Abstract
On the basis of task-related imaging studies in normal human subjects, it has been suggested that two attention systems exist in the human brain: a bilateral dorsal attention system involved in top-down orienting of attention and a right-lateralized ventral attention system involved in reorienting attention in response to salient sensory stimuli. An important question is whether this functional organization emerges only in response to external attentional demands or is represented more fundamentally in the internal dynamics of brain activity. To address this question, we examine correlations in spontaneous fluctuations of the functional MRI blood oxygen level-dependent signal in the absence of task, stimuli, or explicit attentional demands. We identify a bilateral dorsal attention system and a right-lateralized ventral attention system solely on the basis of spontaneous activity. Further, we observe regions in the prefrontal cortex correlated with both systems, a potential mechanism for mediating the functional interaction between systems. These findings demonstrate that the neuroanatomical substrates of human attention persist in the absence of external events, reflected in the correlation structure of spontaneous activity.
Keywords: blood oxygen level-dependent signal, functional MRI, functional connectivity, orienting, synchrony
Attention is not a unitary function: Limitations of resources and the need for selection arise at different levels of processing and in different cognitive domains including perception, action, language, and memory (1, 2). A major advance has been the recognition that separate neural mechanisms/systems mediate different aspects of attention (3). One of the better studied forms of attention is visual orienting, i.e., the ability to select stimuli for action. On the basis of behavioral, neuroimaging, lesion, and electrophysiological studies, a model has been proposed that suggests that different attentional operations during sensory orienting are carried out by two separate frontoparietal systems, a dorsal attention system and a ventral attention system (for review, see ref. 4). The dorsal system is bilateral and composed of the intraparietal sulcus (IPS) and the junction of the precentral and superior frontal sulcus (frontal eye field, FEF) in each hemisphere. It is involved in voluntary (top-down) orienting and shows activity increases after presentation of cues indicating where, when, or to what subjects should direct their attention (5–12). The ventral system is right-lateralized and composed of the right temporal-parietal junction (TPJ) and the right ventral frontal cortex (VFC). This system shows activity increases upon detection of salient targets, especially when they appear in unexpected locations (5, 7, 13, 14). Activity increases also are observed in the ventral system after abrupt changes in sensory stimuli (15), at the onset and offset of task blocks (16), and at the end of a completed trial (17). Although we refer to the core regions of these systems as if they were homogeneous elements (e.g., IPS or FEF), each contains multiple functional subcomponents (5–7, 18, 19). However, for each region, there appears to be functionality common across its subcomponents, providing the basis for the classification into dorsal and ventral attention systems (4).
These two systems appear to cooperate and interact during normal behavior. For example, the attention-capturing effect of salient stimuli is regulated closely by ongoing internal goals. Distracters that are part of a task set (e.g., a red hat when searching for a friend in the crowd wearing a red sweater) are much more attention-grabbing than distracters that are not (e.g., a green hat). Correspondingly, the ventral system reacts more strongly to task-relevant than irrelevant distracters (20) and exhibits activity decreases when subjects search for a difficult target (6). A functional interaction between the dorsal and ventral system has been proposed such that task-relevant signals from the dorsal system “filter” stimulus-driven signals in the ventral system, whereas stimulus-driven “circuit-breaking” signals from the ventral system provide an interrupt to the dorsal system, reorienting it toward salient stimuli (4, 21). Although the mechanism of this functional interaction in unknown, it has been hypothesized to occur between the IPS in the dorsal system and the TPJ in the ventral system (4).
It is important to note that the functional anatomy of this dorsal/ventral model of human attention is inferred from the results of conventional task-response studies (4). In these studies, a task or stimulus is used to manipulate attention in a particular way, multiple trials or epochs are averaged, and brain regions showing a significant response to the task or attentional variable are identified. This task-based approach to studying brain function has a strong precedent (22, 23) and is motivated, in part, by the conceptualization of the brain as a system primarily responding to task demands. However, there is an alternative perspective for understanding brain function that lends itself to a very different experimental approach. This alternative perspective suggests that the brain is active even in the absence of task, primarily driven by internal dynamics, with external events modulating rather than determining the activity of the system (24–28). The importance of this alternative view is supported by the observation that most of the brain’s energy consumption is in support of intrinsic functional activity (29). In this perspective, tasks or stimuli are not needed to observe the functional organization of the brain, rather it can be seen through patterns of ongoing spontaneous activity.
