Abstract
Objective
To investigate the association between care co-ordination and use of the Emergency Department (ED) in older managed care enrollees.
Design
Nested case-control with 103 cases (used the ED) and 194 controls (did not use the ED).
Patients and methods
Older patients with multiple chronic illnesses enrolled in a care management programme of a large group-model health maintenance organisation with more than 50,000 members over the age of 64. Better care co-ordination was defined as timely follow-up after a change in treatment; fewer decision-makers involved with the care plan; and a higher patient-perceived rating of overall care co-ordination. Logistic regression was used to assess the relationship between ED use (the outcome variable) and measures of care co-ordination (the predictor variables).
Results
Self-reported care co-ordination was not significantly different between cases and controls for any of the four classifications of inappropriate ED use. Similarly, no differences were found in the number of different physicians or medication prescribers involved in the patients' care. Four-week follow-up after potentially high-risk events for subsequent ED use, including changes in chronic disease medications, missed encounters, and same day encounters, did not differ between subjects with inappropriate ED use and controls.
Conclusion
Existing measures of care co-ordination were not associated with inappropriate ED use in this study of older adults with complex care needs. The absence of an association may, in part, be attributable to the paucity of validated measures to assess care co-ordination, as well as the methodological complexity inherent in studying this topic. Future research should focus on the development of new measures and on approaches that better isolate the role of care co-ordination from other potential variables that influence utilisation.
Keywords: care co-ordination; emergency department; health services, managed care
Introduction
Older adults with chronic illness often have complex care needs. For many of these individuals, care co-ordination is needed to ensure that different components of the health delivery system (e.g. different professionals and different institutions) do not function independently of one another, or worse, at cross purposes. The growth of home health care, sub-acute care, and disease management programmes has further added to the challenge of providing care that is co-ordinated and not fragmented [1–3]. Poor care co-ordination may manifest as the treatment of one provider interfering with the treatment of another, medication errors, and overuse of costly services such as hospital and emergency care [4–6].
The essential elements of care co-ordination required to reduce fragmentation are not well understood. The fact that the terms care co-ordination and care continuity are often used interchangeably further contributes to this lack of understanding [7]. Although closely related, the two terms refer to different health care constructs [8]. Care co-ordination refers to the primary practitioner's ability to integrate care from multiple practitioners as well as provide follow-up through subsequent visits. In contrast, care continuity refers to continuous care by a practitioner over time. Traditionally, these constructs have been assessed from the perspective of the patient. For the purpose of this article, better care co-ordination was defined as timely follow-up after a change in treatment, fewer decision-makers involved with the care plan, and a higher patient-perceived rating of overall care co-ordination.
A review of the care co-ordination literature reveals a paucity of rigorously conducted interventions that have attempted to link care co-ordination with important health events [9, 10]. Studies conducted to date have shown that well-co-ordinated care can lead to improvements in interprovider communication, information transfer, and the provision of clinical preventive services [11–13].
Patients who receive care that is poorly co-ordinated can be identified in multiple health care settings. However, such deficiencies in care co-ordination may be most pronounced in the setting of the emergency department (ED) [5, 14, 15]. Cost and utilisation studies have demonstrated that older patients with complex care needs account for disproportionate use of emergency services [5, 15]. Emergency visits in this population have been associated with subsequent functional decline, utilisation, and mortality [16–19]. Thus, the ED settting may be particularly valuable from the standpoint of examining whether care co-ordination is associated with increased utilisation in this population.
In a different sample than the one reported herein, we previously demonstrated that older patients who received monthly primary care group visits made fewer ED visits [20]. That study, however, did not afford the opportunity to identify which elements of care were responsible for the reduced ED utilisation.
In the present study, we examined whether older patients who did not visit the ED were more likely to have received better care co-ordination compared with those who visited the ED. We further assessed whether the emergency department visit was “appropriate” or “inappropriate”. We hypothesised that older adults who did not visit the ED received better care co-ordination than those who did visit the ED.
Methods
Setting
Kaiser Permanente, Colorado Region, is a large group-model health maintenance organisation that serves approximately 355,000 members in the Denver metropolitan area. Over 50,000 members are over the age of 64. Although Kaiser Colorado owns and manages its outpatient facilities, it contracts for hospital, skilled nursing and home health care. It has a fully integrated computerised medical record and maintains comprehensive administrative data on the pharmacy and medical service utilisation of its members.
