.1074/jbc.273.42.27396. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke AM, Shao H, Kaye J. A role for p21ras/MAP kinase in TCR-mediated activation of LFA-1. J Immunol. 1998;161:5800–5803. [PubMed] [Google Scholar]
- O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortlepp S, Stephens PE, Hogg N, Figdor CG, Robinson MK. Antibodies that activate β2 integrins can generate different ligand binding states. Eur J Immunol. 1995;25:637–643. doi: 10.1002/eji.1830250302. [DOI] [PubMed] [Google Scholar]
- Pacifici R, Roman J, Kimble R, Civitelli R, Brownfield CM, Bizzarri C. Ligand binding to monocyte α5β1 integrin activates the α2β1 receptor via the α5 subunit cytoplasmic domain and protein kinase C. J Immunol. 1994;153:2222–2233. [PubMed] [Google Scholar]
- Petruzzelli L, Maduzia L, Springer TA. Activation of lymphocyte function-associated molecule-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) mimicked by an antibody directed against CD18. J Immunol. 1995;155:854–866. [PubMed] [Google Scholar]
- Picker LJ, Treer JR, Nguyen M, Terstappen LWMM, Hogg N, Yednock T. Coordinate expression of β1 and β2 integrin “activation” epitopes during T cell responses in secondary lymphoid tissue. Eur J Immunol. 1993;23:2751–2757. doi: 10.1002/eji.1830231105. [DOI] [PubMed] [Google Scholar]
- Porter JC, Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters α4β1- and α5β1-mediated function. J Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C, Alon R, Yauch RL, Masumoto A, Burkly LC, Chen C, Springer TA, Lobb RR, Hemler ME. Defining extracellular integrin α-chain sites that affect cell adhesion and adhesion strengthening without altering soluble ligand binding. Mol Biol Cell. 1997;8:2647–2657. doi: 10.1091/mbc.8.12.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MK, Andrew S, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC. Antibody against the Leu-CAM β-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- Shimizu Y, Hunt SW. Regulating integrin-mediated adhesion: one more function for PI 3-kinase? Immunol Today. 1996;17:565–573. doi: 10.1016/s0167-5699(96)10061-x. [DOI] [PubMed] [Google Scholar]
- Springer TA. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Bates PA, Harvey J, Bennett RI, Hogg N. Integrin LFA-1 α subunit contains an ICAM-1 binding site in domains V and VI. EMBO J. 1994;13:1790–1798. doi: 10.1002/j.1460-2075.1994.tb06447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Hogg N. The I domain of integrin LFA-1 interacts with ICAM-1 domain 1 at residue Glu-34 but not Gln-73. J Biol Chem. 1998;273:3358–3362. doi: 10.1074/jbc.273.6.3358. [DOI] [PubMed] [Google Scholar]
- Stephens P, Romer JT, Spitali M, Shock A, Ortlepp S, Figdor CG, Robinson MK. KIM127, an antibody that promotes adhesion, maps to a region of CD18 that includes cysteine-rich repeats. Cell Adhes Commun. 1995;3:375–384. doi: 10.3109/15419069509081292. [DOI] [PubMed] [Google Scholar]
- Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity versus avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Stewart MP, Cabanas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Stewart MP, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida J, Ueki S, Takada Y, Saito Y, Takagi J. The “ligand-induced conformational change” of α5β1 integrin. Relocation of α5 subunit to uncover the β1 stalk region. J Cell Sci. 1998;111:1759–1766. doi: 10.1242/jcs.111.12.1759. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Heije K, Figdor CG. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Hogervorst F, Verhoeven AJ, van Seventer G, te Velde AA, Borst J, Keizer GD, Figdor CG. Activation of LFA-1 through a Ca2+-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991;112:345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human α11 integrin. J Biol Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- Weber KSC, York MR, Springer TA, Klickstein LB. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines. J Immunol. 1997;158:273–279. [PubMed] [Google Scholar]
- Yauch R, Felsenfest DP, Kraeft S-K, Chen LB, Sheetz MP, Hemler ME. Mutational evidence for control of cell adhesion through integrin diffusion/clustering, independent of ligand binding. J Exp Med. 1997;186:1347–1355. doi: 10.1084/jem.186.8.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Vandevert C, Goldbach EG, Shaw G, Ellis DK, Liaw C, Fritz LC, Tanner LI. α4β1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]