Abstract
Aims
The clinical effectiveness of buprenorphine–naloxone (bup-nx) and clonidine for opioid detoxification in in-patient and out-patient community treatment programs was investigated in the first studies of the National Institute of Drug Abuse Clinical Trials Network.
Design
Diagnostic and Statistical Manual version IV (DSM IV)-diagnosed opioid-dependent individuals seeking short-term treatment were randomly assigned, in a 2:1 ratio favoring bup-nx, to a 13-day detoxification using bup-nx or clonidine.
Methods
A total of 113 in-patients (77 bup-nx, 36 clonidine) and 231 out-patients (157 bup-nx, 74 clonidine) participated. Supportive interventions included appropriate ancillary medications and standard counseling procedures guided by a self-help handbook. The criterion for treatment success was defined as the proportion of participants in each condition who were both retained in the study for the entire duration and provided an opioid-free urine sample on the last day of clinic attendance. Secondary outcome measures included use of ancillary medications, number of side effects reported and withdrawal and craving ratings.
Findings
A total of 59 of the 77 (77%) in-patients assigned to the bup-nx condition achieved the treatment success criterion compared to eight of the 36 (22%) assigned to clonidine, whereas 46 of the 157 (29%) out-patients assigned to the bup-nx condition achieved the treatment success criterion, compared to four of the 74 (5%) assigned to clonidine.
Conclusion
The benefits of bup-nx for opioid detoxification are supported and illustrate important ways in which clinical research can be conducted in community treatment programs.
Keywords: Buprenorphine, clonidine, detoxification, opiate dependence, treatment
INTRODUCTION
The US National Institute on Drug Abuse (NIDA) established a Clinical Trial Network (CTN) in 1999 as a group of university-based regional research training centers, each linked in partnership to a number of community-based treatment programs (CTPs), in response to a National Academy of Science recommendation (Lamb, Greenlick & McCarty 1998). The architecture of the CTN was intended to accelerate the pace of treatment research on drug abuse and its application to real-life treatment settings, thereby translating science-based research knowledge into treatment at the community level. An initial task of the CTN was to determine how best to integrate an array of research-based behavioral and pharmacological therapies into community treatment programs. The anticipated imminent approval of buprenorphine for treatment of opioid dependence made it a logical priority.
Buprenorphine, a partial mu-opioid agonist that had been in development for more than two decades, promised to significantly change opioid pharmacotherapy in the United States (cf. Bickel & Amass 1995; Boatwright 2002; Johnson et al. 2003). For nearly 100 years, and until the Food and Drug Administration (FDA) approved buprenorphine for opioid dependence treatment, physicians in the United States had been prohibited from prescribing an opioid-based treatment in their usual practice setting for their opioid-addicted patients. More than three decades of experience with methadone had amply demonstrated the effectiveness of opioid-based treatment for such patients, but because methadone was available only through specially licensed opioid treatment programs (OTPs), and therefore outside mainstream medicine, most physicians had no access to this effective clinical tool. The anticipated availability of buprenorphine coincided with increasing reports of a growing number of new heroin users, particularly among the young. Introduction of sublingual buprenorphine combined with naloxone (bup-nx) to protect against injection use promised new treatment possibilities for individuals who, until now, would not seek treatment for opioid abuse. An evaluation of bup-nx for short-term withdrawal from opioids at the community clinic level seemed timely and feasible, and provided a good opportunity to conduct a field test of this new treatment (Amass et al. 2004).
Experience with methadone treatment demonstrates consistently that opioid addiction is a chronic disorder and that treatment should be long term (Hser et al. 2001). However, most of the treatment facilities outside OTPs in the United States provided only short-term opioid detoxification as an early medical stabilization and engagement strategy for entry into drug-free treatment. Despite unpleasant and limiting side-effects (Gowing et al. 2003), clonidine (used off-label) was the only non-opioid medication used with any consistency. Prior studies evaluating buprenorphine for opioid detoxification suggested it might be an ideal medication to help patients withdraw from opioids (Amass et al. 1994; Cheskin, Fudala & Johnson 1994; Bickel et al. 1997; Becker et al. 2001).
It was clear that CTPs would value the opportunity to gain clinical experience with bup-nx as the wait for FDA approval ensued. With this in mind, a protocol was written to closely reflect actual clinical practice, and keep to a minimum the conventional requirements of a controlled clinical trial (e.g., without blinding or the use of placebo control). At the same time, it was considered essential to retain some fundamental research procedures to facilitate study conduct (e.g., prescribed medication regimens) and to permit interpretation of results (e.g., random assignment to condition). Clonidine was identified as the non-opioid ‘treatment as usual’ comparison as it was already widely used by CTPs in the United States, and clonidine and buprenorphine had been addressed previously in treatment studies in both the United States and other countries (e.g., Cheskin et al. 1994; Nigam, Ray & Tripathi 1993; Fingerhood et al. 2001; Lintzeris et al. 2002). For ease of understanding and execution, the study was written as separate protocols for both in-patient and out-patient programs.
Based on earlier reports, the current studies hypothesized that patients assigned to bup-nx would be significantly more likely to complete the detoxification treatment and to provide an opioid-free urine sample on the last day of treatment compared to patients assigned to the clonidine condition in both the in-patient and out-patient samples.
