Abstract
Synthesis of the first of a projected series of bryostatin analogues has been accomplished in 26 steps and 2.2% overall yield. In this letter, we detail two approaches to the structural core of these tricyclic macrolactone bryostatin analogues. The key features of the route include BITIP-catalyzed asymmetric allylation reactions and Mukaiyama aldol reactions, a chelation-controlled allylation, pyran annulation reactions, and macrolactonization.
In the previous paper, we described the synthesis of a potential C9-C27 intermediate for a projected synthesis of bryostatin 1.1 However, at this point we elected to delay our efforts on the synthesis of bryostatin 1 itself in favor of investigations of the synthesis of truncated analogues using the same basic overall strategies. Biological evaluation of bryostatin analogues has shown very encouraging results.2 In particular, the efforts of Wender and co-workers in the bryostatin analogue area have highlighted what can be achieved in the area of simplified analogue design. Several interesting facets from Wender's studies have also shed light on the structure–activity relationships of these compounds.3 It was our hope that the syntheses of bryostatin analogues would not only serve as highly advanced “model” studies for the synthesis of bryostatin 1 itself but also give access to structurally simplified analogues of the bryostatins that retained or exceeded the levels of activity exhibited by the natural materials. Synthetic access to such structures and the attendant tuning of structure could in turn provide a better understanding of the mechanism of action of these agents and better define structure–activity relationships in this area.
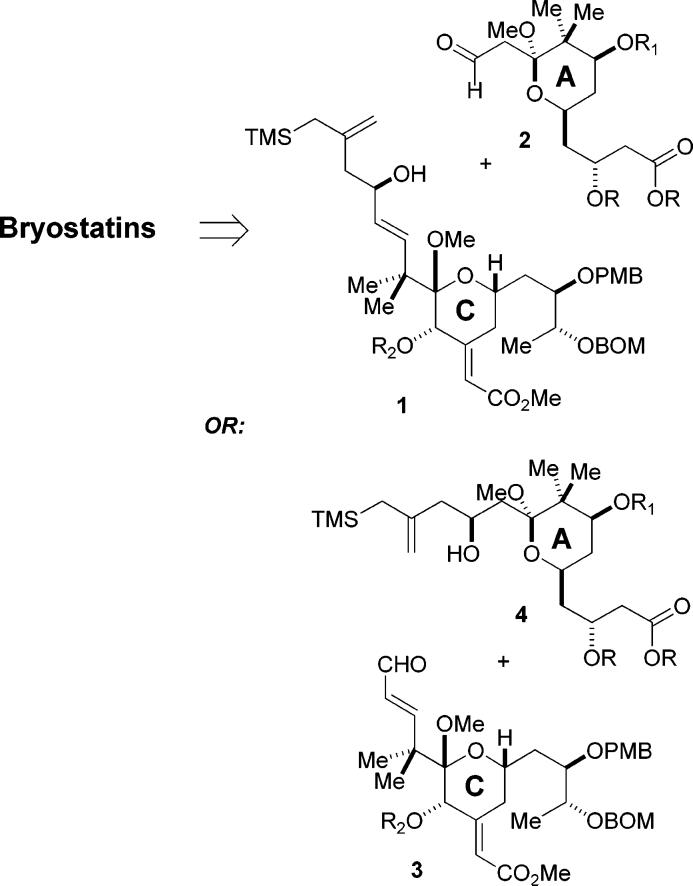
In the previous article, we demonstrated that a C-ring enal could be sucessfully coupled with a simple hydroxyallylsilane using the pyran annulation reaction to incorporate the B-ring carbons in one synthetic step. However, as we gained more experience with this reaction, it became clear that it was a particulary robust and high-yielding process that might be implemented in a more strategic role. In particular, we were intrigued by the possibility of using this process to bring together subunits corresponding to rings A and C with concomitant formation of the B-ring, according to the strategy shown in Scheme 1. As indicated, either the A- or C-ring intermediates could play the role of the hydroxy allylsilane. In addition, the same approach could be applied in the service of analogue synthesis, which would serve as an advanced model study for the synthesis of bryostatin 1 itself. We describe herein the implementation of this strategy for the synthesis of the tricyclic macrolactone core of the bryostatins.
Scheme 1.