Subjects
Study subjects were selected from an existing cohort of patients over the age of 64 enrolled in an outpatient care management programme. Criteria for entry into the care management programme included multiple chronic illnesses, a history of high utilisation, or primary care physician referral. Patients with dementia or those who disenrolled before study group assignment were excluded from this study.
The population of persons enrolled in an existing outpatient care management programme was selected for this study for three primary reasons. First, by virtue of being enrolled in this programme, these patients had already been identified as being at risk for high utilisation and problems of care fragmentation. In a general population of older adults, most would be of low risk for utilisation and problems of care fragmentation and therefore have much less need for effective care co-ordination. Second, before the initiation of this study, wide variation in the frequency and intensity of involvement between patients and care managers had been observed. This wide variation suggested that such potentially important differences could be captured using an appropriate research design. Third, all enrollees of the care management programme were required to periodically complete a health status survey which provided the critical variables needed to facilitate comparison and risk adjustment.
Subjects provided informed consent prior to participation. This study was approved by the University of Colorado Combined Institutional Review Board and Kaiser Permanente's Research Committee.
Study design
A nested case-control design was employed which consisted of embedding a case-control approach within an existing cohort study [21]. This design allowed for the simultaneous assessment of the predictor or exposure variable (care co-ordination) and the outcome variable (ED use). Cases (n=104) and controls (n=193) were selected from the 790 patients enrolled in a prospective quality improvement study that examined an existing cohort of subjects enrolled in a case management programme. The primary study outcome, use of the ED, was assessed using an automated surveillance system created to monitor ED use among the subjects enrolled in the care management programme. Subjects who visited the ED were designated as cases. For each case, two controls were selected from the remaining subjects who had not visited the ED. Controls were matched on duration of enrollment in the case management programme. Subjects designated as controls were not eligible to become cases (i.e. no cross-over).
Data collection and measurement
Table 1 illustrates the measures employed in this study. Our intent was to assess care co-ordination broadly and from a number of different perspectives. Sources of these data included a self-reported health status survey, a telephone survey conducted by a trained research assistant, and abstraction of health plan utilisation and pharmacy administrative data.
Table 1.
Study measures, construct, and data source
| Measure | Construct | Data Source(s) | Reference |
|---|---|---|---|
| Emergency department use | Outcome | Surveillance of admissions | — |
| Health status data | Co-variates | Baseline survey | — |
| Chronic disease score | Co-morbidity Co-variates | Pharmacy data | 23 |
| Care co-ordination | Care co-ordination | Telephone survey | 25 |
| Number of different physicians involved with care | Care co-ordination | Administrative claims data | 27,28 |
| Number of different prescribers involved with care | Care co-ordination | Administrative claims and pharmacy data | 27,28 |
| Percent of changes in one or more chronic disease medications which resulted in a follow-up visit within 28 days | Care co-ordination | Administrative claims and pharmacy data | — |
| Percent of missed ambulatory encounters which resulted in a follow-up visit within 28 days | Care co-ordination | Administrative claims data | — |
| Percent of same-day ambulatory encounters which resulted in a follow-up visit within 28 days | Care co-ordination | Administrative claims data | — |
Co-variates were abstracted from a self-report health status survey administered prior to initiation of the study. This survey included questions on demographic information, prevalence of chronic health conditions, functional status (physical function and self-care), use of skilled home nursing services, and prior ED use.
In addition, pharmacy data were used to derive a co-variate for co-morbidity, the Chronic Disease Score (CDS) [22]. The CDS is constructed using weighted pharmacy data as a proxy for overall disease burden. The CDS has been associated with physician-rated patient disease severity and patient-rated health status. Scores above three have been found to predict adverse events such as hospitalisation and mortality [22, 23].
Measures of care co-ordination were derived from both self-report and administrative data. Patient-perceived care co-ordination and care continuity were assessed within 5 days of study enrollment via a telephone-based survey that used validated scales of a measure developed by Flocke and colleagues [24]. The two self-reported measures are provided in Appendix 1. In addition, the survey assessed subjects' informal support and the duration of their relationship with the health plan and primary physician. The grammatical level of the survey was approximately sixth grade and took 10–15 minutes to complete.
Administrative data were used to evaluate the effect of multiple decision-makers on use of the ED. The number of different physicians involved in the subjects' care and the number of different practitioners prescribing medication were assessed over the six months prior to study enrollment.
