Abstract
Recent research has provided increasing support for the origins of anatomically and genetically “modern” human populations in Africa between 150,000 and 200,000 years ago, followed by a major dispersal of these populations to both Asia and Europe sometime after ca. 65,000 before present (B.P.). However, the central question of why it took these populations ≈100,000 years to disperse from Africa to other regions of the world has never been clearly resolved. It is suggested here that the answer may lie partly in the results of recent DNA studies of present-day African populations, combined with a spate of new archaeological discoveries in Africa. Studies of both the mitochondrial DNA (mtDNA) mismatch patterns in modern African populations and related mtDNA lineage-analysis patterns point to a major demographic expansion centered broadly within the time range from 80,000 to 60,000 B.P., probably deriving from a small geographical region of Africa. Recent archaeological discoveries in southern and eastern Africa suggest that, at approximately the same time, there was a major increase in the complexity of the technological, economic, social, and cognitive behavior of certain African groups, which could have led to a major demographic expansion of these groups in competition with other, adjacent groups. It is suggested that this complex of behavioral changes (possibly triggered by the rapid environmental changes around the transition from oxygen isotope stage 5 to stage 4) could have led not only to the expansion of the L2 and L3 mitochondrial lineages over the whole of Africa but also to the ensuing dispersal of these modern populations over most regions of Asia, Australasia, and Europe, and their replacement (with or without interbreeding) of the preceding “archaic” populations in these regions.
Keywords: archaeology, DNA, modern humans, Palaeolithic
Our understanding of the origins of modern human populations (i.e., Homo sapiens) has made massive strides in the past two decades. We now know from studies of both the DNA patterning of present-day world populations and surviving skeletal remains that populations that were essentially “modern” in both a genetic and an anatomical sense had emerged in Africa by at least 150,000 years ago (1–7). We also know that these populations had dispersed from Africa to most other parts of the world by at least 40,000 years ago, where they demographically replaced the preexisting “archaic” populations, such as the European Neanderthals (1–3, 8–19). However, some of the most central questions as to exactly how and why this dramatic population dispersal and replacement took place have never been clearly resolved.
Two critical issues are posed by this recent research. First, if we now know that populations that were essentially modern in both genetic and anatomical terms had already emerged in Africa by at least 150,000 years ago, why did it take these populations a further 100,000 years to disperse to other regions of the world (1, 2, 8, 10–12)? And second, what were the crucial evolutionary and adaptive developments that allowed these populations to colonize a range of entirely new and alien environments and to successfully compete with, and replace, the long-established, and presumably well adapted, archaic populations in these regions (2, 8, 13, 14, 17)?
As noted earlier, the answer to these questions seems to lie partly in the results of recent DNA research among different geographical groups of present-day African populations and partly in a number of striking new archaeological discoveries at sites in southern and eastern Africa.
The African DNA Evidence
Demographic reconstructions based on DNA studies of present-day human populations are notoriously problematic and controversial, with the data from African populations being no exception. Debates over the rates of mutation of different genetic loci, the effects of adaptive selection on DNA patterns, and the potential complications of demographic dispersals and back migrations between different regions, all serve to complicate the surviving fingerprints of demographic history in ways that have still to be fully resolved (2, 18–22). Evidence from mitochondrial DNA (mtDNA), even though reflecting only a small segment of the total human genome, has the advantage of unusually rapid mutation rates, descent predominantly, if not entirely, through the female lineage, and apparently few, if any, effects of environmental selective forces (2, 3, 8, 11). In the present context, therefore, it is interesting to see that two separate approaches to the analysis of mtDNA patterns in present-day African lineages point strongly to an episode of rapid population growth in the ancestral Africa populations centered broadly within the time range from ca. 60,000 to 80,000 years ago, i.e., some 100,000 years after the inferred most recent common ancestor (MRCA) of mitochondrially modern populations in Africa.
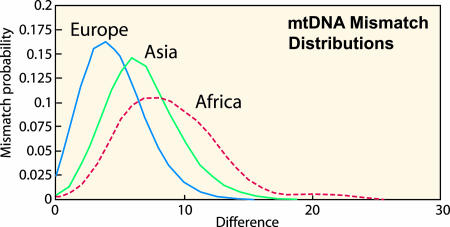
Evidence for this pattern was first recognized by Harpending, Rogers, Sherry, and others (23–25) from studies of so-called mtDNA “mismatch” distributions (i.e., frequency distributions of genetic differences between pairs of individuals within a population), which revealed a clearly defined peak in African populations dated broadly to ≈80,000 years before present (B.P.). This peak was followed by equally sharply defined peaks in Asian and European populations at ≈60,000 and 40,000 B.P. (see Fig. 1). Clearly, the precise age of these inferred population expansions depends on the accuracy of the assumed mutation rate of mtDNA (2, 3, 8), but the evidence as a whole points strongly to a major and apparently rapid increase in African population numbers much earlier than that experienced in either Asia or Europe and apparently involving expansion by means of a demographic “diffusion wave” (15) from a relatively small population nucleus (probably confined to a fairly small region of Africa) to other parts of the continent (23–25).
Fig. 1.
mtDNA “mismatch” distributions of present-day African, Asian, and European populations, showing the frequency distribution of differences between pairs of individuals in the three populations. The modes of the three distributions clearly reflect a much earlier demographic expansion of African populations (ca. 80,000 B.P.), than those in Asia (ca. 60,000 B.P.) and Europe (ca. 40,000 B.P.) (23–25).