Recent experiments in both animals and humans have lent support to this alternative perspective on brain function (25–28, 30–35). For example, orientation columns in the visual cortex can be observed even in the absence of visual input (27). Similarly, low frequency (<0.1 Hz) spontaneous fluctuations of the functional MRI blood oxygen level-dependent (BOLD) signal show spatially specific coherence patterns in the resting human brain. Correlations have been noted between regions commonly modulated together by task paradigms such as somatomotor, visual, auditory, language, task-negative/default, and task-positive regions including the IPS and FEF (28, 30–34). In addition to these positive correlations, negative or anticorrelations have been noted between regions routinely modulated in opposite directions by attention demanding tasks (28, 35).
These experimental results and the idea that the brain may be driven primarily by internal dynamics raise new questions regarding the neuronal basis of human attention. Specifically, is the dorsal/ventral model of human attention limited to the description of task-response patterns, or does it reflect a more fundamental functional architecture represented in ongoing patterns of spontaneous activity? To address this issue, we examine spontaneous BOLD fluctuations in recently acquired resting state data by using four regions defined on the basis of previously published studies of attention: IPS and FEF in the dorsal attention system and TPJ and VFC in the ventral attention system. First, we determine whether these four regions can be partitioned into a dorsal and ventral system on the basis of spontaneous activity. Second, we determine whether there are differences in the laterality of the systems associated with each seed region. Finally, we address the question of how these two systems might interact by testing the hypothesized link between the IPS and TPJ and describing regions correlated with both dorsal and ventral seed regions.
Results
Resting-State Correlation Maps Associated with Attentional Seed Regions.
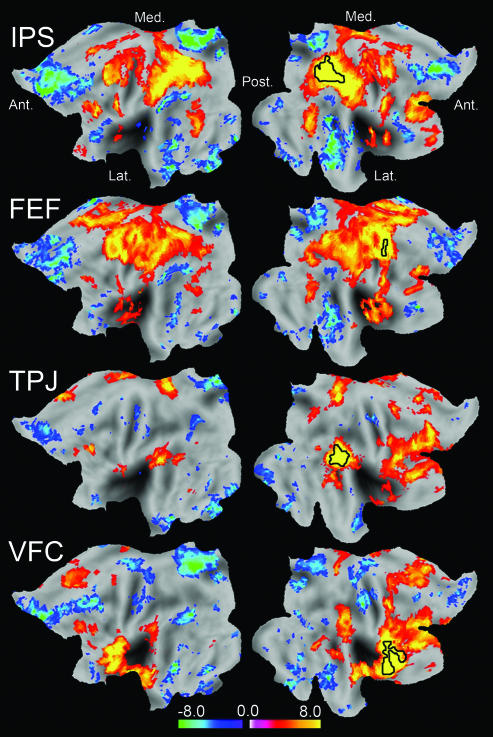
We conducted a metaanalysis of several previous task-based studies of attention (5–7, 13, 14) and defined four regions of interest: IPS and FEF in the dorsal attention system and TPJ and VFC in the ventral attention system (Figs. 6 and 7, which are published as supporting information on the PNAS web site). These regions of interest then were used to analyze resting-state functional MRI data acquired during three conditions: visual fixation on a crosshair, eyes open during low-level illumination, and eyes closed (28). Correlation maps were generated for each of the four regions and each of the three resting-state conditions by using random effects analysis across the population. Maps of voxels whose time course was significantly correlated or anticorrelated (P < 0.01) with the time course of each seed region are shown for the fixation condition in Fig. 1. Two observations are of particular importance to the present investigation. First, the IPS and FEF correlation maps appear similar and the TPJ and VFC correlation maps appear similar, but the IPS/FEF correlation maps appear quite distinct from the TPJ/VFC correlation maps. The second observation is that correlations with the IPS and FEF are largely bilateral, whereas correlations with the TPJ and VFC are much more unilateral and largely limited to the right hemisphere. These findings are independent of the specific size or shape of the seed regions (Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 1.