Three additional administrative measures of care co-ordination were employed to examine follow-up care, a critical component of care co-ordination. We hypothesised that follow-up care would be particularly important during time periods when subjects may be at increased risk for subsequent adverse events, such as after urgent ambulatory visits, missed appointments, or changes in chronic disease medications. These measures were constructed using administrative encounter and pharmacy data, and were examined during the 6 months prior to study enrollment. Ambulatory encounters were categorised as either attended or failed, and scheduled or same-day (i.e. patient was seen within 24 hours of contacting the delivery system). We then assessed the percent of failed or same day visits that resulted in a follow-up visit within 28 days. A third measure assessed the percent of chronic disease medication changes (i.e. either initiation or dosage adjustment) that resulted in a follow-up visit within 28 days. Pharmacy profiles were examined for all subjects and chronic disease medications were identified. The list of chronic disease medications is provided in Appendix 2.
We further categorised ED visits among cases into “appropriate” versus “inappropriate”. To our knowledge, there is no accepted classification scheme for this purpose. We developed a taxonomy of four scenarios under which the question of appropriate use of the ED could reasonably be raised (Table 2 ). The first scenario, ambulatory care sensitive diagnosis, refers to those patients who were discharged from the ED with one of 10 conditions proposed by the Institute of Medicine (IOM) [25]. The IOM list includes both acute and chronic diagnoses for which improved ambulatory care could avert hospital use. The list includes: asthma, chest pain, congestive heart failure, chronic obstructive pulmonary disease, diabetes, hypertension, cellulitis, dehydration, pneumonia, and urinary tract infection. The second scenario, discharged from the ED, refers to those patients for whom hospital admission was not indicated. The third scenario, walked into the ED, refers to those patients whose presenting condition was not of a severity to preclude the ability to walk into the ED. The fourth scenario, use during clinic hours, refers to those patients who presented to the ED during regular clinic hours.
Table 2.
Classification of “inappropriate” emergency department (ED) visits.
| Name | Description |
|---|---|
| Ambulatory sensitive diagnosis | Case discharged from the ED with one of 10 ambulatory care |
| Sensitive diagnoses as proposed by the Institute of Medicine | |
| Discharged from the ED | Case discharged from the ED to home without hospital admission |
| Walked into the ED | Case's mode of arrival to ED was classified by ED staff as “walk-in” (as opposed to arriving by ambulance) |
| ED use during clinic hours | Case utilised the ED during normal clinic operating hours |
Statistical analysis
Unadjusted comparisons of baseline demographic, health status, and utilisation as well as care co-ordination measures were analysed using Student's t-test, Fisher's exact test, and Chi-Squared techniques. Logistic regression was used to assess the relationship between ED use (the dependent or outcome variable) and measures of care co-ordination (the independent or predictor variables), controlling for age, gender, chronic conditions, co-morbidity, functional status, informal caregiver support, use of skilled home health nursing services, and prior ED use. Informal care support and use of skilled home health nursing services were believed to represent factors that may serve to reduce the need for ED use. Analyses were first conducted comparing controls to all cases and then comparing controls to cases that met criteria for each of the four scenarios reflecting the appropriateness of ED use. Because of the case-control study design, odds ratios are provided for primary outcomes rather than risk ratios.
Results
Co-variates
Table 3 compares cases and controls with respect to co-variates, including demographic, co-morbid, and utilisation parameters. No significant differences were found between the two groups for age, gender, living with spouse, duration of relationship with the health plan or regular physician, and whether an informal caregiver helped the patient obtain care. Despite their enrollment in a care management programme, 21–25 percent of all subjects were not aware that they had a care manager.
Table 3.
Baseline comparison of demographics, co-morbidity, and prior utilisation
| Characteristics | Case (n=103) | Control (n=194) | p Value |
|---|---|---|---|
| Average age | 78 | 79 | 0.737 |
| Female (%) | 67 | 67 | 0.999 |
| Living with spouse (%) | 48 | 41 | 0.270 |
| Years with health plan | 11.3 | 11.1 | 0.529 |
| Years with regular physician | 4.1 | 3.5 | 0.184 |
| Can identify care manager (%) | 79 | 75 | 0.554 |
| Informal caregiver (%) helps get care | 39 | 50 | 0.068 |
| Self-rated health (1–5; 5=worse health) | 3.8 | 3.5 | 0.031 |
| Heart disease (%) | 51 | 35 | 0.013 |
| Lung disease (%) | 45 | 32 | 0.042 |
| Chronic Disease Score (CDS)* | 7.9 | 6.5 | 0.011 |
| ED utilisation (%) in prior 6 months | 66 | 45 | 0.001 |
| Home health visits (%) | 21 | 12 | 0.040 |
*Scores above 3 are significantly associated with subsequent hospitalisation and mortality. The CDS ranged from 0 to 19 among cases, and 0 to 17 among controls.