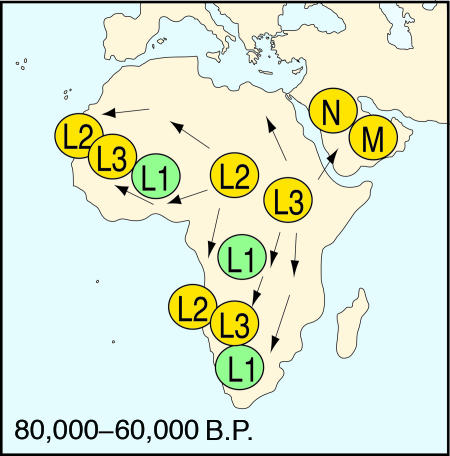
More recently, strong support for this pattern has been provided by detailed mtDNA “lineage-analysis” studies of modern African populations by Watson, Forster, Salas, Kivisild, Macaulay, and others (2, 8, 9, 26–28). Once again, the precise timing of these lineage expansions depends on the assumed mutation rate of mtDNA, but, in all of these studies, there is evidence for what Forster and Matsumura (28) have recently described as a “remarkable expansion” of the distinctive L2 and L3 mitochondrial lineages dating broadly to between ca. 80,000 and 60,000 B.P. (2, 8, 9, 26–28) (Fig. 2). As in the case of the mismatch analyses, the evidence points to an expansion centered initially in one small area of Africa (most probably in eastern or southern Africa) followed by an expansion to other regions, apparently reaching western Africa by at least 30,000–40,000 B.P., and perhaps across the mouth of the Red Sea to the adjacent parts of southern Asia by ≈60,000–65,000 B.P. (2, 8, 9, 28). Whether this dispersal of the L2 and L3 lineages reflects an actual dispersal of discrete human populations, or simply a rapid expansion in these specific mitochondrial types amongst the existing African populations, remains perhaps more debatable. But in either case, it is clear that some significant demographic or cultural factors must have promoted these lineage expansions at roughly the same time as the mtDNA mismatch analyses point to a rapid increase in total population numbers from some localized geographical source. A similar expansion in African populations has also been claimed from some studies of DNA microsatellite data, although with less specific age estimates (3).
Fig. 2.
Inferred patterns of geographical dispersal of the L2 and L3 mtDNA lineages in Africa between ca. 80,000 and 60,000 B.P., according to Forster (2). Later dispersals of the M, N, and R lineages into Asia and Europe after 65,000 B.P. derive from the L3 lineage.
Archaeological Evidence
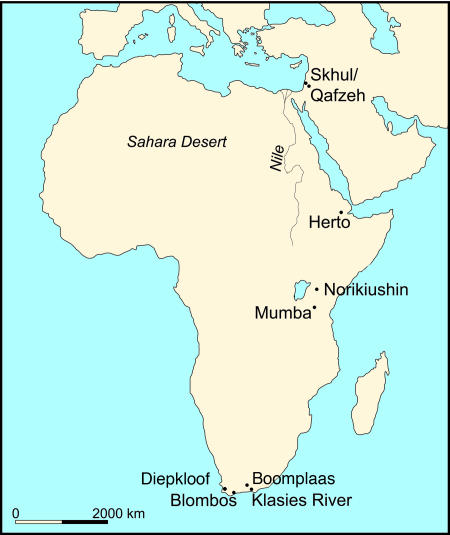
The central question is what could have caused this apparently dramatic expansion in African populations ≈60,000–80,000 B.P., and it is here that recent archaeological research in southern and central Africa becomes central to the interpretation of the demographic data. The most relevant evidence at present comes from a number of sites located close to the southern tip of Africa in Cape Province, most notably from Blombos Cave and Klasies River on the southern coast and those of Boomplaas Cave and Diepkloof, further to the north and west (29–40) (Fig. 3). These are backed up by a number of rather less well documented sites in eastern and central Africa (34, 41–43). The general time range of these sites is that of the African Middle Stone Age (MSA) extending from ≈250,000 to 40,000 B.P., and coinciding broadly with the Middle Palaeolithic (or Mousterian) periods in Europe and Asia (44, 45). But the relevant evidence from the so-called “Still Bay” levels in the Blombos Cave and the ensuing “Howiesons Poort” levels at Klasies River, Boomplaas, and Diepkloof, can be dated specifically to the later stages of the MSA, between ca. 75,000 and 55,000 B.P. (35, 46, 47).
Fig. 3.
Map of archaeological sites and early anatomically modern human remains in Africa and Israel, referred to in text.