Retrosynthetic Analysis of the Bryostatins
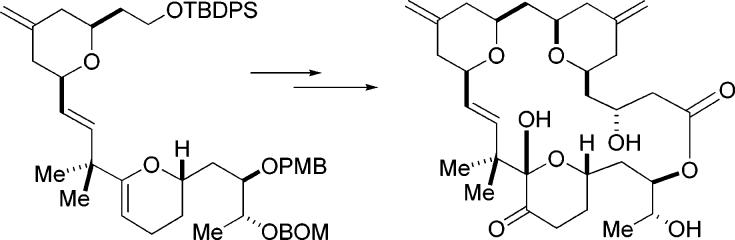
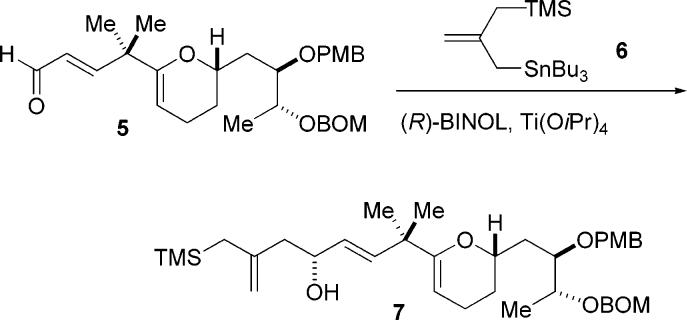
The dissection in which ring C played the role of the hydroxy allylsilane component was examined first. In the preceding paper, we described the preparation of the C-ring enal intermediate 5. To obtain the β- hydroxy allylsilane, our plan was to utilize our catalytic asymmetric allylation (CAA) reaction with this enal.4 However, the execution of this simple step was complicated due to the presence of sensitive functionalities on the C-ring. We were unable to obtain an acceptable yield using a catalytic amount of the BITIP catalyst with this enal and the 2-(trimethylsilylmethyl) allylstannane reagent 6. However, success was obtained using a large excess of the BITIP reagent. The product 7 was obtained in modest (67%) yield due to some decomposition during the reaction.
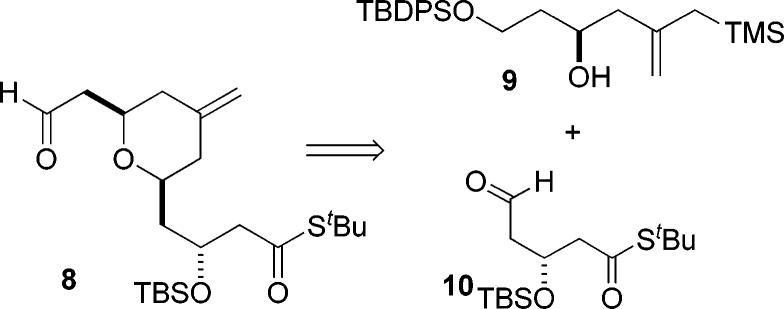
The reaction partner needed for this annulative approach to fragment coupling is an aldehyde incorporating the A-ring pyran. As indicated in Scheme 3, we envisioned that the A-ring pyran 8 could itself be accessed by a pyran annulation between the simple hydroxy allylsilane 9 and aldehyde 10. We chose to use a thiol ester as the precursor to the carboxylate at C1 since we anticipated that it could be selectively hydrolyzed under mild and specific conditions to liberate the carboxylic acid needed for esterification with the C26 hydroxyl group of the C-ring fragment. In addition, using this approach, the catalytic asymmetric Mukaiyama aldol reaction using BITIP catalysis would provide a very convenient means to set the C3 stereocenter.5
Scheme 3.