Z score maps showing voxels significantly correlated or anticorrelated with seed regions in the IPS, FEF, TPJ, and VFC during resting fixation (P < 0.01, random effects). Data are displayed on the flattened left hemisphere (Left) and the flattened right hemisphere (Right) of an average human brain with anterior (Ant.), posterior (Post.), medial (Med.), and lateral (Lat.) directions noted. The seed regions are outlined in black. The IPS and FEF correlation maps are similar to each other and largely bilateral, whereas the TPJ and VFC correlation maps are similar to each other and right lateralized. Prominent anticorrelations also are present, especially in the IPS and FEF maps, and have been discussed in ref. 16.
Qualitative observations from these resting-state correlation maps were supported by quantitative analyses, the results of which are shown for each resting-state condition in Figs. 9 and 10, which are published as supporting information on the PNAS web site. Because results of these quantitative analyses were similar in the three resting-state conditions, we averaged across them, the results of which are described below and shown in Fig. 2.
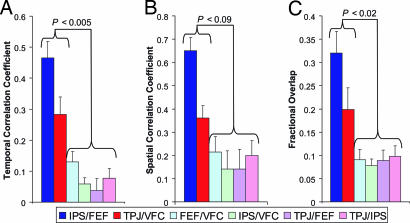
Fig. 2.
Seed regions can be partitioned into an IPS/FEF system and a TPJ/VFC system on the basis of spontaneous activity. (A) Temporal correlation coefficient between the regional time courses from each pair of regions. (B) Spatial correlation coefficient between each pair of resting state correlation maps. (C) Percent overlap of significant positively correlated voxels for each pair of resting-state correlation maps. Displayed values are averaged across subjects and resting state conditions. P values represent the least significant pairwise comparison (two-tailed paired t test) between the IPS/FEF or TPJ/VFC and all other columns.
Quantitative Partitioning of Regions on the Basis of Spontaneous Activity.
The first set of quantitative analyses addressed the issue of whether the four attentional seed regions were related equally in their spontaneous activity or whether they should be partitioned into two systems. Three analyses were used to address this issue (Fig. 2). The first analysis examined the correlation coefficient between the time courses of all possible pairs of regions (Fig. 2A). All region pairs were not correlated equally (F(5,45) = 23.70; P < 0.0001), with the IPS/FEF correlation and TPJ/VFC correlation being significantly stronger than the correlation between any other region pair (P < 0.005, paired t test). The second and third analyses determined the similarity of the voxelwise correlation maps associated with two different seed regions. The first of these similarity measures computed the spatial correlation coefficient between all pairs of resting state correlation maps (Fig. 2B). All map pairs were not equally correlated (F(5,45) = 15.54; P < 0.0001), with the IPS/FEF and the TPJ/VFC correlation maps being the most similar. The second similarity measure computed the fractional overlap between two correlation maps, i.e., the number of voxels significantly correlated with both seed regions divided by the number of voxels significantly correlated with either seed region (Fig. 2C). All map pairs were not equally overlapped (F(5,45) = 17.04; P < 0.0001), with the IPS/FEF and the TPJ/VFC pairs showing significantly more overlap than any other pair of correlation maps (P < 0.02). Taken together, these analyses demonstrate that the four attention regions are not equally related in their spontaneous BOLD fluctuations but rather should be partitioned into two distinct systems consisting of the IPS/FEF and the TPJ/VFC.
Quantification of the Lateralization of Resting State Correlation Maps.
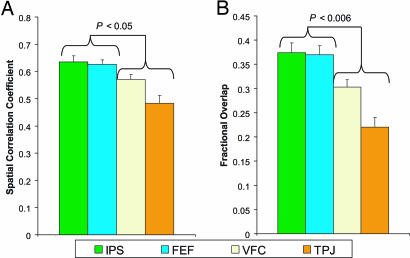
The second set of statistical analyses determined whether the correlation maps associated with some regions were more lateralized than others (Fig. 3). To make this comparison, we used the spatial correlation coefficient (Fig. 3A) and the fractional overlap measures (Fig. 3B) discussed above, but instead of comparing two maps associated with different seed regions, we compared the correlation map associated with a single seed region to that same map flipped about the midline (y axis). If the correlation map is bilaterally symmetric, then flipping the image about the midline will result in little change, and the spatial correlation and fractional overlap will be high. There was a significant difference in laterality by using both the spatial correlation coefficient [F(3,27) = 28.28; P < 0.0001] and the fractional overlap [F(3,27) = 39.79; P < 0.0001] measures. The correlation maps associated with the IPS and FEF seed regions were significantly more bilateral than those associated with the VFC or the TPJ (spatial correlation, P < 0.05; overlap, P < 0.006; Fig. 3).