METHOD
Study design
Twelve CTPs participated in either the in-patient or out-patient study (six CTPs in each) comparing bup-nx and clonidine in an open label, randomized 13-day detoxification regimen. To maximize opportunities for community practitioners to work with bup-nx, patients were assigned randomly to bup-nx or clonidine conditions using a 2 : 1 ratio in favor of buprenorphine. Other aspects of treatment outcome pertaining to patients assigned to the bup-nx arm of these trials are reported elsewhere (Amass et al. 2004).
Participants
Participants were enrolled from 26 February 2001 to 30 July 2002 for the in-patient study, and 9 January 2001 to 26 February 2002 for the out-patient study. Participants included treatment-seeking adults at least 18 years old and in good general health, who met Diagnostic and Statistical Manual version IV (DSM-IV) criteria for opioid dependence, and were in need of medical management for opioid withdrawal. Potential participants were excluded if they had a serious psychiatric or medical condition that would make participation medically hazardous (e.g., suicidal behavior, uncontrolled diabetes); had a known allergy or sensitivity to buprenorphine, naloxone or clonidine; were receiving medications contraindicated with clonidine or had a systolic blood pressure < 100 mmHg or pulse < 56 beats per minute (bpm). They were also excluded if they had been enrolled in a methadone or levo-alpha-acetyl-methadol (LAAM) treatment program or had participated in another investigational drug study within 30 days of study enrollment, or if they could not remain in the area for the duration of active treatment. Co-dependence on other drugs (e.g., cocaine, alcohol or benzodiazepines, or other depressants or stimulants) did not exclude individuals from participation unless immediate medical attention was required to manage these disorders. Female participants were excluded if pregnant or lactating, and were required to have a negative pregnancy test prior to randomization.
Participants were assessed at one time-point. However, if a participant met all inclusionary criteria, but had a positive methadone urine drug test result, he/she could return on a subsequent day, produce a negative urine drug screen and be allowed to enroll in the study. Additionally, participants who received treatment with methadone or LAAM in the 30 days prior to screening could return at 31 days and participate in the current study, provided that this was done within 14 days of the original consent to participate in the study. A total of 25 participants in the in-patient study and 46 participants in the out-patient study were dropped prior to randomization because they met exclusion criteria as determined by baseline assessment following informed consent.
All individuals not eligible for participation in the current study during initial screening, or following baseline screening, were referred to standard treatment services within the CTP or another local treatment facility as determined appropriate by the CTP staff. Available treatment resources may have included a wide variety of treatment programs and recovery resources, and was predicated on the specific clinical site. The study was approved by each of the participating Institutional Review Boards and the UCLA Human Subjects Protection Committee. All participants provided written informed consent prior to any study procedures.
Sample characteristics
A total of 344 participants were randomized: 113 to the in-patient study (77 bup-nx; 36 clonidine) and 231 to the out-patient study (157 bup-nx; 74 clonidine). As per study design, the samples were randomized on a 2:1 ratio (bup-nx:clonidine).
No statistically significant differences across groups were found for gender, race/ethnicity, age, employment, education or years of heroin use was (all comparisons P > 0.05). The percentage of female participants was lower in the out-patient (27% bup-nx, 31% clonidine) than in the in-patient study (39% bup-nx, 42% clonidine). More than half the in-patient sample was Caucasian (56%), with 19% African American and 16% Hispanic. In the out-patient sample, 40% were Caucasian, 37% were African American and 20% Hispanic. From 70 to 94% of participants worked full- or part-time across groups, and all groups had at least a high school education. Out-patients were somewhat older (39 years) than in-patients (36 years) and had a longer history of heroin use (more than 9 years) compared to their in-patient peers (less than 7 years). No differences in other substance dependence diagnoses were found across treatment groups for either the in-patient or out-patient studies, except that more participants in the clonidine group met diagnostic criteria for cannabis dependence than in the bup-nx group (13.9% versus 2.6%, P < 0.05). Percentages of dependence diagnoses for in-patient and out-patient participants, respectively, are: alcohol, 12% 5.2%; cannabis, 6.2%, 6.1%; cocaine, 22.1%, 17.3%; nicotine, 33.6%, 32%; and benzodiazepines, 4.4%, 0.9%. All other substance dependence diagnoses occurred at less than 1%.
Study medications
Bup-nx combination tablets in dosages of 2 mg buprenorphine/0.5 mg naloxone tablet(s) and/or 8 mg buprenorphine/2.0 mg naloxone were provided in child-proof blister packs from Reckitt Benckiser (Hull, UK) and supplied by the National Institute on Drug Abuse through the Research Triangle Institute. Clonidine tablets (CAT-APRES®—clonidine hydrochloride USP, 0.1 mg tablet for oral dosing) and clonidine transdermal therapeutic systems (CATAPRES-TTS®—1, 0.1 mg) were purchased commercially from Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA.
Procedure
Screening and intake
After providing informed consent and prior to randomization, participants completed a 2–3-h screening to collect information on demographics, medical history, physical examination, laboratory evaluation and HIV-risk and drug abuse history using the DSM-IV criteria checklist (American Psychiatric Association 1994) and the Addiction Severity Index (ASI) (McLellan et al. 1992). To assess withdrawal and craving, both observer (Clinical Opiate Withdrawal Scale (COWS); Wesson, Ling & Jara, 1999) and self-report (Adjective Rating Scale for Withdrawal (ARSW); Bickel et al. 1988a, 1988b; Amass et al. 2000) withdrawal ratings were administered. The Visual Analogue Scale (VAS) was used to rate severity of craving. Once randomized, participants completed daily self-report measures and received their daily study medications, and the prescribed counseling sessions for up to 13 consecutive days, as well as the psychosocial treatment provided customarily at the clinical sites.