Both groups appeared to have a significant burden of functional decline and co-morbid conditions. Cases rated their health status significantly lower than controls on a scale from 1 to 5 (5=worse health) (3.8 vs. 3.5, p=0.031). When compared to controls, cases had a higher prevalence of heart disease (51% vs. 35%, p=0.013) and lung disease (45% vs. 32%, p=0.042). Similarly, cases had a significantly higher level of co-morbidity as measured by the chronic disease score (CDS) (7.9 vs. 6.5, p=0.011). When examining utilisation of health care in the 6 months prior to enrollment, cases were significantly more likely to have used the ED (66% vs. 45%, p=0.001) and home skilled nursing services (21% vs. 12%, p=0.040).
Self-reported and administrative measures comparing all cases with controls
Table 4 compares patient-assessed and administrative measures of care co-ordination between the controls and all cases. Overall, controls and cases rated their care co-ordination and care continuity highly. However, these ratings were not significantly different between the two groups in either the unadjusted or the adjusted analyses.
Table 4.
Unadjusted and adjusted* care co-ordination measures comparing all ED users with controls
| Measure | Case (n=103) | Control (n=194) | Unadj. p-value | Adjusted* odds ratio | 95% CI |
|---|---|---|---|---|---|
| Patient Reported: | |||||
| Care co-ordination (avg) (1–5, 5=poor co-ordination) | 2.0 | 1.9 | 0.406 | 1.101 | (0.813, 1.491) |
| Administrative | |||||
| Number of physicians involved with care | 3.7 | 3.6 | 0.676 | 0.957 | (0.847, 1.082) |
| Number of practitioners prescribing medications | 4.1 | 3.9 | 0.467 | 0.951 | (0.833, 1.084) |
| % of changes in chronic disease medications which resulted in a follow-up visit within 28 days | 74 | 74 | 0.948 | 1.003 | (0.993, 1.014) |
| % of missed ambulatory encounters which resulted in a follow-up visit within 28 days | 63 | 59 | 0.345 | 0.998 | (0.990, 1.006) |
| % of same-day ambulatory encounters which resulted in a follow-up visit within 28 days | 46 | 40 | 0.241 | 1.001 | (0.993, 1.008) |
*Adjusted for age, gender, heart disease, lung disease, self-reported health status, chronic disease score, informal care support, home health visits, and prior ED use.
Cases and controls did not differ in number of different physicians involved with their care (3.7 vs. 3.6; OR=0.957; 95% CI 0.847, 1.082) or the number of different practitioners prescribing a medication (4.1 vs. 3.9; OR=0.951; 95% CI 0.833, 1.084) (Table 4). Similarly, no differences were found between cases and controls with respect to changes in chronic disease medication (OR=1.003; 95% CI 0.993, 1.014), missed ambulatory encounters (OR=0.998; 95% CI 0.990, 1.006), or percent of same day encounters (OR=1.001; 95% CI 0.993, 1.008) that resulted in 28-day follow-up.
Self-reported and administrative measures comparing classifications of “inappropriate” cases with controls
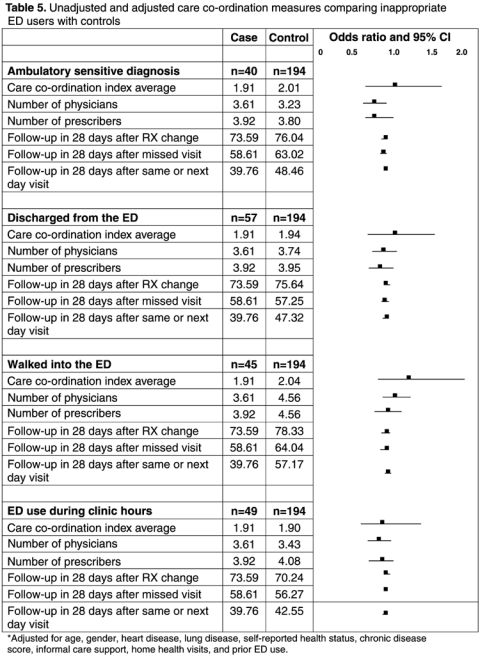
The results of analyses comparing controls with the four classifications of inappropriate ED use are illustrated in Table 5. Self-reported measures of care co-ordination and care continuity did not differ between controls and any of the four classifications of cases. In each case, the adjusted 95 percent confidence interval (CI) included 1.0. Similarly, no significant differences were found amongst measures derived from administrative data. These included number of physicians, number of providers, and follow-up after changes in medications, missed appointments, or same-day appointments. In each case, the adjusted 95 percent confidence interval included 1.0.