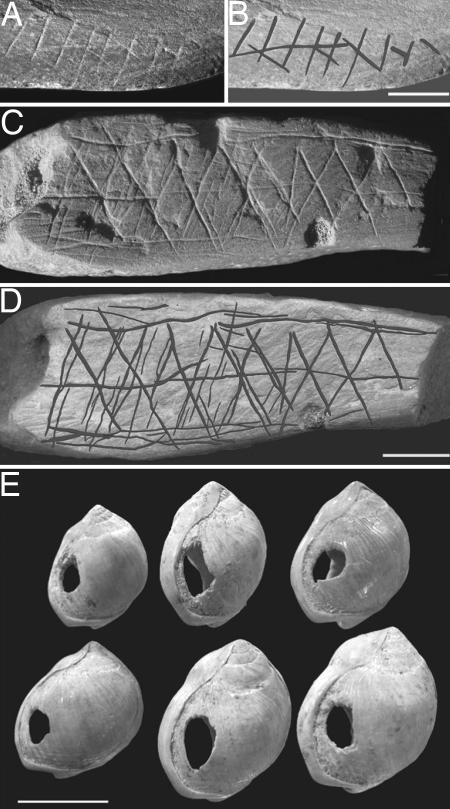
Although the archaeological assemblages from these sites have traditionally been attributed to the MSA, they reveal a number of radical technological and cultural features that collectively contrast sharply with those of the earlier African MSA sites, and which show many resemblances to those that appear in Europe and western Asia with the arrival of the first anatomically and genetically modern populations at ≈45,000–50,000 B.P., the period of the so-called “Upper Palaeolithic revolution” (17, 45, 48–50). These assemblages include, for example, new patterns of blade technology, produced by means of “soft hammer” techniques of flaking (29–32, 51); new forms of both specialized skin working tools (end-scrapers) and tools for the controlled shaping of bone and wooden artefacts (so-called burin forms) (32, 35); a range of extensively shaped bone tools, apparently used as both tips of throwing spears and sharply pointed awls for skin working (36, 37); new forms of carefully shaped stone inserts, probably used as tips and barbs of either hafted throwing spears or conceivably wooden arrows (30–32, 34, 51); large numbers of perforated estuarine shells, evidently used as personal ornaments of some kind (39); and large quantities of imported red ochre, including two pieces from the Blombos cave with carefully incised and relatively complex geometrical designs on their surfaces (38). These designs represent the earliest unambiguous forms of abstract “art” so far recorded (Figs. 4 and 5). Equally significant in these sites is the evidence for the large-scale distribution or exchange of both high-quality stone for tool production and the recently discovered shell beads from the Blombos cave, in both cases either transported or traded over distances of at least 20–30 km (31, 39). All of these features show a striking resemblance to those which characterize fully modern or “Upper Palaeolithic” cultures in Europe and western Asia, which first appeared with the initial arrival of anatomically and behaviorally modern populations at ≈45,000–50,000 B.P., i.e., some 20,000 years later than their appearance in the African sites (17, 45, 48–50). As Henshilwood (35) has recently commented, the combination of these behavioral innovations in the Still Bay and succeeding Howiesons Poort levels at these South African sites seems to reflect “a dynamic period of diverse technological behavior not previously seen in the African Middle Stone Age.”
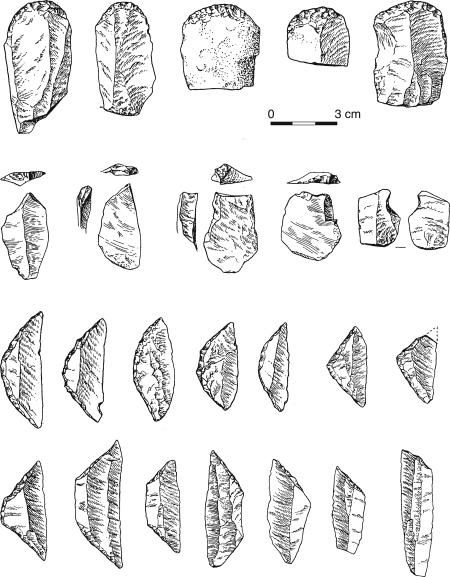
Fig. 4.
Stone tools from the MSA Howiesons Poort levels at Klasies River (South Africa) dated to ca. 65,000 B.P., showing closely similar forms of blades, end scrapers, burins, and small, hafted segment forms to those found in European and Asian Upper Palaeolithic sites from ca. 45,000 B.P. onwards (32).
Fig. 5.
Fragments of red ochre incised with a complex geometrical design (A–D), and a series of deliberately perforated shells of Nassarius kraussianus (E) from the MSA levels of the Blombos Cave (South Africa), dated to ca. 75,000 B.P. (38, 39). [A–D reproduced with permission from Henshilwood et al. (38) (Copyright 2002, AAAS). E reproduced with permission from Henshilwood et al. (104) (Copyright 2004, AAAS).]
Population Expansion
The critical importance of these new archaeological discoveries is that they may provide the explanation for the major expansion in African populations, which is reflected so clearly in the recent mtDNA evidence, dated broadly to between 80,000 and 60,000 B.P. The precise cultural and demographic mechanisms that underlay the population expansion inevitably remain more hypothetical. At least four aspects of the archaeological data, however, could be significant in this context. The first is that the character of the artefacts recovered from both the Blombos Cave and the Howiesons Poort levels at Klasies River and elsewhere would appear to reflect the emergence of more complex forms of hunting equipment, apparently involving the construction of several different forms of hunting weapons (i.e., the sharply pointed bone spear heads and the bifacial leaf-point forms from the Blombos cave, and the appearance of composite, multiple-component hafted weapons in the Howiesons Poort levels at Klasies River and other sites) (31, 34–37) (Fig. 4). The possibility has been suggested that some of these forms could well have served as the tips and barbs of wooden arrows, based on comparisons with similar artefacts recovered from both later African Stone Age sites and much later Mesolithic contexts in Europe (34, 40). Even without inferring the use of archery equipment, however, it is reasonable to assume that the introduction of more effective hunting weapons would have substantially increased the efficiency and productivity of hunting activities and, therefore, the overall productivity of the food resources available to the human groups (31, 34). The second and potentially equally important suggestion, which has been mooted by Deacon (29, 31), is that the dense accumulations of burnt plant remains in the Howiesons Poort levels at Klasies River (together with identifiable remains of root crops, such as Watsonia, in the later MSA levels at the Strathalan B site, further to the north) could reflect either the increased use of these particular plant resources or even the deliberate burning of the local fynbos vegetation, which has been shown to increase the annual productivity of these root crops by between five- and ten-fold (31). For the present, the latter suggestion remains speculative, but, if this were to be supported by further research, one could see this effectively as an early form of plant food management strategies, potentially analogous to those used in later Mesolithic and early agricultural communities, or in the recently reported 26,000-year-old processing of seed remains from the Ohalo II site in Israel (52). A third suggestion advanced by Henshilwood (35, 36) is that the Still Bay levels at Blombos cave may provide evidence for the first systematic exploitation of marine fish, and perhaps sea birds, as parts of the human food supply. Finally, Deacon, Ambrose, and others (31, 35, 42, 43) have argued that the large-scale movements of high-quality stone and imported shell ornaments recorded from these sites may reflect increased trading and exchange networks between adjacent human groups, which could have acted as a further critical mechanism to ensure regular access and distribution of essential food supplies, especially during seasonal or other episodes of food scarcity.