Prior to randomization and dosing on day 1, a urine drug screen was done to ensure methadone-negative status (also benzodiazepines in out-patient settings) to mitigate potential drug interactions with bup-nx and to minimize risks of precipitated withdrawal. If the drug screen was positive for methadone (and/or benzodiazepine in out-patient settings), the participant was rescheduled for randomization at a later date when all requirements for induction were met. Urine drug screens were conducted on-site using Accutest® 10-MultiDrug Screen (JANT Pharmacal, Encino, CA, USA) to determine eligibility for participation and induction onto study medication. Urine samples were collected using FDA-approved collection devices for monitoring specimen integrity at baseline and at four time-points during the detoxification. Urine drug screens collected during treatment were analyzed centrally (North-west Toxicology, Inc., Salt Lake City, UT, USA).
Medication procedure
Bup-Nx induction and administration
To facilitate a comfortable transition, participants were instructed not to use heroin or other opioids for at least 6 h and to be in mild withdrawal before receiving their first dose of the bup-nx combination tablet. Physicians or study coordinators at each site took participants’ history before administering the first dose of bup-nx to document the time and date of last drug use and verify patient withdrawal status.
The bup-nx combination tablets were administered sublingually using a 3-day rapid induction schedule (Amass et al. 2004). Participants were instructed to hold the tablet(s) under their tongue until dissolved. On day 1 participants received an initial dose of two tablets, each containing 2 mg buprenorphine (bup) and 0.5 mg naloxone (nx). An additional two tablets were provided 1–2 h following the initial dose unless clinically contraindicated, as judged by the study physicians. Physicians were encouraged to provide participants the full 8 mg bup/2 mg nx dose for day 1. Dosage was fixed throughout the remaining 12 days, increasing in a stepwise manner to 16 mg bup/4 mg nx on day 3 and tapering to 2 mg bup/.05 mg nx by days 12/13.
Clonidine induction and administration
The clonidine detoxification schedule reflected current clinical practice at the participating clinic sites. In out-patient sites, detoxification procedures were started by Wednesday of each week to ensure that a steady-state level was attained before the weekend when fewer medical staff were available. Patients were encouraged to drink fluids prior to receiving the first dose of clonidine, and vital signs were obtained to ensure appropriate blood pressure (systolic > 100 mmHg) and heart rate > 56 (bpm). On day 1, participants received oral clonidine, 0.05–0.1 mg every 4–6 h for 24 h (not to exceed 0.6 mg total). If the oral dose was well tolerated, a clonidine transdermal patch (clonidine TTS, 0.1 mg) delivering medication for 7 days was applied, with number of patches adjusted by weight (< 110 lbs = 1–3 patches; 110–200 lbs = 2–4 patches; > 200 lbs = 2–6 patches). Oral clonidine was discontinued on day 3, and if the patch was well tolerated no additional oral clonidine was administered. A new patch(es) was applied on day 7. By day 13, clonidine was discontinued and remaining patches removed. Clonidine doses were adjusted as clinically indicated and, where applicable, take-home doses were allowed for Saturday, Sunday and scheduled holidays. Participants were told to swallow all pills and/or to wear their clonidine patch(es) until instructed otherwise.
Ancillary medications
A limited number of prescription and over-the-counter ancillary medications were provided for relief of specific withdrawal symptoms such as anxiety, restlessness, insomnia, nausea, vomiting, diarrhea and muscle, bone and joint pain (see Amass et al. 2004 for further details). Patients were prohibited from using any other drugs than those provided by the study physician. Over-the-counter medications provided included: acetaminophen (650 mg), ibuprofen (200 mg), loperimide (2 mg) and diphenhydramine (25 mg). Ancillary prescription medications included: oxazepam (15, 30 mg tablets or capsules); lorazepam (0.5, 1, 2 mg tablets); phenobarbital (15, 16, 30 mg tablets, 16 mg capsules); hydroxyzine hydrochloride (50 mg tablets); methocarbamol (500, 750 mg tablets); trimethobenzamide (250 mg capsules, 100/200 mg suppositories); Donnatal (tablets or capsules: 0.0194 mg atropine sulfate, 0.0065 mg scopolamine hydrobromide, 0.1037 mg hyosyamine hydrobromide or sulfate, and 16 mg phenobarbital); zolpidem tartrate (5, 10 mg tablet); trazadone hydrochloride (50, 100, 150, 300 mg tablets); and doxepin hydrochloride (50 mg tablets).
Counseling procedures
A self-help detoxification handbook prepared by the first author (Ling & Obert 2000) was distributed to all participants for use in conjunction with the standard counseling program in accordance with standard policies and procedures at each study site. This handbook utilizes a format adapted from the Matrix Model of Addiction Treatment manualized guidebook (Rawson et al. 2001). Addressed are descriptions of opiates and addiction, and materials and strategies are provided to assist the patient in avoiding relapse utilizing cognitive-behavioral, relapse prevention, and Matrix Model treatment tools. Counseling procedures to guide in the use of the handbook were also provided to clinical personnel at each site.
Compensation
Participants received $25 in gift certificates for completing the screening interview. All medications and counseling services were provided at no cost to participants during the 13-day detoxification.
Study measures
Self-report measures
Addiction Severity Index (ASI; McLellan et al. 1992). An abbreviated version of the ASI was administered prior to randomization to characterize this sample along demographic, medical, employment, alcohol, drug, legal and psychiatric domains.