Retrosynthesis of the A-Ring Aldehyde
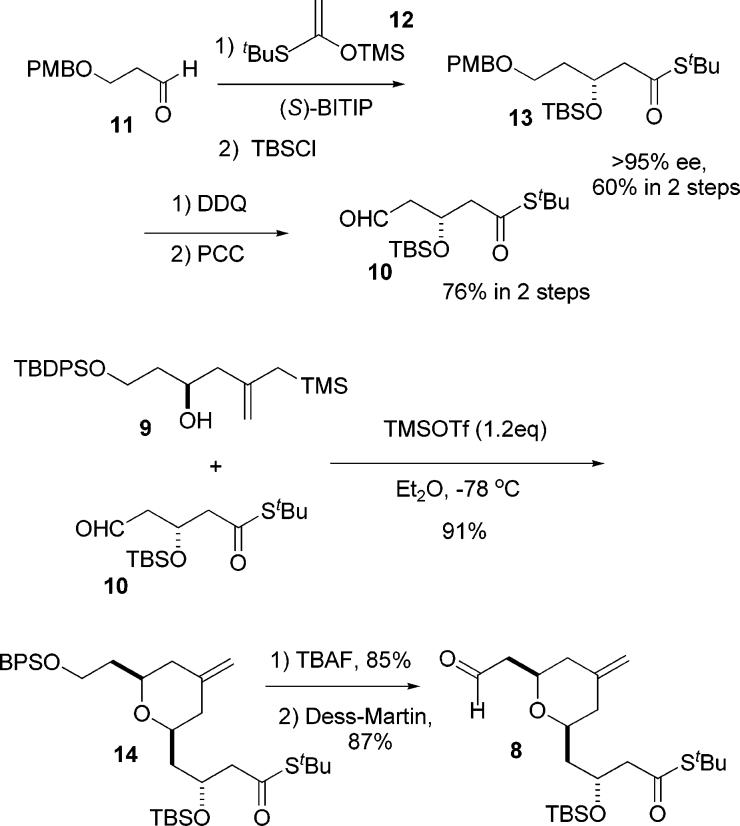
The synthesis of the aldehyde component, 10, began with the BITIP-catalyzed Mukaiyama aldol reaction of the PMB-protected β-hydroxy aldehyde 11 with thiosilyl ketene acetal 12. The resulting alcohol was protected as the TBS ether to give 13 in 60% overall yield and in 95% ee. The PMB ether was then removed under standard conditions using DDQ in a methylene chloride–water mixture. Immediate oxidation of the crude alcohol so produced with PCC afforded the desired aldehyde 10 in 76% yield from the PMB ether 11. Thus, aldehyde 10 was obtained in four simple steps from readily available starting materials (Scheme 4).
Scheme 4.
Synthesis of the A-Ring Pyran-aldehyde
The hydroxy allylsilane 9 was prepared using stannane 6 and (R)-BITIP catalyst to set the stereocenter at C9. This reaction proceeded in good yield (81%) and excellent selectivity (95% ee) on a 3–5 g scale.
These two fragments were then coupled via the pyran annulation reaction to give the desired pyran 14 in excellent (91% isolated) yield.6 Fortunately, this reaction went smoothly, despite the possibility of potentially complicating side reactions such as β-elimination or cleavage of the tert-butyl thiol ester. Selective deprotection of the BPS group and oxidation of the resulting alcohol then afforded the desired A-ring aldehyde 8 in good yield. As shown in Scheme 4, the synthesis of the A-ring aldehyde was completed in seven steps and in 31% overall yield.
Coupling of the C1–C11 and C12–C27 Fragments Using the Pyran Annulation Reaction
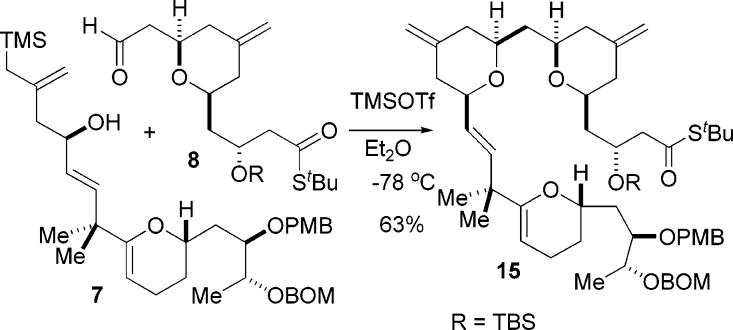
With the synthesis of the A-ring aldehyde 8 accomplished, we turned our attention to the coupling of these fragments by reacting C-ring hydroxy silane 7 with A-ring aldehyde 8 in the presence of TMS triflate. We were pleased to find that the annulation reaction gave us the desired product 15 in good yield (63% isolated yield) despite the presence of potentially problematic acid-sensitive functionality such as the enol ether of the C-ring pyran (Scheme 5).7
Scheme 5.
Union of Rings A and C via Pyran Annulation
Sequential Annulation Approach to the Bryostatin Core
Although this synthesis of the tricyclic core of bryostatin analogues is flexible, we were concerned about the use of excess chiral promotor in the sequence of reactions leading to 15. Thus, we decided to investigate an alternative strategy for the synthesis of the tricycle 15. In this alternative approach, the tricyclic material was envisioned to arise from two sequential annulation reactions starting from the enal 5. The first would use the same hydroxy allylsilane as in our previous report;1 the second would use a more complex hydroxy allylsilane component to bring in carbons 1–8 with the required stereocenters in this fragment already established. Thus, we would use the same BC-ring intermediate prepared earlier1 in a second annulation with the more complex β-hydroxy allylsilane 19.