Clearly, all of these possibilities will require further analysis and testing in the course of future research. But the implication seems clear that many of the behavioral innovations reflected in the southern African archaeological records between ca. 80,000 and 60,000 B.P. could have led to a substantial increase in the carrying capacity of the environment for human populations and, accordingly, to a major expansion in human population numbers and densities. Even allowing for the imprecisions in current DNA dating estimates, the apparent coincidence between these major behavioral changes and the estimated timing of the population expansions reflected strongly in both the mtDNA mismatch and lineage-analysis data seems hard to ignore. It should be emphasized that there is no necessary implication that population numbers in Africa as a whole increased dramatically at this time. Indeed, it could be that total population numbers in Africa decreased significantly at this time, owing to the onset of extremely dry conditions in many parts of Africa between ca. 60,000 and 30,000 B.P. (31, 44). The point is simply that increased levels of technological efficiency and economic productivity in one small region of Africa could have allowed a rapid expansion of these populations to other regions and an associated competitive replacement (or absorption) of the earlier, technologically less “advanced,” populations in these regions (2, 16, 23, 53, 54).
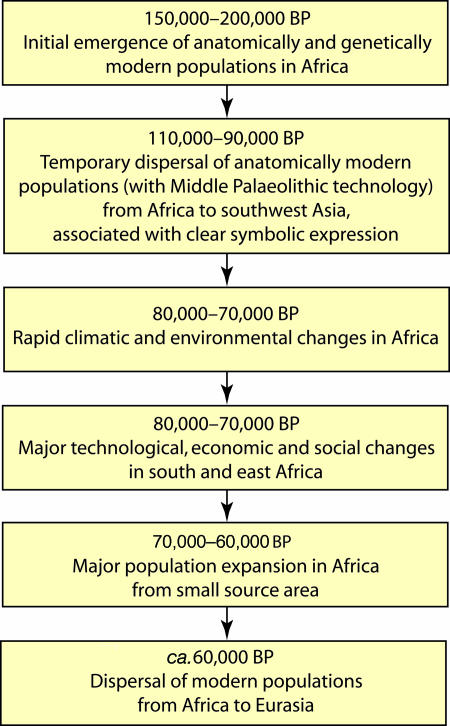
Any attempt to define the precise point of origin of these behavioral innovations, and the associated demographic expansion event, immediately encounters the relative sparsity of well documented archaeological sites in many regions of subSaharan Africa, especially in the more central and eastern areas of Africa, which are potentially crucial to the current debates over modern human origins (31, 34). Clearly, we must be aware of falling into the obvious trap of assuming these developments must have occurred initially within South Africa, simply because this area is where the relevant archaeological evidence is at present most fully investigated and best documented (i.e., the “drunk looking for keys under the street lamp” syndrome!). In this context, it should be recalled that industries conforming closely to the South African Howiesons Poort variations are well represented over large areas of central and southern Africa (to the south of the Zambezi) and apparently extending northwards into parts of East Africa—such as at the site of Mumba in Tanzania (34, 41) and the recently excavated site of Norikiushan in Kenya (S. Ambrose, personal communication) >4,000 km to the north of the South African sites. The main problem at present lies in the accurate dating of these sites in relation to the South African localities (34). On present evidence, it is impossible to exclude the possibility that the Howiesons Poort technologies, or indeed those of the preceding Still Bay, could have emerged in certain parts of, say, eastern or central Africa, before they subsequently appeared in the South African sites. In this case, the developments in South Africa could be seen more as a reflection of events in other parts of Africa than their initial point of origin. But, in any event, the sheer scale of the geographical distribution of the Howiesons Poort-like technologies could be seen as a further potential reflection of a major episode of population dispersal within subSaharan Africa centered broadly within the time range from ca. 70,000 to 55,000 B.P. (31, 34, 35). It is equally tempting to suggest that it was precisely this new, integrated complex of so-called modern behavioral features embodied in the Howiesons Poort and preceding Still Bay technologies that led directly to the widespread geographical expansion of the southern African populations not only to other areas of Africa (as reflected in the widespread dispersal of the L2 and L3 mitochondrial lineages; see Fig. 2) but also to the adjacent areas of Asia and Europe, sometime after 70,000 B.P. (1, 2, 8, 16, 17, 42) (Fig. 6).†
Fig. 6.
Summary of the model proposed here for modern human origins and dispersal from Africa.