Clinical Opiate Withdrawal Scale (COWS; Wesson et al. 1999; Wesson & Ling 2003). A clinical observer assesses 11 signs of clinical withdrawal symptoms.
Adjective Rating Scale for Withdrawal (ARSW; Bickel et al. 1988a, 1988b; Amass et al. 2000). This 16-item self-report scale measured subjective severity of opioid withdrawal and symptoms at baseline and during treatment.
Visual Analogue Scale (VAS; Kaplan et al. 1985; Childress, McLellan & O’Brien 1986). Participants were asked to place a mark across the line at the point that corresponds to their immediate craving for opioids. Anchors included 0 mm—‘no cravings’ to 100 mm—‘most extreme cravings possible.
Objective measures
Treatment retention. Defined as the number of days from first dose to the last dose received.
Ancillary medications. Measured by the number of patients who were provided with ancillary medications for any purpose.
Serious adverse events and adverse events. Adverse events that resulted in overnight hospitalization or death, were immediately life-threatening, involved any permanent or substantially disabling event or congenital anomaly, or were judged by investigators or the Data and Safety Monitoring Board to be serious or that would suggest a significant hazard, contraindication, side-effect or precaution were recorded as ‘serious adverse events’ and distinguished from adverse events. The mean number of adverse events reported for each of the treatment days was calculated based on the total number of adverse events reported by participants assigned to each group on each day of evaluation divided by the number of participants present on that day. An overall mean number of adverse events per treatment day was then calculated for each group. The total number of serious adverse events reported for each group was also summarized.
Urine drug screening. Urine samples were collected daily. Urine drug screening results were coded qualitatively as positive or negative for metabolites of illicit opioids (cutoff = 300 ng/ml).
Data analysis. The primary outcome measure for determining the relative success of the treatment conditions was the proportion of patients who successfully completed the detoxification schedule (present on the last day to receive medication) and provided an opioid-free urine sample on that last day (days 13/14). Although the detoxification period was 13 days, participants who presented to clinic on day 14 (e.g., if 13 occurred on a weekend or a holiday) and provided a urine sample free of opioid metabolites were also considered successes. This composite outcome index reflects the probability of being both retained in treatment and abstaining from illicit opioid use, represented by the number of participants providing an opioid-free urine sample on days 13/14 divided by the number of participants randomized to the respective condition. Participants not meeting the composite ‘success’ criterion were considered ‘failures’. This aggregate composite was selected over multivariate approaches because it reflects what clinicians ultimately consider important in treating heroin abusers, i.e., the success criterion reflects both retention and opioid abstinence.
During the study design period, targeted sample sizes were computed for both treatment groups. Early termination of the study protocols, however, served to decrease the intended sample size. In the course of the study, the NIDA Data and Safety Monitoring Board (DSMB) recommended that the study be halted prior to collecting the full complement of subjects for the following reasons: the large enrollment status, the consistency of findings overwhelmingly favorable toward the bup-nx condition and the consideration that additional participant enrollment would not yield meaningful new information.
Initial sample size and power calculations were made based on the primary outcome measure, treatment response. For a χ2 test, assuming a 2:1 bup:clonidine randomization ratio, a total sample of 360 was estimated to be sufficient to detect a 15% difference in the percentage of responders in the two groups with a two-tailed alpha of 0.05 and power of 80%. Thus, the proposed number of patients in the bup-nx arm was 240 and 120 in the clonidine arm. Actual recruitment due to early study termination resulted in the randomization of a total of 113 (77 bup-nx, 36 clonidine) in the in-patient study. This sample size was sufficient to detect a difference of approximately 30% in the primary outcome measure, using χ2 with two-tailed alpha = 0.05 and power = 0.80 [e.g., a 20% response for clonidine versus 49% for bup-nx (Cohen 1988; Elashoff 2002)]. For comparing means, the actual sample size was sufficient to detect a medium effect (e.g., d = 0. 57 standard deviation units difference) between groups. In the out-patient study, early study termination resulted in the randomization of 231 (157 bup-nx, 74 clonidine). The actual sample size was sufficient to detect a difference of approximately 19% in the primary outcome measure, using χ2 with a two-tailed alpha = 0.05, and power = 0.80, assuming response rates of about 20% for the clonidine group versus 39% for bup-nx (Cohen 1988; Elashoff 2002). Note that because observed response rates were more extreme than those assumed originally (5% clonidine rather than the assumed 10%), this yielded somewhat greater power, allowing detection of a somewhat smaller difference of 13%. For comparing means, the actual sample size was sufficient to detect a medium effect (e.g., d = 0.40 standard deviation units difference) between groups. Observed effect sizes are provided.
Analyses were conducted separately in parallel fashion for in-patient and out-patient protocols using both intent-to-treat and treatment completer samples. Differences in demographic and drug use history variables between conditions at baseline were evaluated separately using t-tests and χ2. The difference in the proportion of participants who achieved the treatment success criterion between the medication conditions was analyzed using χ2. Substantially high rates of dropout for the clonidine conditions in both in-patient and out-patient studies complicated comparison of subjective ratings across time. Hence an analysis using the intent-to-treat sample required calculating the grand mean for each condition for the COWS, ARSW and VAS scales. These grand means were compared between conditions using a t-test for each of the conditions. Subjective ratings using the sample of participants who completed the study (completer sample) for the COWS, ARSW and VAS craving scales were compared between conditions using ANCOVAs (baseline covaried) in parallel fashion for the in-patient and out-patient samples. Treatment retention between conditions was tested using Wilcoxon’s (Gehan) survival analysis. Univariate indices of retention included comparisons of the average number of days retained in the trial by condition (t-test) and the percentage of participants who completed the trial by condition (χ2). Similarly, reports of adverse events were calculated based on the mean number of events (weighted equally) per participant at each evaluation over the study and the grand means for each condition were compared using a t-test. Reports of serious adverse events were too infrequent for statistical comparison, but the totals for the two conditions are presented for descriptive purposes. All statistical tests utilized a two-tailed alpha level of P < 0.05.