Fig. 3.
Resting-state correlation maps associated with the IPS and FEF regions are significantly more bilateral than those associated with the TPJ and VFC regions. (A) Spatial correlation coefficient between each resting state correlation map and that same map flipped about the midline. (B) Fractional overlap of significant positively correlated voxels between each resting state correlation map and that same map flipped about the midline. Displayed values are averaged across subjects and resting state conditions. P values show the least significant pairwise comparison (two-tailed paired t test) between the IPS or FEF and the TPJ or VFC.
Dorsal and Ventral Attention Systems Defined on the Basis of Spontaneous activity.
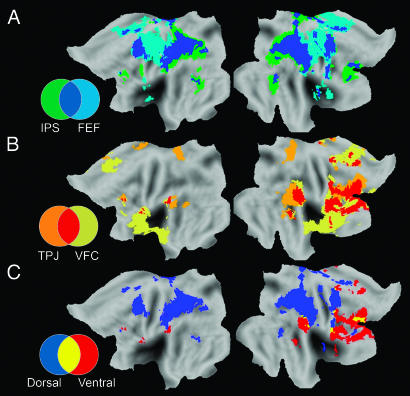
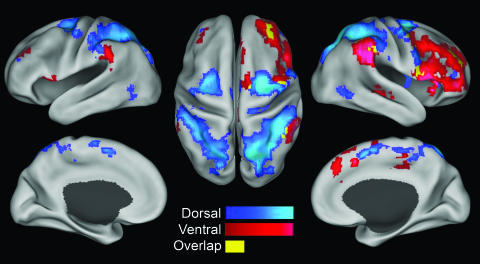
Because results from one resting-state condition alone are susceptible to potential confounds (i.e., attention modulation during fixation or eye movements), we collapsed across conditions to find those voxels significantly correlated (P < 0.01) with each seed region regardless of whether the subject was fixating, resting with eyes open, or resting with eyes closed (Fig. 4). Results for the IPS and FEF seed regions are displayed together along with the overlap in these distributions (Fig. 4A), and the results for the TPJ and VFC are displayed together along with the overlap in these distributions (Fig. 4B). The dorsal system is defined as those voxels significantly correlated with both the IPS and FEF seed regions in all three resting state conditions (Fig. 4A, blue) and the ventral attention system as those voxels significantly correlated with both the TPJ and VFC seeds in all three conditions (Fig. 4B, red). These intrinsically defined dorsal and ventral attention systems are displayed together on a flat map in Fig. 4C and on the surface of an inflated brain in Fig. 5. The values for the images shown in Fig. 5 were obtained by averaging the correlation maps from all three resting-state conditions associated with the IPS and FEF seed regions (dorsal system, blue scale) or the TPJ and VFC seed regions (ventral system, red scale) then masking with the respective conjunction map. Regions of overlap in these intrinsically defined dorsal and ventral attention systems are shown in yellow (Figs. 4C and 5). Talairach coordinates for peak foci in the dorsal and ventral system and the center of mass of regions of overlap are listed in Table 1, which is published as supporting information on the PNAS web site.
Fig. 4.
Conjunction of thresholded correlation maps across fixation, eyes-open, and eyes-closed resting-state conditions. (A) Voxels significantly correlated (P < 0.01) with the IPS (green), the FEF (cyan), and both the IPS and FEF (defined as the dorsal attention system, blue) in all three resting-state conditions. (B) Voxels significantly correlated (P < 0.01) with the TPJ (orange), the VFC (dark yellow), and both the TPJ and VFC (defined as the ventral attention system, red) in all three resting-state conditions. (C) The IPS/FEF dorsal attention system from A (blue), the TPJ/VFC ventral attention system from B (red), and the overlap between them (yellow).
Fig. 5.
Intrinsically defined dorsal and ventral attention systems and the overlap between them. Voxels in the dorsal system (blue scale) were significantly correlated (P < 0.01) with both the IPS and FEF regions in all three resting state conditions (fixation, eyes open, and eyes closed). Voxels in the ventral system (red scale) were significantly correlated with both the TPJ and VFC regions in all three resting-state conditions. Voxels significantly correlated with all four regions in all three conditions are shown in yellow. Data are displayed on the lateral and medial surfaces of the left hemisphere (Left), the dorsal surface (Center), and the lateral and medial surfaces of the right hemisphere (Right).