The Mechanisms of Behavioral Change
The pivotal question, of course, is what caused these radical changes in the technology, economy, and social patterns of African groups ≈80,000–70,000 B.P.? Here we have two fairly stark alternatives. First, we could suggest, as Klein (44, 55) has done, that the emergence of distinctively modern patterns of culture and technology was due to a sudden change in the cognitive capacities of the populations involved, entailing some form of neurological mutation (although, according to the model advanced here at ≈80,000 B.P. and not at ca. 40,000–50,000 B.P., as Klein himself has suggested). Or alternatively (and more prosaically), we could look for an interpretation in terms of some major shift in the adaptive and selective pressures to which the human populations were subjected, perhaps precipitated by some major episode of climatic and environmental change. In this context, the obvious candidate would be the sharp oscillations between wetter and drier climatic conditions that marked the transition from oxygen isotope stage 5 to stage 4, as reflected in the deep-sea core and ice-core climatic records (56). In subSaharan Africa, there is evidence that this transition resulted in changes in annual rainfall by up to 50% (57). To groups occupying the more arid regions of Africa (especially around the margins of the Kalahari and Sahara deserts), the impact of these climatic changes on all aspects of human economic, technological, and social adaptations could have been dramatic, as Deacon, Ambrose, and others (29, 31, 42, 43) have emphasized. A further potentially significant factor could have been the climatic and associated environmental effects of the Mount Toba volcanic “supereruption” in Sumatra, dated to ≈73,000 B.P., as Ambrose (58) has argued very effectively [but see Oppenheimer (59) and Gathorne-Hardy & Harcourt-Smith (60) for an opposing view]. It would, in short, be possible to see changes in human technology, subsistence, settlement patterns, and associated patterns of social and even symbolic communication as a fairly direct response to the new environmental challenges that emerged at this time (53, 54, 61, 62). Significantly, all these major environmental changes fall within the time range of ca. 80,000–70,000 B.P., precisely the time when the archaeological evidence indicates that technological and other behavioral changes were occurring most rapidly.
Human Cognitive Evolution
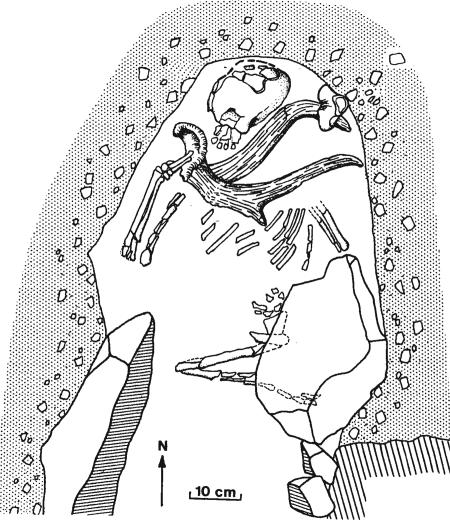
Even if we accept that the pattern of behavioral changes in southern Africa can be explained more parsimoniously in terms of adaptive environmental processes than by changes in human cognitive capacities, we cannot escape the evidence for significant changes in at least some aspects of human cognitive behavior associated broadly with the emergence of our own species (63–68). One aspect of the current evidence that is potentially highly informative in this context lies in the evidence for a precocious and apparently short-lived expansion of anatomically modern populations from northern Africa into the immediately adjacent areas of southwest Asia at ≈110,000–90,000 B.P. (1, 69–72). This expansion is best reflected in the large samples of typically (if relatively robust and variable) anatomically modern skeletal remains from the two sites of Skhul and Qafzeh in northern Israel (Fig. 3). Three features of these finds are especially significant. The first is that at least two of the skeletons in these sites occurred in the form of clearly ceremonial or ritualistic burials, associated with seemingly unmistakably intentional grave offerings (a large deer antler lying directly on top of one of the Qafzeh skeletons and a complete boar’s jaw said to be “clasped in the arms” of one of the burials at Skhul) (72–75) (see Fig. 7). Secondly, that, at least in the case of the Qafzeh burials, the remains were associated with a number of deliberately perforated seashell ornaments, together with large quantities of used and apparently heat-treated fragments of red ochre, almost certainly used as coloring pigments (76, 77). And, thirdly, that, despite these clearly “symbolic” aspects of the archaeological material, the stone tool assemblages found in association with both the Skhul and Qafzeh remains were of typically Middle Palaeolithic or MSA in form, without any trace of the distinctively modern or Upper Palaeolithic technological features recorded at the later African MSA sites of Klasies River, Blombos, and elsewhere (71, 72).
Fig. 7.
Burial of an anatomically modern human skeleton at the Qafzeh Cave (Israel), accompanied by a large deer antler and dated to ca. 90,000–100,000 B.P. (73, 74).
The clear implication of these finds is that, whilst the human populations represented at Skhul and Qafzeh were essentially modern in both anatomical terms and in terms of clearly symbolic behavioral patterns, the levels of technology associated with these populations were still of strictly archaic, Middle Palaeolithic form (71, 72). Viewed in these terms, it is equally interesting that the early incursion of these anatomically modern populations into southwest Asia seems to have been a very localized and short-lived event, apparently confined to this southwest Asian region, and followed by a reestablishment of the earlier Neanderthal populations within these regions from at least 70,000 B.P. onwards, as reflected by the typically Neanderthal remains recovered from the later Mousterian levels at the Kebara cave, Tabun, Amud and Shanidar (1, 71, 72, 78). In other words, it would seem that whatever the intellectual and symbolic capacities of these early anatomically modern populations, their levels of technological and socioeconomic organization were not sufficient to withstand competition from the long-established Neanderthal populations of Eurasia during the later (and colder) stages of the Middle Palaeolithic sequence (71, 72, 78).
Mosaic Evolution
The obvious and seemingly inescapable conclusion is that the patterns of cultural and technological development associated with the evolution of fully modern populations were strongly mosaic in character, with the emergence of several explicitly symbolic aspects of culture apparently preceding any major change in either the stone-tool or bone-tool components of the associated technologies (40). In Africa itself, there may be further evidence for this symbolic behavior in the indications of apparently ritualistic treatment of the two early anatomically modern skulls recently discovered at Herto in Ethiopia, dated to ≈ 160,000 B.P., and again associated with characteristically archaic MSA stone tool technology (6, 79).