RESULTS
Treatment ‘success’
Significantly more in-patients assigned to bup-nx (77%) were present on days 13/14 and provided urine samples free of metabolites of illicit opioids compared to those in-patients assigned to clonidine (8/36 or 22%; χ12 = 30.1, P < 0.0001; odds ratio = 11.9; Table 1). Significantly more out-patients assigned to bup-nx (46/157 or 29%) were present on days 13/14 and provided urine samples free of metabolites of illicit opioids compared to out-patients assigned to the clonidine (5%; χ12 = 16.9, odds ratio = 7.7, P < 0.0001).
Table 1.
Percentages (n) of participants who provided urine samples free of metabolites of illicit opioids by study, treatment group and treatment day.
|
In-patient protocol |
Out-patient protocol |
|||
|---|---|---|---|---|
| Bup-Nx n = 77 | Clonidine n = 36 | Bup-Nx n = 157 | Clonidine n = 74 | |
| Day 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Day 3 or 4 | 67.5 (52) | 44.4 (16) | 23.6 (37) | 6.8 (5) |
| Day 7 or 8 | 81.8 (63) | 36.1 (13) | 35.7 (56) | 8.1 (6) |
| Day 10 or 11 | 72.7 (56) | 27.8 (10) | 33.1 (52) | 6.8 (5) |
| Day 13 or 14 | 76.6 (59) | 22.2 (8) | 29.3 (46) | 5.4 (4) |
Subjective ratings of withdrawal
Comparison of the summary COWS measure (computed for each subject as mean of COWS scores across available observations) for the in-patient groups indicates that the bup-nx group had significantly fewer withdrawal symptoms documented by the COWS (M = 3.8, SD = 2.2) compared to the clonidine group (M = 7.4, SD = 3.6; F1110 = 48.8, P < 0. 001; η2 = 0.31). Comparing groups of treatment completers, both bup-nx (M = 3.5, SD = 1.8) and clonidine groups (M = 3.7, SD = 1.9) produced similar COWS summary scores (NS; η2 = 0.001). The summary ARSW scores for patients was 23.6 (SD = 14.2) for bup-nx and 48.9 (SD = 29.8) for the clonidine group, a statistically significant difference (F1106 = 53.6, P < 0. 001; η2 = 0.34). Comparison between groups of treatment completers showed significant differences between bup-nx and clonidine groups on the ARSW summary scores (M = 22.5, SD = 11.5 versus M = 30.5, SD = 11.2, respectively; F1,69 = 4.1, P = 0. 05; η2 = 0.06).
A similar pattern of results was observed in out-patients for the subjective reports of withdrawal and craving. Comparisons of the summary COWS measure shows that the bup-nx group had significantly fewer withdrawal symptoms (M = 4.0, SD = 3.0) compared to the clonidine group (M = 5.1, SD = 3.2, F1,227 = 9.3, P < 0.01, η2 = 0.04). For the completer sample, the bup-nx group reported significantly fewer withdrawal symptoms(M = 3.1, SD= 2.2) than the clonidine conditions (M = 4.2, SD = 2.8; F1,107 = 4.7, P < 0.05, η2 = 0.04). The summary ARSW score was significantly lower for the bup-nx condition (M = 29.7, SD = 24.8) compared to the clonidine condition (M = 41.6, SD = 33.7, F1,218 = 20.1, P < 0.001, η2 = 0.08). Analysis of the completer sample also showed significantly lower ARSW withdrawal scores for the bup-nx condition (M = 23.2, SD = 17.9) than the clonidine condition (M = 36.2, SD = 24.2, F1.104 = 8.7, P < 0.01, η2 = 0.08).
Craving ratings
Analysis of craving scores in the in-patient study using the VAS showed statistically significant differences for the groups in the in-patient study with the bup-nx group producing lower mean craving ratings (M = 29.1, SD = 19.1) than the clonidine group (M = 51.5, SD = 28.4, F1109 = 25.1, P < 0.001; η2 = 0.19). No statistically significant differences were found between mean craving ratings for the bup-nx and clonidine completer groups (M = 24.5, SD = 15.3 versus 23.4, SD = 13.0, respectively; η2 = 0.001).
Analysis of the VAS craving scores for the out-patient group showed that ratings for the bup-nx condition were significantly lower (M = 37.7, SD = 20.8) compared to the clonidine condition (M = 57.1, SD = 23.3, F1,224 = 46.7, P < 0.0001, η2 = 0.17). Analysis of the completer sample showed similarly lower mean craving ratings for bup-nx (M = 30.3, SD = 17.1) compared to clonidine (M = 49.6, SD = 19.9, F1,106) =15.4, P < 0.0001, η2 = 0.13).