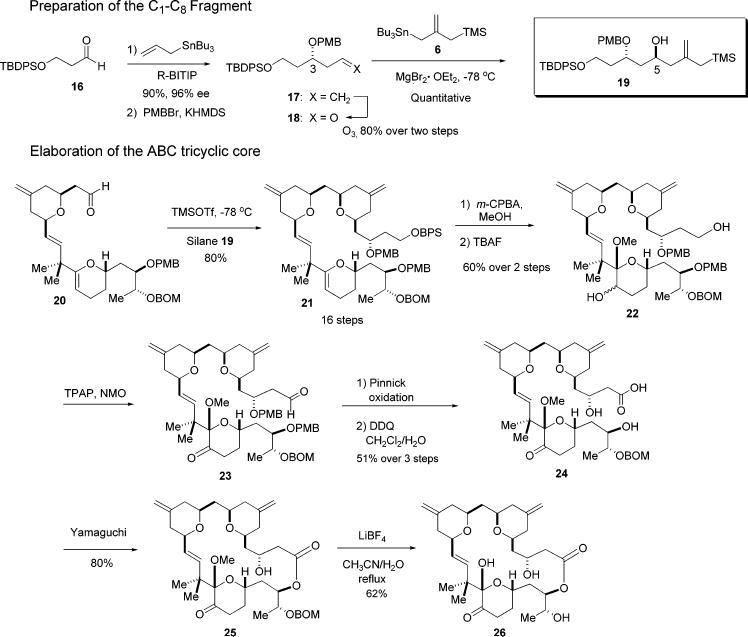
The synthesis of the C1–C8 fragment began with readily available aldehyde 16 (Scheme 6). A catalytic asymmetric allylation (CAA) reaction using allyltri-n-butylstannane and our normal CAA conditions afforded the homoallylic alcohol in excellent yield and with excellent selectivity (90%, 96% ee). Protection of this alcohol as the PMB ether was best accomplished using PMBBr and KHMDS to give 17. Oxidative cleavage of the alkene then gave the desired PMB-aldehyde 18 in good yield (80% over two steps).
Scheme 6.
Completion of the Bryostatin Core Structure
Incorporation of the allylsilane in this instance was accomplished with the desired stereochemical result at C5 by simply using a chelation-controlled addition with 1,3-asymmetric induction; asymmetric catalysis was unnecessary. Thus, as expected,8 reaction of the β-OPMB-aldehyde 18 with silyl stannane 6 using MgBr2•Et2Oat −78 °C proceeded with complete stereoselectivity at C5, and in quantitative yield (Scheme 6). With the desired β-hydroxy allylsilane 19 in hand, the pyran annulation event was found to occur smoothly. Reaction of this silane with aldehyde 20 and TMS triflate, in ether at −78 °C, afforded the desired tricyclic C1–C27 subunit 21 in good yield (80%) and with all requisite stereocenters now in place (Scheme 6).9
Elaboration of the ABC Tricyclic Core
The C-ring glycal was elaborated first. Treatment of the ABC tricycle 21 with m-CPBA in MeOH,10 followed by oxidation with TPAP–NMO, afforded the desired ketone at C20. It is noteworthy that considerable experimentation was required to determine a suitable order of functional group manipulations en route to the seco-acid. We first deprotected the PMB ethers at C3 and C25, followed by the deprotection of the BPS ether at C1. Many attempts to selectively oxidize the primary C1 hydroxyl in the presence of the free secondary hydroxyls at C3 and C25 using PhI(OAc)2 and TEMPO gave only a trace of the desired product and mainly unidentified decomposition products. A successful sequence elaborated C1 to the acid prior to removal of the PMB ethers. Thus, the BPS ether at C1 was removed first, by reaction with TBAF. The resulting diol 22 was then oxidized to keto-aldehyde 23 using TPAP–NMO. This material was then subjected to a Pinnick oxidation11 to give the carboxylic acid. Finally, the PMB ether protecting groups at C3 and C25 were removed to give the desired dihydroxy acid 24 in good yield (80%).