If so, what, if anything, might this evidence tell us tell us about the patterns of human cognitive and neurological evolution associated with the emergence of fully anatomically and genetically modern populations? If explicit symbolism is accepted as an index of essentially modern cognitive capacities and with associated patterns of essentially modern, complex language [as most archaeologists and palaeoanthropologists tend to assume (30, 63, 66, 68, 80–87)], then these capacities were clearly in place by at least 100,000–150,000 B.P. and could well have emerged in direct association with the evolution of anatomically and genetically modern populations at this time. Viewed in these terms, the subsequent elaboration of these symbolic patterns and the emergence of a range of new technological, economic, and social patterns reflected in the archaeological evidence from Blombos, Klasies River, and elsewhere, could be seen simply as a gradual working out of these new cognitive capacities under the stimulus of various kinds of environmental, demographic, or social pressures, in much the same way as that reflected in the later emergence of fully agricultural communities (53, 54, 61, 68, 88).
The alternative, of course, would be to visualize the trajectory of human cognitive evolution as an inherently more complex process, involving potentially a series of successive and cumulative changes in brain capacities, dependent on a succession of genetic mutations affecting various aspects of brain function and organization (63–67, 89, 90). Recent studies of the Microcephalin and FOXP2 genes (63, 64) have now effectively demonstrated the possibility of such mutations, potentially at various points since the emergence of genetically modern humans. Clearly, if there had been a further genetic mutation involving cognitive capacities ≈80,000 B.P., this could provide a further potential explanation for the emergence of significantly new patterns of technology, social organization, and symbolic expression reflected in the archaeological evidence from the African sites.
The problem of adequately testing these speculations against hard archaeological data is, of course, one of the notorious dilemmas in studies of human cognitive evolution, epitomized by Renfrew’s (91) notion of the “Sapient paradox.” In other words, how do we formulate plausible archaeological tests for the emergence of new behavioral capacities, as opposed to the gradual elaboration and increasing complexity of technological and other behavioral patterns for which the necessary cognitive potentials had already long existed (68, 82, 84, 92)? One thing, however, is certain: If the evolutionary trajectories of the Eurasian Neanderthals and the African ancestors of modern populations had been separate over a span of at least 300,000 years [as all of the current genetic and skeletal evidence suggests (1, 12, 78, 93)], then the possibility of some significant changes in human neurological and cognitive capacities over this time range can in no way be ruled out. Even if the cognition of Neanderthals and other archaic populations was not “inferior” to that of modern humans, it could have been significantly different (66, 67, 80–83).
The Out of Africa Diaspora
The final, and most controversial, issue at present is exactly when and how these anatomically and genetically modern populations first spread from Africa to other parts of Asia and Europe. Here there are two main possibilities. The first is that the initial expansion occurred via North Africa and the Nile valley, with subsequent dispersals to both the west into Europe and to the east into Asia (69–71, 78, 94, 95). The second is that the initial dispersal was from Ethiopia, across the mouth of the Red Sea, and then either northward through Arabia or eastward along the south Asian coastline to Australasia—the so-called “southern” or “coastal” route (28, 69, 70, 96). The strongest evidence at present for the second hypothesis is provided by the mtDNA lineage-analysis patterns. These point strongly to the conclusion that there was only a single (successful) dispersal event out of Africa, represented exclusively by members of the L3 lineage and probably carried by a relatively small number of at most a few hundred colonists (2, 8, 28, 97). This lineage rapidly diversified into the derivative M, N, and R lineages, which are particularly well represented in modern Asian populations and which are estimated to have arrived and diversified further in southern Asia by at least 50,000 B.P. and possibly as early as 65,000 B.P. in Malaysia and the Andaman islands (8, 9, 28, 97). A similar conclusion has been drawn from recent studies of the Y chromosome evidence (97). This evidence would also conform well with the clear peak in the mtDNA distributions of Asian populations, dated broadly to ≈60,000 B.P. (23–25) (Fig. 1). This model, of course, would mean that the subsequent dispersals of anatomically and behaviorally modern populations into southwest Asia and Europe must have reached these areas substantially later, via western or central Asia (2, 8, 97).
The main problem posed by this scenario at present lies in the sparsity of well documented and well dated archaeological evidence for the early modern human colonization of Asia prior to ca.45,000 B.P., when we know that early colonists had reached parts of northern and southern Australia, best represented by the archaeological and skeletal finds from Lake Mungo in New South Wales (98–100). But clearly the spotlight is now directed strongly onto southern Asia to secure more direct evidence for this hypothetical early dispersal route (101, 102). Future discoveries in both mitochondrial and Y chromosome DNA research and, above all, archaeology, are awaited to provide the crucial tests for this hypothesis of the origins and dispersal of our own species (103).
Acknowledgments
I thank P. Forster, S. Matsumra, C. Stringer, H. Harpending, A. Rogers, T. Kivisild, P. Underhill, C. Marean, P. Endicott, A. Brooks, S. Ambrose, G. Sampson, O. Bar Yosef, C. Henshilwood, J.-J. Hublin, and M. Petraglia for discussions of points raised in the paper. I also thank D. Kemp for assistance with the illustrations, P. Forster for Fig. 2, and C. Henshilwood for Fig. 5. Research funds were provided by the British Academy and Corpus Christi College (Cambridge University, U.K.).
Abbreviation
- MSA
Middle Stone Age.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Note that claims for a reemergence of MSA-like technologies after the Howiesons Poort industries in South Africa (31) are not directly relevant to this model, because it is likely that by this time (ca. 50,000–55,000 B.P.) the initial dispersal from Africa had already taken place (8, 9, 28). Exactly what these post-Howiesons Poort MSA industries represent remains to be clarified.