We have defined the dorsal and ventral attention systems on the basis of a conjunction across resting-state conditions; however, it is important to know whether these systems would be different were we to consider each condition independently. We performed this analysis and found that the intrinsically defined dorsal system, ventral system, and overlap are consistent across resting-state conditions (Fig. 11, which is published as supporting information on the PNAS web site).
Regions of Overlap Between the Dorsal and Ventral Attention Systems.
To reduce the chance of false positives, regions of overlap <10 voxels were not considered (this number corresponded to a natural break in the data). The largest region of overlap was in the right middle frontal gyrus with additional regions in the inferior frontal gyrus and inferior parietal cortex. We performed several analyses to determine whether these overlap regions could be attributed to nonneuronal factors such as averaging, spatial resolution, or locally coherent noise (see Supporting Text and Figs. 12 and 13, which are published as supporting information on the PNAS web site). These factors appear to contribute to the overlap region in the inferior parietal cortex but not the other two, suggesting that the prefrontal cortex (middle and inferior frontal gyrus) may be an important locus of functional interaction between dorsal and ventral attention systems.
Discussion
The original model of the dorsal and ventral attention systems was based on patterns of neuronal activity observed in response to external tasks or stimuli. In the current study, we determine whether this functional organization is present even in the absence of task, stimuli, or explicit attentional demands. Using this approach, we have shown that attentional regions can be partitioned into a bilateral dorsal attention system and a right-lateralized ventral attention system solely on the basis of spontaneous activity. These systems appear to be largely segregated in their spatial topography, with only small regions of overlap in the prefrontal cortex.
Interpreting Spontaneous Activity.
A prominent difference between the current analysis and previous studies on the neuroanatomical substrates of attention is that the current study was conducted at rest, in the absence of task, stimuli, or explicit attentional demands. Although the exact nature of the spontaneous BOLD fluctuations measured in the present analysis is unknown, the specific topography of these fluctuations and previous observations by using local field-potential recordings (36) suggest that they reflect underlying changes in neuronal activity. Specifically, they may relate to electrophysiological fluctuations in the power of higher frequency bands (e.g., gamma 30–80 Hz) (36, 37). A question important for interpretation of the current results is the origin of this neuronal activity. Are spontaneous BOLD fluctuations simply a reflection of spontaneous behavior/cognition during the unconstrained resting state (38)? For example, subjects might be intermittently attending to the scanner noise resulting in coherent fluctuations in attention regions.
Although unconstrained behavior cannot be ruled out as the source of some of the spontaneous BOLD activity, it is unlikely to be the predominant source for several reasons. First, similar topography of BOLD correlations has been observed across very different behavioral states including different resting conditions (28), low-level task performance (39, 40), and even light anesthesia (41). The use of three different resting state conditions in the current study controls for specific behavior such as eye movements or attentional demands during fixation. Second, coherent spontaneous fluctuations have been observed within systems associated with specific behavior in the absence of that behavior such as in the motor system in the absence of movement (30, 40, 42). Third, task-evoked activity due to a specific behavior seems to be distinct from and superimposed on top of underlying spontaneous activity (40, 42). This finding suggests that unconstrained behavior in the scanner would result in BOLD modulations that are in addition to, not the source of, spontaneous coherent BOLD fluctuations. Finally, coherent spontaneous fluctuations are present continuously across all brain regions. It is difficult to imagine a behavior that would simultaneously modulate every known brain system, each in its own coherent fashion.
We should note that although explicit behavior is unlikely to be the primary source of coherent spontaneous activity, this observation does not rule out an influence of spontaneous activity on behavior. For example, coherent spontaneous activity appears to be an important source of trial-to-trial variability in measured brain responses (42, 43) and may relate to the trial-to-trial variability commonly observed in human behavior (44). The extent to which coherent spontaneous fluctuations in attentional systems account for fluctuations in vigilance or attentional performance will be an important topic for future studies.
Dorsal and Ventral Attention Systems Defined on the Basis of Spontaneous Activity.