Table 5.
Unadjusted and adjusted care co-ordination measures comparing inappropriate ED users with controls
Correlations between self-report and administrative measures
Finally, we examined correlations between self-report and administrative data derived care co-ordination measures. Pearson correlations (r) ranged from 0.00 to 0.28, suggesting that the two types of measures were likely measuring distinct aspects of care co-ordination.
Discussion
Main findings
We hypothesised that older adults that did not visit the ED would be more likely to have received better care co-ordination than those who did, and that this relationship would be even stronger in comparison to cases who visited the ED “inappropriately”. Both cases and controls that participated in this study had a high burden of illness and multiple different providers involved with their care. Both groups provided high ratings for their care co-ordination and continuity. However, these measures were not significantly different between cases and controls. Four-week follow-up after changes in chronic disease medications, missed appointments, or same day visits, was also found to be relatively high at 74%, 59–63%, and 40–46%, respectively. Once again, however, the results were not significantly different between the two groups.
Placing these findings in context with those in the literature
The developers of the self-reported care co-ordination measure used in this study demonstrated significant associations between this measure and two important aspects of care: forced discontinuity (when a patient can no longer see his or her primary practitioner due to insurance reasons) and delivery of clinical preventive services [11, 12, 24]. Given that these measures had been shown to reflect clinically meaningful change, we chose to employ them in our study. However, all three of those studies were conducted in younger populations with a lesser burden of illness. In two of the studies, the mean age was 42 years, the mean number of problems was 2.3, and the average health status rating was 3.8 on a scale from 1 to 5 (where 5 represented not at all limited) [12, 24]. Care co-ordination scores in this population averaged over one point higher than in our study population. In the third study, the average age was 34, the mean number of health problems was 2, and the care co-ordination ratings were approximately three-quarters of a point higher than in our study sample [11]. Thus, differences between patient populations may, in part, account for why this self-reported measure did not reflect the anticipated findings in the present study.
Administrative measures of care co-ordination also did not significantly differ between study groups. We accounted for the number of different decision-makers involved in subjects' care by examining the number of different physicians and prescribers involved in the subjects' care. Our approach was modelled after previous work in this area. Raddish and colleagues examined the number of providers involved in an individual's care and found that having more providers was associated with greater resource utilisation and cost [26]. Roblin and colleagues found that patients between the ages of 18–64 years who visited three or more different physicians over a 90-day time period were nearly 50% more likely to be hospitalised than those who did not [27]. We analysed this same variable in our study and were unable to show an association with ED use or hospitalisation (not reported).
We also evaluated 28-day follow-up after a change in chronic disease medication, a missed ambulatory encounter, or a same-day ambulatory encounter. We believed that more attentive follow-up would likely reduce ED use through early detection of disease exacerbation, monitoring of treatment and side effects, and reinforcing adherence to the treatment plan. Little is known regarding what the appropriate follow-up period should be under these circumstances [28]. Schwartz and colleagues surveyed primary care physicians and found wide variation in the recommended revisit interval for persons with chronic conditions [29]. Examining these same measures for 14- and 21-day intervals led to similar findings and these are not reported herein.
What comprises an “inappropriate” emergency visit?
Currently there is no accepted taxonomy for distinguishing whether an emergency department visit was “appropriate” or “inappropriate”. We proposed an informed approach to this question that examined discharge diagnosis, hospitalisation, mode of arrival to the ED, and time of day. These four scenarios are not mutually exclusive. Cross tab analyses ranged from 9.8 percent of cases had an Ambulatory Sensitive diagnosis and were categorised as a “walk-in” by the ED staff to 33.0 percent of cases who were not admitted to the hospital and were categorised as a “walk-in”. However, less than three percent of cases were found to meet all four classifications.
This classification scheme has not been validated and has limitations. In particular, it is not clear whether ED visits can be appropriately judged retrospectively as the care seeking behaviour of the individual cannot be fully evaluated. For example, the older person who seeks care for chest discomfort unlikely is aware that his or her discharge diagnosis will be acute bronchitis or esophagitis as opposed to an acute myocardial infarction. Furthermore, there is also an important temporal component that cannot be adequately assessed retrospectively. There are many common clinical situations for which seeking care in the ED early in the course of illness presentation (e.g. dysuria) may be classified as “inappropriate” as it could have been managed effectively in an ambulatory setting. However, left unattended, the course of illness may advance to the point (e.g. urosepsis) that no clinician would argue that seeking care in the ED was “appropriate”.