Treatment retention
Figure 1 shows the proportion of patients retained at each of the evaluation points for both studies. Both bup-nx groups had significantly better retention than the clonidine groups over the entire study period (in-patient: Wilcoxon (1) = 29.7, P < 0.0001; out-patient: Wilcoxon (1) = 32.8, P < 0.0001). Participants in the in-patient protocol assigned to the bup-nx condition averaged 12.6 days (SD = 3.2) compared to 6.7 days (SD = 4.8) for participants in the clonidine condition (t111 = 7.7, P < 0.0001, η2 = 0.35). Similarly, participants in the out-patient protocol assigned to the bup-nx condition averaged 11.3 days (SD = 4.2) compared to 7.1 days (SD = 5.3) for the clonidine condition (t229 = 6.4, P < 0.0001, η2 = 0.15).
Figure 1.
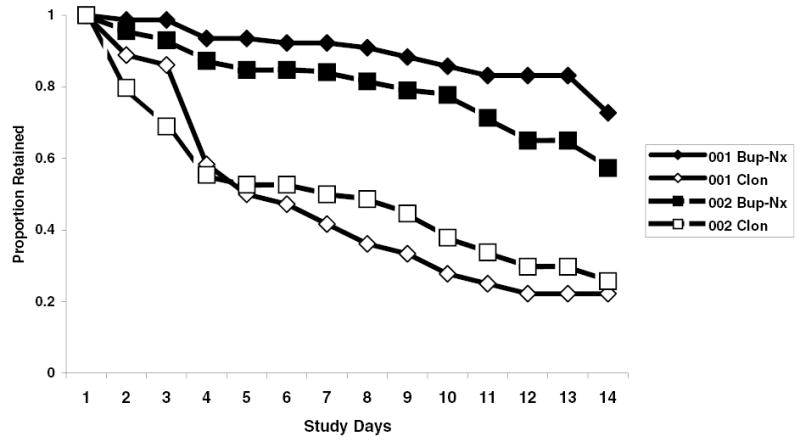
Proportion of patients retained over time for the in-patient (diamond symbols) and out-patient (square symbols) protocols as a function of assignment to bup-nx (closed symbols) or clonidine (open symbols)
When possible, patients were surveyed to ascertain why they were dropped from the study or voluntarily terminated treatment. In the in-patient study, six patients were administratively withdrawn, 10 transferred to another treatment program, one was hospitalized, one moved from the area, one was in a controlled environment, four could no longer attend clinic, 17 no longer attended clinic for reasons not specified, one developed a sensitivity to the study medication and one patient died. In the out-patient study, 35 patients were administratively withdrawn, two were hospitalized for a medical condition, 38 transferred to other treatment programs, three moved from the treatment area, one was in a controlled environment, eight could no longer attend clinic and 104 no longer attended clinic for reasons not specified. The reasons provided were not mutually exclusive.
Use of ancillary medications
Comparison of the number of symptoms for which medications were prescribed in the in-patient study showed similar levels of medication usage for the bup-nx (M 2.7 doses, SD = 1.2) and clonidine groups (M = 2.9 doses, SD = 1.3). Of those who completed treatment, those assigned to the bup-nx group averaged similar doses (M = 2.8 doses, SD = 1.2) of ancillary medications, as did those in the clonidine group (M = 2.6 doses, SD = 1.2, NS). For the out-patient protocol, no significant differences were found between the number of symptoms for which medications were prescribed for the bup-nx (M = 2.0, SD = 1.9) and clonidine conditions (M = 2.3, SD = 2.0). Significant differences were observed between conditions in the analysis of the completer sample, with 1.7 (SD = 1.8) reported for the bup-nx condition and 3.2 (SD = 2.0) for the clonidine condition (t109 = −3.1, P < 0.01).
Adverse events
For the in-patient protocol, the mean number of reported adverse events per participant per day was significantly different across conditions, with an average of 1.5 (SD = 0.8) adverse events for the bup-nx condition and 2.4 (SD = 1.6) for the clonidine condition (t111 = −3.9, P < 0.0001). Analyses of the completer sample for the in-patients showed no significant differences between the bup-nx and clonidine conditions (M = 1.6, SD = 0.8; M = 2.0, SD = 1.3, respectively). In the out-patient study, the mean number of adverse events per participant per day was significantly different across conditions, with an average of 0.7 (SD = 0.8) for the bup-nx condition and 1.2 for the clonidine group (SD = 1.6, t224 = −3.0, P < 0.001). In the analysis of the completer sample, the bup-nx condition averaged 0.6 events (SD = 0.6) compared to 1.1 events for the clonidine group (SD = 0.8, t109 = −3.7, P < 0.0001).
Serious adverse events
Few serious adverse events occurred in either protocol. In the in-patient study, four serious adverse events occurred in each condition, including a death in each condition. Respiratory failure was the cause of death for a participant in the bup-nx condition, whereas bacterial endocarditis was the cause of death for a participant in the clonidine condition. Neither death was attributed to study medication. The three other adverse events reported in the bup-nx group include suicidal behavior for two participants and severe vomiting in one. The three additional adverse events reported in the clonidine group include severe vomiting in one participant, a motor vehicle accident in another and cellulitis in yet another. In the out-patient sites, 18 serious adverse events occurred, with 14 occurring in the bup-nx group and four in the clonidine group. No deaths occurred in either of the out-patient groups. The adverse events reported in the bup-nx group include 10 cases of continued substance abuse/overdose, two cases of depression, one case of severe vomiting and case of spine surgery. The adverse events reported in the clonidine group include one participant each reporting continued substance abuse, nausea/vomiting, kidney stones and pneumonia.