The main finding in the current study is that the functional organization of the dorsal and ventral attention systems is represented in the correlation structure of spontaneous activity. First, we showed that spontaneous activity is more correlated between regions within the dorsal or ventral system than between systems. Second, we demonstrated that correlations in the dorsal system are bilateral, whereas those in the ventral system are lateralized to the right hemisphere. Finally, we constructed voxelwise maps of the dorsal and ventral systems defined solely on the basis of spontaneous activity, largely reproducing the spatial topography of the dorsal and ventral attention systems hypothesized on the basis of task-activation paradigms.
Our intrinsically defined dorsal system is consistent with the task-based model in that it includes IPS and FEF and is largely bilateral. However, in contrast to the model, the intrinsically defined dorsal attention system extends beyond IPS and FEF and includes a midline supplementary motor area (SMA)/pre-SMA region and MT+. This extended system is consistent with previous resting-state correlation studies (28, 45) and has been dubbed by us the task-positive network (28). Both SMA/pre-SMA and MT+ commonly show activity increases along with IPS and FEF in response to an attentional cue and during working memory (46), visual search (6), and target detection. However, SMA/pre-SMA and MT+ typically have not been considered part of the dorsal attention system for various reasons. For example, cue-related activity in MT+ tends to be more transient than that in the IPS or FEF (4, 8, 46). Similarly, spatially specific attention effects have been observed in parts of IPS and FEF but not in other areas (5, 10, 46). The fact that IPS and FEF seem to share some functionality not present in the more extended network may relate to the stronger intrinsic correlation between these two regions (see Fig. 1).
The intrinsically defined ventral attention system also is broadly consistent with the original task-based model of attention. It is largely right-lateralized and includes TPJ and VFC as well as other right frontal areas. Although previous task-based studies have reported right lateralization of this network (4, 15, 16, 47), we directly test and quantify this laterality on the basis of spontaneous activity. It should be noted that the finding of a highly lateralized system on the basis of spontaneous activity is uncommon. The vast majority of intrinsically defined systems identified to date are predominantly bilateral, including the somatomotor, visual, auditory, task-negative/default, and task-positive/dorsal attention system (28, 30, 31, 33, 34). The only other report of an intrinsically lateralized system is the left-lateralized language system, which includes Broca’s and Wernike’s areas (32). Interestingly, Broca’s and Wernike’s areas are, to a large extent, the left hemisphere homologues of the right VFC and right TPJ. The presence of lateralization in resting activity is important because it suggests that hemispheric lateralization in function is not induced by task processing but is sculpted more fundamentally in the pattern of spontaneous activity. Whether these functional asymmetries relate to anatomical asymmetries that have been reported in inferior parietal cortex (48) is an interesting topic for future work.
An important result of the present analysis is that the intrinsically defined dorsal and ventral attention systems are largely segregated in their spatial distribution. This segregation distinguishes them from other resting state relationships that could have been observed. For example, regions routinely modulated together during task conditions tend to be strongly correlated in their spontaneous activity and regions modulated in opposite directions tend to be anticorrelated (28). The fact that the dorsal and ventral systems are largely segregated may be important for the flexibility observed in the task-response patterns of these two systems, allowing them to be modulated together, independently, or in opposite directions.
Regions of Overlap Between the Dorsal and Ventral Attention Systems.
Although the intrinsically defined dorsal and ventral attention systems are largely segregated in their spatial topography, the original model and task-related findings suggest that there must be some mechanism of information exchange between them. As noted in the introduction, the ventral system responds more strongly to task-relevant distracters than irrelevant ones (termed contingent reorienting), and the dorsal system exhibits an activity increase in addition to the ventral system when a reorientation of attention is needed. This information exchange has been hypothesized to occur between the right IPS and right TPJ (4). However, the intrinsic correlation between these two regions was no stronger than that between the FEF/TPJ, IPS/VFC, or FEF/VFC. Consistent with this finding, recent lesion studies suggest that contingent reorienting remains intact and is even enhanced after IPS damage (S. Shomstein, personal communication), suggesting that the IPS is not required for top-down information to reach the ventral attention system.