Possible explanations for lack of association
There are multiple possible explanations for why we did not find an association between measures of care coordination and ED use. First, both groups rated their care co-ordination relatively highly. This concentration of responses on one end of the measurement spectrum suggests that there may have been psychometric limitations with regard to the responsiveness of the self-reported measures. Second, the findings of this study raise some questions as to whether older patients with complex care needs can reliably evaluate the level of care co-ordination that they receive. All cases and controls had a designated care manager, yet 20–25 percent of subjects were unaware of this fact, a finding that supports this argument. Third, cases and controls may have been too similar in that they were both recruited from a care management programme. Power calculations were based on the ability to detect a difference of 0.5 points on the care co-ordination or continuity measures with 80 percent certainty. Thus, there was 20 percent chance that we did not detect a meaningful difference. Fourth, care co-ordination may be more difficult to measure than anticipated. Despite the diverse approaches we used to assess this construct, our measures may not have adequately captured it. As one example, there may be a point at which the burden of illness outweighs the disadvantage of having multiple providers. Finally, care co-ordination may be only a minor factor in explaining ED use, or alternatively, care co-ordination may be most critical around particular events that were not evaluated in our study, such as during the transition period following ED or hospital discharge.
Study strengths
There are multiple strengths of our study. The nested case-control design allowed for simultaneous assessment of both the exposure (i.e. care co-ordination) and the outcome of interest (i.e. use of the ED). This approach minimised biases attributed to loss to follow-up or recall. Study subjects suffered from a significant burden of illness, co-morbidity, and functional impairment, suggesting that they likely required care from multiple providers and were consequently at high-risk for fragmented care and associated high utilisation. In other words, they represented an appropriate target population requiring well-co-ordinated care [30, 31]. We examined multiple dimensions of care co-ordination, using both self-report and administrative measures. The fact that correlations between self-report and administrative measures were quite low suggests that the measures evaluated distinct dimensions of care co-ordination.
Study limitations
There are a number of important limitations to consider when interpreting these findings. The first limitation is that of generalisability. Subjects were drawn from a large group-model health maintenance organisation. It is unknown whether our results would have differed had we studied patients in other settings. Second, it is unknown whether visiting the ED influenced cases' reporting of their perception of care co-ordination. A longitudinal design may be more suited to this question, however, attrition would likely affect the findings. Lastly, case-mix adjustment is critically important to this type of study design. Although every attempt was made to apply rigorous methodology, it is possible that unaccounted confounders influenced our findings.
Conclusion and potential next steps
In summary, the different measures of care co-ordination employed in this study were not associated with ED use in this population of older adults with complex care needs. The results of the study speak to the methodological complexity of understanding the relationship between programme co-ordination, quality of care and efficient use of services. Many health care delivery systems have implemented programmes that attempt to address care co-ordination for select patient populations. At present, our ability to assess the effectiveness of these efforts appears to be constrained by an incomplete understanding of what are the essential elements of care co-ordination and how can they best be measured.
Further investigation is needed to better define what aspects of care co-ordination are most important, when these are most needed, and with what intensity. This might include examining time periods or events when care co-ordination is most critical, such as the transition period following hospital or ED discharge, changes in chronic disease status, or the hand-off of care between practitioners, such as from a specialist to a primary care physician.
Acknowledgments
Dr. Coleman was a Hartford/Jahnigen Center of Excellence Faculty Awardee and Pfizer/American Geriatrics Society Fellow during the conduct of this study. This work was presented at the annual meeting of the American Geriatrics Society in Chicago, 2001. Funding Sources: The American Geriatrics Society/Pfizer Health Outcomes Fellowship (an unrestricted career development award to Dr. Coleman) and the John A. Hartford Foundation.
Appendix 1: Wording of self-report survey measures
Co-ordination of care (see ref. 24 Flocke et al.):
Scale ranges from 1=Strongly Agree to 5=Strongly Disagree
This doctor knows when I'm due for a check-up.
This doctor keeps track of all my health care.
This doctor always follows up on a problem I've had, either at the next visit or by phone.
This doctor always follows up on my visits to other health care providers.
This doctor helps me interpret my lab tests, X-rays or visits to other doctors.