DISCUSSION
This national study provides strong evidence that a representative community-based sample of opioid-dependent participants receiving a short-term episode of bup-nx are significantly more likely to complete their detoxification episodes and to be free of illicit opioids at that time and to report fewer subjective withdrawal and craving effects during a dose taper as compared to participants receiving clonidine. This is particularly true for in-patients treated with bup-nx. Although significantly more out-patients treated with bup-nx achieved the treatment success criterion than those treated as out-patients with clonidine, these rates were considerably lower than for the in-patients. There was no reliable evidence that either condition showed differential effects on use of ancillary medication. Findings showed overwhelmingly that the medications administered were safe, with few adverse events observed at any level of severity.
Paradoxically, the strengths of this study are also its weaknesses. That is, the purpose of the Clinical Trials Network is to investigate the usefulness of new treatments for drug dependence. In the current study, because the efficacy of bup-nx for the treatment of opioid abuse has been established, the effectiveness of using bup-nx in a real-world setting was the study’s primary aim. Consistent with the aims of this study, stringent empirical control was unnecessary. Instead a design was adopted that approximated the experiences in addiction medicine settings. Although the less stringent, open-label design and results presumably limit some of the internal validity of the study, the overwhelmingly positive findings for the bup-nx condition compared to the clonidine condition provide strong indications that this effectiveness trial achieved its goal. The study design yields results that clearly generalize to treatments in real-world clinical settings. In fact, clinicians were instrumental in designing this study. It was they who emphasized that the primary clinically relevant outcome measure reflect the ability of a medication to enable participants to complete treatment while abstaining from illicit opioid use.
The results showed bup-nx to be convincingly superior. Of the 77 participants in the in-patient protocol assigned to bup-nx, 59 can be defined according to the strict study criteria as treatment successes compared to only eight of the 36 participants assigned to clonidine. The number needed to treat (NNT) to attain one treatment success for bup-nx was 77/59 or 1.31 compared to 36/8 or 4.50 for clonidine. In other words, bup-nx was nearly 3.5 times more effective clinically than clonidine. This ratio was larger in the out-patient protocol, in which the NNT for bup-nx was 157/46 or 3.4 versus 74/4 or 18.5 for clonidine, showing bup-nx to be nearly 5.5 times more effective than clonidine in this setting.
The superiority of bup-nx would have been called into question if participants in the bup-nx group experienced more withdrawal symptoms or craving, or suffered more adverse or serious adverse events. Examination of these outcomes suggests that this was not the case. Participants assigned to bup-nx experienced fewer and less intense symptoms of withdrawal and craving than those taking clonidine. While there is no indication that participants assigned to receive bup-nx were less likely to take ancillary medications than those taking clonidine, bup-nx was quite simply superior to clonidine under the conditions of this study and confirms findings from numerous other published reports (Gowing et al. 2003).
Finally, although this study found that bup-nx holds great appeal both for clinicians and for patients, the data raise questions as to the adequacy of short-term detoxification as a treatment strategy. Only the bup-nx condition in in-patient settings resulted in a majority of participants achieving the treatment success criteria. Opioid addiction is a chronic relapsing disorder, with relapse to drug use a frequent occurrence even in short-term detoxification episodes. Relapses to injection use of illicit opioids during or following repeated detoxification episodes carry substantial potential for injury associated with uncontrolled drug use and include overdose, infectious disease and death. While bup-nx demonstrates a significant and relevant improvement over standard pharmacotherapy for opioid dependent patients attempting detoxification, the vast majority of patients do not achieve this goal. Treatment options, including opioid agonist therapy may assist those who fail short-term detoxification attempts in reaching treatment goals.
Acknowledgments
This publication was supported by a series of grants from NIDA as part of the Cooperative Agreement on the Clinical Trials Network (University of California, Los Angeles: U10 DA13045; Oregon Health Sciences Center: U10 DA13036; New York University School of Medicine: U10 DA13046; University of Pennsylvania: U10 DA13043; Wayne State University: U10 DA13710; University of Cincinnati: U10 DA13732; University of Miami Center for Family Studies: U10 DA13720; Research Foundation for Mental Hygiene, Inc., New York State Psychiatric Institute Division: U10 DA13035). We thank the NIDA CTN staff, especially Betty Tai, Jack Blaine, Ming Shih and Carmen Rosa. We thank Nora Chiang and Moo Park, of NIDA’s Division of Treatment Research and Development for their patience and help bringing this project to life. The following individuals are thanked for their invaluable assistance with these studies: Joe Hass and Ben Weltin from McKesson HBOC; Clare Keany, Mindy Blum, Frank Flammino, Luna Yojay, Dave Bennett and Richard A. Rawson, from the UCLA Integrated Substance Abuse Programs; Nancy Waite-O’Brien, Michelle Buckman and Steven Ey, from the Betty Ford Center; Steve Ruh and Suzette Gelacio from Aegis Medical Systems, Inc.; Lynn Kunkel, Sara Lamb and Anna Sosnowski from the Oregon Health and Science University; Bradley M. Anderson and Lucile Gauger, from the Kaiser Permanente North-west Department of Addiction Medicine; Joseph Kann, Reesa Laws, Frances Lynch and Suzanne E. Gillespie from the