As an alternative to the IPS/TPJ link, regions correlated with both the dorsal and ventral attention systems were found in the right middle frontal gyrus, inferior frontal gyrus, and inferior parietal lobe. Although the region in the parietal lobe may be due to spatial blurring, the two regions in the prefrontal cortex are not, and therefore may represent, important sites of functional interaction between the two systems. This observation is not without precedent, because the currently identified prefrontal cortex regions have been implicated in sustained attention, vigilance, and both maintaining and updating of a task set (49–51). Furthermore, damage to the prefrontal cortex is associated with behavior consistent with a disruption in the link between the dorsal and ventral systems. Specifically, an inability of the ventral system to distinguish between relevant and irrelevant stimuli could cause increased distractibility, and an inability of the dorsal system to receive reorienting signals could cause an increase in perseveration. Both distractibility and perseveration are hallmarks of prefrontal cortex damage (50).
Why Task-Related Activity Patterns Might be Reflected in Spontaneous Activity.
The current study demonstrates that the dorsal/ventral attention model defined on the basis of externally imposed tasks with specific attentional demands is reflected in the correlation structure of ongoing spontaneous activity. An important question is why task-evoked and spontaneous activity patterns are so similar. One possibility is that spontaneous activity serves as a record or memory of previous use, showing correlations between regions that have been modulated together in a task-dependent manner (27, 52). Another possibility is that spontaneous activity serves to organize and coordinate neuronal activity (53–55), and this coordination is more prominent between regions that commonly work in concert. Finally, spontaneous activity may represent a dynamic prediction regarding expected use (56, 57), with correlations occurring between regions likely to be used together in the future. These possibilities are not mutually exclusive, and all may be relevant to understanding why systems defined on the basis of task-related activity patterns, such as the dorsal and ventral attention systems, can be observed in patterns of spontaneous neuronal activity in the absence of task.
Methods
Functional MRI data were acquired in 10 normal subjects during three resting conditions and has been used previously in a study of anticorrelated functional networks (28). The data were preprocessed (28) and seed-based correlation analyses were performed by using seed regions identified through a metaanalysis of previous task-based studies of attention (5–7, 13, 14). Analyses of temporal correlation, spatial correlation, and overlap were used to demonstrate a partitioning of these regions into two systems. Extended methodological details can be found in Supporting Text.
Supplementary Material
Acknowledgments
We thank Linda Larson-Prior and John Zempel for help with data acquisition. This work was supported by National Institutes of Health Grants NS06833, F30 NS054398-01, MH7192-06, and NS048013.
Abbreviations
- BOLD
blood oxygen level-dependent
- FEF
frontal eye field
- IPS
intraparietal sulcus
- SMA
supplementary motor area
- TPJ
temporal-parietal junction
- VFC
ventral frontal cortex
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Pashler H. E. Cambridge, MA: MIT Press; 1998. The Psychology of Attention. [Google Scholar]
- 2.Alport A. The Foundations of Cognitive Science. In: Posner M. I., editor. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 3.Posner M. I., Petersen S. E. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 4.Corbetta M., Shulman G. L. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 5.Corbetta M., Kincade J. M., Ollinger J. M., McAvoy M. P., Shulman G. L. Nat. Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 6.Shulman G. I., McAvoy M., Cowan M. C., Astafiev S. V., Tansy A. P., D’Avossa G., Corbetta M. J. Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- 7.Astafiev S. V., Shulman G. I., Stanley C. M., Snyder A. Z., Van Essen D. C., Corbetta M. J. Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shulman G. I., Ollinger J. M., Akbudak E., Conturo T. E., Snyder A. Z., Petersen K. F., Corbetta M. J. Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastner S., Pinsk M. A., De Weerd P., Desimone R., Ungerleider L. G. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 10.Hopfinger J. B., Buonocore M. H., Mangun G. R. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 11.Corbetta M., Tansy A. P., Stanley C. M., Astafiev S. V., Snyder A. Z., Shulman G. I. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Giesbrecht B., Woldorff M. G., Song A. W., Mangun G. R. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 13.Astafiev S. V., Stanley C. M., Shulman G. L., Corbetta M. Nat. Neurosci. 2004;7:542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- 14.Kincade J. M., Abrams R. A., Astafiev S. V., Shulman G. I., Corbetta M. J. Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downar J., Crawley A. P., Mikulis D. J., Davis K. D. Nat. Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 16.Fox M. D., Snyder A. Z., Barch D. M., Gusnard D. A., Raichle M. E. NeuroImage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Shulman G. I., Tansy A. P., Kincade M., Petersen S. E., McAvoy M. P., Corbetta M. Cereb. Cortex. 2002;12:590–600. doi: 10.1093/cercor/12.6.590. [DOI] [PubMed] [Google Scholar]