This doctor communicates with the other health providers I see.
This doctor always knows about care I have received at other places.
Continuity of care/accumulated knowledge (see ref. 24 Flocke et al.)
Scale ranges from 1=Strongly Agree to 5=Strongly Disagree
This doctor and I have been through a lot together.
This doctor understands what is important to me regarding my health.
This doctor does not know my medical history very well.
This doctor clearly understands my health needs.
This doctor always takes my beliefs and wishes into account in caring for me.
This doctor knows a lot about me as a person (such as my hobbies, job, etc.).
Appendix 2: Chronic disease medications
Albuterol
Amlodipine
Atenolol
Beclomethasone
Clonidine
Digoxin
Diltiazem
Felodipine
Flunisolide
Fluticasone
Furosemide
Glyburide
Hydralazine
Hydrochlorothiazide
Insulin
Ipratropium Bromide
Isosorbide Dinitrate
Lisinopril
Losartan
Metolazone
Metoprolol
Nifedipine
Nitroglycerin
Potassium Chloride
Propranolol
Reserpine
Sotalol
Theophylline
Triamterene
Triamterene and Hydrochlorothiazide
Troglitazone
Verapamil
Contributor Information
Eric A. Coleman, Divisions of Geriatric Medicine and Health Care Policy and Research, University of Colorado Health Sciences Center, Denver, Colorado, USA, Clinical Research Unit, Colorado Permanente and Kaiser Health Plan, Denver, Colorado, USA.
Theresa B. Eilertsen, Divisions of Geriatric Medicine and Health Care Policy and Research, University of Colorado Health Sciences Center, Denver, Colorado, USA.
David J. Magid, Clinical Research Unit, Colorado Permanente and Kaiser Health Plan, Denver, Colorado, USA, Departments of Emergency Medicine and Preventive Medicine and Biometrics, University of Colorado Health Sciences Center, Denver, Colorado, USA.
Douglas A. Conner, Clinical Research Unit, Colorado Permanente and Kaiser Health Plan, Denver, Colorado, USA.
Arne Beck, Clinical Research Unit, Colorado Permanente and Kaiser Health Plan, Denver, Colorado, USA.
Andrew M. Kramer, Divisions of Geriatric Medicine and Health Care Policy and Research, University of Colorado Health Sciences Center, Denver, Colorado, USA.
References
- 1.Shaughnessy P, Crisler K, Schlenker R, et al. Measuring and assuring the quality of home health care. Health Care Financing Review. 1994;16(1):35–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Harvell J. Subacute care: its role and the assurance of quality. In: Newcomer RJ, Wilkinson A, editors. Annual Review of Gerontology and Geriatrics: Focus on Managed Care and Quality Assurance. New York: Springer; 1996. pp. 37–59. [Google Scholar]
- 3.Harris J. Disease management: new wine in new bottles. Annals of Internal Medicine. 1996;124(9):838–42. doi: 10.7326/0003-4819-124-9-199605010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington DC: National Academy Press; 2001. [Google Scholar]
- 5.Parboosingh E, Larsen D. Factors influencing frequency and appropriateness of utilisation of the emergency room by the elderly. Medical Care. 1987;25(12):1139–47. doi: 10.1097/00005650-198712000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Clancy C, Eisenberg J. Emergency medicine in population-based systems of care. Annals of Emergency Medicine. 1997;30(6):800–3. doi: 10.1016/s0196-0644(97)70052-0. [DOI] [PubMed] [Google Scholar]
- 7.Sparbel KJH, Anderson MA. Integrated literature review of continuity of care. Journal of Nursing Scholarship. 2000;32(1):17–24. doi: 10.1111/j.1547-5069.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Defining primary care: an interim report. Washington DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 9.Feguson J, Weinberger M. Case management programmes in primary care. Journal of General Internal Medicine. 1998;13(2):123–6. doi: 10.1046/j.1525-1497.1998.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacala J, Boult C, Hepburn K, et al. Case management of older adults in health maintenance organisations. Journal of the American Geriatrics Society. 1995;43(5):538–42. doi: 10.1111/j.1532-5415.1995.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 11.Flocke S, Stange K, Zyzanski S. The impact of insurance type and forced discontinuity on the delivery of primary care. Journal of Family Practice. 1997;45(2):129–35. [PubMed] [Google Scholar]