References
- 1.Stringer C. Philos. Trans. R. Soc. London B. 2002;357:563–579. doi: 10.1098/rstb.2001.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster P. Philos. Trans. R. Soc. London B. 2004;359:255–264. doi: 10.1098/rstb.2003.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tishkoff S. A., Williams S. M. Nat. Rev. Genet. 2002;3:611–621. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- 4.Prugnolle F., Manica A., Bailloux F. Curr. Biol. 2005;15:159–160. doi: 10.1016/j.cub.2005.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran S., Deshpande O., Roseman C. C., Rosenberg N. A., Feldman M. W., Cavalli-Sforza L. L. Proc. Natl. Acad. Sci. USA. 2005;92:15942–15947. doi: 10.1073/pnas.0507611102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White T. D., Asfaw B., DeGusta D., Gilbert H., Richards G. D., Suwa G., Clark Howell F. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- 7.McDougall I., Brown F. H., Fleagle J. G. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 8.Kivisild T., Shen P., Wall D., Do B., Sung R., Davis K., Passarino G., Underhill P. A., Scharfe C., Torroni A., et al. PLoS Genetics. 2006;172:373–387. doi: 10.1534/genetics.105.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macaulay V., Hill C., Achilli A., Rengo C., Clarke D., Meehan W., Blackburn J., Semino O., Scozzari R., Cruciani T., et al. Science. 2005;308:1034–1036. doi: 10.1126/science.1109792. [DOI] [PubMed] [Google Scholar]
- 10.Underhill P., Passarino G., Lin A. A., Shen P., Lahr M. M., Foley R. A., Oefner P. J., Cavalli-Sforza L. L. Ann. Hum. Genet. 2001;65:43–62. doi: 10.1046/j.1469-1809.2001.6510043.x. [DOI] [PubMed] [Google Scholar]
- 11.Richards M., Macaulay V., Hickey E., Vega E., Sykes B., Guida V., Rengo C., Sellitto D., Cruciani F., Kivisild T., et al. Am. J. Hum. Genet. 2000;67:1251–1276. [PMC free article] [PubMed] [Google Scholar]
- 12.Krings M., Stone A., Schmitz R. W., Krainitzki H., Stoneking M., Pääbo S. Cell. 1997;90:19–30. doi: 10.1016/s0092-8674(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 13.Serre D., Langaney A., Chech M., Teschler-Nicoa M., Paunovic M., Mennecier P., Hofreiter M., Possnert G., Pääbo S. PLoS Biol. 2004;2:E57. doi: 10.1371/journal.pbio.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currat M., Excoffier L. PLoS Biol. 2004;2:e421. doi: 10.1371/journal.pbio.0020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eswaran V., Harpending H., Rogers A. R. J. Hum. Evol. 2006 doi: 10.1016/j.jhevol.2005.02.006. in press. [DOI] [PubMed] [Google Scholar]
- 16.Mellars P. Nature. 2004;432:461–465. doi: 10.1038/nature03103. [DOI] [PubMed] [Google Scholar]
- 17.Mellars P. Nature. 2006;439:931–935. doi: 10.1038/nature04521. [DOI] [PubMed] [Google Scholar]
- 18.Harpending H., Eswaran V. Science. 2005;309:1995–1997. doi: 10.1126/science.309.5743.1995b. [DOI] [PubMed] [Google Scholar]
- 19.Ray L., Currat M., Berthier P., Excoffier L. Genome Res. 2006;15:1161–1167. doi: 10.1101/gr.3708505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobling M. A., Hurles M. E., Tyler-Smith C. Human Evolutionary Genetics: Origins, Peoples, and Disease. New York: Garland; 2004. [Google Scholar]
- 21.Goldstein D. B., Chikhi L. Annu. Rev. Genomics Hum. Genet. 2002;3:129–152. doi: 10.1146/annurev.genom.3.022502.103200. [DOI] [PubMed] [Google Scholar]
- 22.Barbujani G., Goldstein D. B. Annu. Rev. Genomics Hum. Genet. 2004;5:119–150. doi: 10.1146/annurev.genom.5.061903.180021. [DOI] [PubMed] [Google Scholar]
- 23.Harpending H. C., Sherry S. T., Rogers A. R., Stoneking M. Curr. Anthropol. 1993;34:483–496. [Google Scholar]
- 24.Sherry S. T., Rogers A. R., Harpending H., Soodyall H., Jenkins T., Stoneking M. Hum. Biol. 1994;66:761–775. [PubMed] [Google Scholar]
- 25.Harpending H., Rogers A. Annu. Rev. Genomics Hum. Genet. 2000;1:361–385. doi: 10.1146/annurev.genom.1.1.361. [DOI] [PubMed] [Google Scholar]
- 26.Watson E., Forster P., Richards M., Bandelt H. J. Am. J. Hum. Genet. 1997;61:691–704. doi: 10.1086/515503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salas A., Richards M., Tomás D. L. F., Lareu M. V., Sobrino B, Sánchez-Diz P., Macaulay V., Carracedo A. Am. J. Hum. Genet. 2002;71:1082–1111. doi: 10.1086/344348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forster P., Matsumura S. Science. 2005;308:965–966. doi: 10.1126/science.1113261. [DOI] [PubMed] [Google Scholar]
- 29.Deacon H. J. In: The Human Revolution: Behavioural and Biological Perspectives on the Origins of Modern Humans. Mellars P. A., Stringer C. B., editors. Edinburgh, U.K.: Edinburgh Univ. Press; 1989. pp. 547–564. [Google Scholar]