- 18.Andersen R. A., Buneo C. A. Ann. Rev. Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 19.Colby C. L., Goldberg M. E. Ann. Rev. Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 20.Serences J. T., Shomstein S., Leber A. B., Golay X., Egeth H. E., Yantis S. Psychol. Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 21.Shulman G. L., Corbetta M. Functional Neuroimaging of Visual Cognition: Attention and Performance XX. In: Kanwisher N., Duncan J., editors. Oxford: Oxford Univ. Press; 2003. pp. 345–362. [Google Scholar]
- 22.Hubel D. H., Wiesel T. N. J. Physiol. (London) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posner M. I., Petersen S. E., Fox P. T., Raichle M. E. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 24.Llinas R. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 25.Fiser J., Chiu C., Weliky M. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 26.MacLean J. N., Watson B. O., Aaron G. B., Yuste R. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 27.Kenet T., Bibitchkov D., Tsodyks M., Grinvald A., Arieli A. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- 28.Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle M. E., Mintun M. A. Annu. Rev. Neurosci. 2006 Apr 20;29 doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 30.Biswal B., Yetkin F., Haughton V., Hyde J. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 31.Greicius M. D., Krasnow B., Reiss A. L., Menon V. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampson M., Peterson B. S., Skudlarski P., Gatenby J. C., Gore J. C. Hum. Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordes D., Haughton V. M., Arfanakis K., Wendt G. J., Turski P. A., Moritz C. H., Quigley M. A., Meyerand M. E. Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe M. J., Mock B. J., Sorenson J. A. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 35.Fransson P. Hum. Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leopold D. A., Murayama Y., Logothetis N. K. Cereb. Cortex. 2003;13:423–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 37.Bruns A., Eckhorn R., Jokeit H., Ebner A. NeuroReport. 2000;11:1509–1514. [PubMed] [Google Scholar]
- 38.Stark C. E. L., Squire L. R. Proc. Natl. Acad. Sci. USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greicius M. D., Menon V. J. Cognit. Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 40.Arfanakis K., Cordes D., Haughton V. M., Moritz C. H., Quigley M. A., Meyerand M. E. Magn. Reson. Imaging. 2000;18:921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 41.Kiviniemi V., Kantola J. H., Jauhiainen J., Hyvarinen A., Tervonen O. NeuroImage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 42.Fox M. D., Snyder A. Z., Zacks J. M., Raichle M. E. Nat. Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 43.Arieli A., Sterkin A., Grinvald A., Aertsent A. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 44.Gilden D. L. Psychol. Rev. 2001;108:33–56. doi: 10.1037/0033-295x.108.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Laufs H., Krakow K., Sterzer P., Egger E., Beyerle A., Salek-Haddadi A., Kleinschmidt A. Proc. Natl. Acad. Sci. USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbetta M., Kincade J. M., Shulman G. I. J. Cognit. Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- 47.Macaluso E., Frith C. D., Driver J. J. Cognit. Neurosci. 2002;14:389–401. doi: 10.1162/089892902317361912. [DOI] [PubMed] [Google Scholar]
- 48.Eidelberg D., Galaburda A. M. Arch. Neurol. (Chicago) 1984;41:843–852. doi: 10.1001/archneur.1984.04050190049013. [DOI] [PubMed] [Google Scholar]
- 49.Desimone R., Duncan J. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 50.Miller E. K., Cohen J. D. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 51.Husain M., Rorden C. Nat. Rev. Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- 52.Foster D. J., Wilson M. A. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 53.Shatz C. J. Proc. Natl. Acad. Sci. USA. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buzsaki G., Draguhn A. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 55.Salinas E., Sejnowski T. J. Nat. Rev. Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olshausen B. A. In: The Visual Neurosciences. Chalupa L. M., Werner J. S., editors. Cambridge, MA: MIT Press; 2003. pp. 1603–1615. [Google Scholar]
- 57.Pouget A., Dayan P., Zemel R. S. Annu. Rev. Neurosci. 2003;26:381–410. doi: 10.1146/annurev.neuro.26.041002.131112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.