Having successfully overcome this particular hurdle, we then faced the critical issue of macrolactonization of the seco-acid in the presence of the hydroxyls at both C3 and C25 and also the removal of the Me and BOM ethers at C19 and C26. Macrolactonization was achieved using a modified Yamaguchi mixed anhydride esterification protocol to give the desired 20-membered lactone 25.12 Finally, the BOM group and methyl ether at C26 and C19 were removed under very mild and specific conditions (LiBF4, aqueous CH3CN/H2O) to give the dihydroxy macrolactone 26 in 62% yield.13
The synthesis of 26 requires 26 steps in total and has a longest linear sequence of 23 steps, considerably less arduous than required for the synthesis of a natural bryostatin. Efforts to elaborate this structure to more closely resemble the naturally occurring structures, as well as to provide biological evaluation of these agents, are in progress and will be reported in due course.
Supplementary Material
Scheme 2.
Synthesis of the C12–C27 Fragment
Acknowledgment
Financial assistance provided by the National Institutes of Health (through Grant GM-28961) is gratefully acknowledged.
Footnotes
Supporting Information Available: Spectral (1H and 13C NMR) data for materials described herein. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Keck GE, Truong AP. Org. Lett. 2005;7:2149. doi: 10.1021/ol050511w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Hale KJ, Hummersone MG, Manaviazar S, Frigerio M. Nat. Prod. Rep. 2002;19:413. doi: 10.1039/b009211h. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Hale KJ, Frigerio M, Hummersone MG, Manaviazar S. Org. Lett. 2003;5:503. doi: 10.1021/ol027393m. [DOI] [PubMed] [Google Scholar]
- 3.Wender PA, Baryza JL, Bennett CE, Bi FC, Brenner SE, Clarke MO, Horan JC, Kan C, Lacote E, Lippa B, Nell PG, Turner TM. J. Am. Chem. Soc. 2002;124:13648. doi: 10.1021/ja027509+. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, De Brabander J, Harran PG, Jimenez J-M, Koehler MFT, Lippa B, Park C-M, Shiozaki M. J. Am. Chem. Soc. 1998;120:4534. [Google Scholar]; (c) Wender PA, Hinkle KW, Koehler MFT, Lippa B. Med. Res. Rev. 1999;19:388. doi: 10.1002/(sici)1098-1128(199909)19:5<388::aid-med6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]; (d) Wender PA, Lippa B. Tetrahedron Lett. 2000;41:1007. [Google Scholar]; (e) Wender PA, Mayweg AVW, VanDeusen CL. Org. Lett. 2003;5:277. doi: 10.1021/ol0272390. [DOI] [PubMed] [Google Scholar]
- 4.(a) Keck GE, Li X-Y, Krishnamurthy D. J. Org. Chem. 1995;60:5998. [Google Scholar]; (b) Keck GE, Krishnamurthy D. Synth. Commun. 1996;26:367. [Google Scholar]; (c) Keck GE, Krishnamurthy D. Org. Synth. 1998;75:12. [Google Scholar]; (d) Keck GE, Abbott DE, Boden EP, Enholm EJ. Tetrahedron Lett. 1983;25:3927. [Google Scholar]
- 5.Keck GE, Krishnamurthy D. J. Am. Chem. Soc. 1995;117:2363. [Google Scholar]
- 6.Keck GE, Covel JA, Schiff T, Yu T. Org. Lett. 2002;4:1189. doi: 10.1021/ol025645d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. This synthetic plan is quite flexible since the annulation can be approached from two different directions to form the B-ring pyran. In fact, we were able to demonstrate that the pyran annulation reaction worked equally well with the roles of the annulation partners reversed.
- 8.(a) Keck GE, Castellino S, Wiley MR. J. Org. Chem. 1986;5:5478. [Google Scholar]; (b) Evans DA, Dart MJ, Duffy JL, Yang MG, Livingston AB. J. Am. Chem. Soc. 1995;117:6619. [Google Scholar]; (c) Evans DA, Dart MJ, Duffy JL, Yang MG. J. Am. Chem. Soc. 1996;118:4322. [Google Scholar]
- 9. Aldehyde 20 was prepared from the corresponding BPS ether by deprotection using TBAF followed by oxidation with TPAP–NMO. For the synthesis of this BPS ether, see ref 1.
- 10.Evans DA, Carter PH, Charette AB, Prunet JA, Lautens M. J. Am. Chem. Soc. 1999;121:7540. [Google Scholar]
- 11.Bal BS, Childers WE, Jr., Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
- 12.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989. [Google Scholar]
- 13.Zakarian A, Batch A, Holton RA. J. Am. Chem. Soc. 2003;125:7822. doi: 10.1021/ja029225v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.