- 12.Flocke S, Stange K, Zyzanski S. The association of attributes of primary care with the delivery of clinical preventive services. Medical Care. 1998;36(8):AS21–30. doi: 10.1097/00005650-199808001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gow P. Care co-ordination improves quality of care at South Auckland Health. Journal of Quality of Clinical Practice. 1999;19:107–10. doi: 10.1046/j.1440-1762.1999.00312.x. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein L. The emergency department: a useful site for CGA. Journal of the American Geriatrics Society. 1996;44(5):601–2. doi: 10.1111/j.1532-5415.1996.tb01451.x. [DOI] [PubMed] [Google Scholar]
- 15.Wofford JL, Schwartz E, Byrum JE. The role of emergency services in health care for the elderly: a review. Journal of Emergency Medicine. 1993;11:317–26. doi: 10.1016/0736-4679(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 16.Currie C, Lawson P, Robertson C, Jones A. Elderly patients discharged from an accident and emergency department: their dependency and support. Annals of Emergency Medicine. 1984;1(4):205–13. doi: 10.1136/emj.1.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denman S, Ettinger W, Zarkin B, Coon P, Casani J. Short-term outcomes of elderly patients discharged from an emergency department. Journal of the American Geriatrics Society. 1989;37(10):937–43. doi: 10.1111/j.1532-5415.1989.tb07278.x. [DOI] [PubMed] [Google Scholar]
- 18.Rowland K, Maitra AK, Richardson DA, Hudson K, Woodhouse KW. The discharge of elderly patients from an accident and emergency department: functional changes and risk of readmission. Age Ageing. 1990;19:415–8. doi: 10.1093/ageing/19.6.415. [DOI] [PubMed] [Google Scholar]
- 19.Sinoff G, Clarfield A, Bergman H, Beaudet M. A two-year follow-up of geriatric consults in the emergency department. Journal of the American Geriatrics Society. 1998;46(6):716–20. doi: 10.1111/j.1532-5415.1998.tb03806.x. [DOI] [PubMed] [Google Scholar]
- 20.Coleman E, Magid D, Beck A, Eilertsen T, Conner D, Kramer A. Reducing emergency visits in older adults with chronic illness: a randomized controlled trial of group visits. Effective Clinical Practice. 2001;2:49–57. [PubMed] [Google Scholar]
- 21.Rodrigues L, Kirkwood BR. Case-control designs in the study of common diseases: updates on the demise of the rare disease assumption and choice of sampling scheme for controls. International Journal of Epidemiology. 1990;19:205–13. doi: 10.1093/ije/19.1.205. [DOI] [PubMed] [Google Scholar]
- 22.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. Journal of Clinical Epidemiology. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 23.Coleman E, Wagner E, Grothaus L, Hecht J, Savarino J, Buchner D. Predicting hospitalization and functional decline in older health plan enrollees: are administrative data as accurate as self-report? Journal of the American Geriatrics Society. 1998;46(4):419–25. doi: 10.1111/j.1532-5415.1998.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 24.Flocke S. Measuring attributes of primary care: development of a new instrument. Journal of Family Practice. 1997;45(1):64–74. [PubMed] [Google Scholar]
- 25.Institute of Medicine. Access to Health Care in America. Washington DC: National Academy Press; 1993. [Google Scholar]
- 26.Raddish M, Horn SD, Sharkey P. Continuity of care: is it cost-effective? American Journal of Managed Care. 1999;5:727–34. [PubMed] [Google Scholar]
- 27.Roblin DW, Juhn PI, Preston BJ, Della Penna R, Feitelberg SP, Khoury A, et al. A low-cost approach to prospective identification of impending high cost outcomes. Medical Care. 1999;37(11):1155–63. doi: 10.1097/00005650-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Chapko MK, Fisher ES, Welch HG. When should this patient be seen again? Effective Clinical Practice. 1999;2(1):37–43. [PubMed] [Google Scholar]
- 29.Schwartz LM, Woloshin S, Wasson JH, Renfrew RA, Welch HG. Setting the revisit interval in primary care. Journal of General Internal Medicine. 1999;14:230–35. doi: 10.1046/j.1525-1497.1999.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boult C, Boult L, Pacala J. Systems of care for older populations of the future. Journal of the American Geriatrics Society. 1998;46(4):499–505. doi: 10.1111/j.1532-5415.1998.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 31.Thornton C, Retchin S, Smith K, Fox PD, Black W, Stapulonis R. Princeton: Mathematica Policy Research Inc; 2001. Constrained innovation in managing care for high-risk seniors in Medicare + Choice risk plans. [Google Scholar]