Kaiser Center for Health Research; Robert Maslansky, from Bellevue Hospital; Svetlana Brodsky and Rhonda Wade, from the NYU School of Medicine; Melissa Chu, Larry Brown, Sidiki Dabo, Anthony McLeod, Michael Blizzard and A.T.M. Yousuf, from the Addiction Research and Treatment Corporation; Trish Dooley, Ivy Pearlstein, Jeff Berman, Glenda Torres, Sylvia Atdjian, Marc Steinberg, Anna O’Kinsky, Ava Stanley, Mary Joan Barr, Michael Centrella, Alina Vrinceanu and Donna Drummond, from the Mercer Trenton Addiction Science Center, Robert Wood Johnson Medical School; Eric Pihlgren, James Pierre, John Hopper, Adrianne Haggins, Tanya Paul and Luanne Beamer, from Wayne State University; Alan Bray, Virginia Ryan, Anne Benion and Vickie Seeley, from SHAR House; Eugene Somoza, Judy Harrer, Rebecca Defevers, Jeff Goldsmith, Peggy Somoza and Julie Jansen from the University of Cincinnati; Stephanie Kapp, Mary Ann Crawford, Steve Fekete and Leela Rau, from Mid-town Community Mental Health Center; Ruth Ann Holzhauser, Cynthia Kohl and Cookie Hart from Maryhaven; Jose Szapocznik, Daniel Santisteban and Roberto Dominguez, from the Center for Family Studies, University of Miami; Fred Sanchez and Michael Sheehan, from Operation PAR; Deborah Orr, Judy Ruiz and Hector Barreto, from the Center for Drug-Free Living; Eric Collins, from Columbia University College of Physicians and Surgeons, Department of Psychiatry; Sahadeo Ramnauth and Timothy Wallace, from Phoenix House; and Lois Levy, from the New York State Psychiatric Institute. We thank the Pharmaceutical Division of the firm Reckitt Benckiser for providing buprenorphine–naloxone to support these studies. We extend our thanks to all of the individuals who participated and made this work possible.
References
- Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. Journal of Addictive Diseases. 1994;13:33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug and Alcohol Dependence. 2000;58:143–152. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, et al. Bringing buprenorphine–-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field Experience. American Journal on Addictions. 2004;13:S42–S66. doi: 10.1080/10550490490440807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Washington, DC: Author.
- Becker AB, Strain EC, Bigelow GE, Stitzer ML, Johnson RE. Gradual dose taper following chronic buprenorphine. American Journal on Addictions. 2001;10:111–121. doi: 10.1080/105504901750227778. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L. Buprenorphine treatment of opiate dependence: a review. Experimental and Clinical Psychopharmology. 1995;3:477–489. [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opiate detoxification with buprenorphine. Journal of Consulting and Clinical Psychology. 1997;65:803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clinical Pharmacology and Therapeutics. 1988a;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. Journal of Pharmacology and Experimental Therapeutics. 1988b;247:47–53. [PubMed] [Google Scholar]
- Boatwright DE. Buprenorphine and addiction: challenges for the pharmacist. Journal of American Pharmacological Association. 2002;42:432–438. doi: 10.1331/108658002763316860. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Fudala PJ, Johnson RE. A controlled comparison of buprenorphine and clonidine for acute detoxification from opioids. Drug and Alcohol Dependence. 1994;36:115–121. doi: 10.1016/0376-8716(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Conditioned responses in a methadone population: a comparison of laboratory, clinic, and nature settings. Journal of Substance Abuse Treatment. 1986;3:173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988) Statistical power analysis for the behavioral sciences Hillsdale, NJ: Lawrence Erlbaum.
- Elashoff, J. (2002) nQuery Advisor (Version 5.0) [Computer software]. Los Angeles, CA.
- Fingerhood MI, Thompson MR, Jasinski DR. A comparison of clonidine and buprenorphine in the outpatient treatment of opiate withdrawal. Substance Abuse. 2001;22:193–199. doi: 10.1080/08897070109511459. [DOI] [PubMed] [Google Scholar]
- Gowing, L., Farrell, M., Ali, R. & White, J. (2003) Alpha 2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database Systemic Review, CD 002024 [DOI] [PubMed]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotic addicts. Archives of General Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain E, Amass L. Buprenorphine: how to use it right. Drug and Alcohol Dependence. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerlau OF. Reactivity to alcohol-related cues: Physiological and subjective responses in alcoholics and non-problem drinkers. Journal of Studies on Alcohol. 1985;46:267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Lamb, S., Greenlick, M. R. & McCarty, D. (1998) Bridging the Gap Between Practice and Research: Forging Partnerships with Community-Based Drug and Alcohol Treatment Washington, DC: Institute of Medicine. [PubMed]
- Ling, W. & Obert, J. L. (2000) Handbook for Recovery from Opiate Dependence Adapted from the Matrix Model, Version 1, (11/16/00). Available from the senior author upon request.
- Lintzeris N, Bell J, Bammer G, Jolley DJ, Rushworth L. A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction. 2002;97:1395–1404. doi: 10.1046/j.1360-0443.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nigam AK, Ray R, Tripathy BM. Buprenorphine in opiate withdrawal: A comparison with clonidine. Journal of Substance Abuse Treatment. 1993;10:391–394. doi: 10.1016/0740-5472(93)90024-v. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Shoptaw S, Miotta K, Frosch DL, Obert JL, et al. Naltrexone for opiate addiction: the evaluation of a manualized psychosocial protocol to enhance treatment response. Drug and Alcohol Review. 2001;20:69–80. [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Wesson, D., Ling, W. & Jara, G. (1999) Buprenorphine in pharmacotherapy of opioid addiction: implementation in office-based medical practice. Translating the experience of clinical trials into clinical practice. Newsletter of the California Society of Addiction Medicine, 25