- 30.Deacon H. J., Wurz S. In: Human Roots: Africa and Asia in the Middle Pleistocene. Barham L., Robson-Brown K., editors. Bristol, U.K.: Western Academic and Specialist Press; 2001. pp. 55–63. [Google Scholar]
- 31.Deacon H. J., Deacon J. Human Beginnings in South Africa: Uncovering the Secrets of the Stone Age. Cape Town, South Africa: David Philip; 1999. [Google Scholar]
- 32.Singer R., Wymer J. The Middle Stone Age at Klasies River Mouth in South Africa. Chicago, IL: Univ. of Chicago Press; 1982. [Google Scholar]
- 33.Parkington J., Poggenpoel C., Rigaud J.-P., Texier P.-J. In: From Tools to Symbols: From Early Hominids to Modern Humans. d’Errico F., Blackwell L., editors. Witswatersrand, South Africa: Wits Univ. Press; 2005. pp. 475–492. [Google Scholar]
- 34.McBrearty S., Brooks A. J. Hum. Evol. 2000;39:453–563. doi: 10.1006/jhev.2000.0435. [DOI] [PubMed] [Google Scholar]
- 35.Henshilwood C. S. In: Combining the Past and Present: Archaeological Perspectives on Society. Oestigaard T., Ansinset N., Saetersdar T., editors. Oxford, U.K.: Archaeopress; 2004. pp. 95–106. [Google Scholar]
- 36.Henshilwood C., Sealy J. C., Yates R. J., Cruz-Uribe K., Goldberg P., Grine F. E., Klein R. G., Poggenpoel C., Van Niekerk K. L., Watts I. J. Archaeol. Sci. 2001;28:421–448. [Google Scholar]
- 37.Henshilwood C. S., d’Errico F., Marean C., Milo R., Yates R. J. Hum. Evol. 2002;41:631–678. doi: 10.1006/jhev.2001.0515. [DOI] [PubMed] [Google Scholar]
- 38.Henshilwood C. S., d’Errico F., Yates R., Jacobs Z., Tribolo C., Duller G. A. T., Mercier N., Sealy J. C., Valladas H., Watts I., Wintle A. G. Science. 2002;295:1278–1280. doi: 10.1126/science.1067575. [DOI] [PubMed] [Google Scholar]
- 39.d’Errico F., Henshilwood C., Vanhaeren M., van Niekerk K. J. Hum. Evol. 2005;48:3–24. doi: 10.1016/j.jhevol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Mellars P. A. In: The Speciation of Modern Homo Sapiens. Crow T. J., editor. London, U.K.: British Academy; 2002. pp. 31–47. [Google Scholar]
- 41.Mehlman M. J. In: Cultural Beginnings: Approaches to Understanding Early Hominid Lifeways in the African Savannah. Clark J. D., editor. Germany: Habelt, Bonn; 1991. pp. 177–196. [Google Scholar]
- 42.Ambrose S. H. J. Archaeol. Sci. 1998;25:377–392. [Google Scholar]
- 43.Ambrose S. H. In: Thinking Small: Global Perspectives on Microlithic Technologies. Elston R., Kuhn S. L., editors. Washington, DC: Am. Anthropol. Assoc; 2004. pp. 9–29. [Google Scholar]
- 44.Klein R. G. Evol. Anthropol. 2000;9:7–36. [Google Scholar]
- 45.Mellars P. A. Curr. Anthropol. 1989;30:349–385. [Google Scholar]
- 46.Feathers J. K. J. Archaeol. Sci. 2002;29:177–194. [Google Scholar]
- 47.Tribolo C., Mercier N., Valladas H. In: From Tools to Symbols: From Early Hominids to Modern Humans. d’Errico F., Blackwell L., editors. Witswatersrand, South Africa: Wits Univ. Press; 2005. pp. 493–511. [Google Scholar]
- 48.Mellars P. A. In: The Human Revolution: Behavioural and Biological Perspectives on the Origins of Modern Humans. Mellars P. A., Stringer C. B., editors. Edinburgh, U.K.: Edinburgh Univ. Press; 1989. pp. 338–365. [Google Scholar]
- 49.Mellars P. A. Evol. Anthropol. 2005;14:12–27. [Google Scholar]
- 50.Bar-Yosef O. Annu. Rev. Anthropol. 2002;31:363–393. [Google Scholar]
- 51.Wurz S. S. Afr. Archaeol. Bull. 1999;54:38–50. [Google Scholar]
- 52.Piperno D. R., Weiss E., Holft I., Nadel D. Nature. 2004;430:670–673. doi: 10.1038/nature02734. [DOI] [PubMed] [Google Scholar]
- 53.Mellars P. A. In: Evolution of Social Behaviour Patterns in Primates and Man. Runciman W. G., Maynard-Smith J., Dunbar R. I. M., editors. London, U.K.: British Academy; 1996. pp. 179–202. [Google Scholar]
- 54.Read D. W., LeBlanc S. A. Curr. Anthropol. 2003;44:59–85. [Google Scholar]
- 55.Klein R. G. The Human Career. Chicago, IL: Univ. of Chicago Press; 1999. [Google Scholar]
- 56.Dansgaard W., Johnsen S. J., Clausen H. B., Dahl-Jensen D., Gundestrup N. S., Hammer C. U., Hvidberg C. S., Steffensen J. P., Sveinbjörnsdottir A. E., Jouzel J., Bond G. Nature. 1993;364:218–220. [Google Scholar]
- 57.Partridge T. C., DeMenocal P. B., Loprentz S. A., Paiker M. J., Vogel J. C. Quat. Sci. Rev. 1997;16:1125–1133. [Google Scholar]
- 58.Ambrose S. H. J. Hum. Evol. 1998;34:623–651. doi: 10.1006/jhev.1998.0219. [DOI] [PubMed] [Google Scholar]
- 59.Oppenheimer S. The Peopling of the World. London, U.K.: Constable; 2003. [Google Scholar]
- 60.Gathorne-Hardy F. J., Harcourt-Smith W. E. H. J. Hum. Evol. 2003;45:227–230. doi: 10.1016/s0047-2484(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 61.Shennan S. Genes, Memes, and Human History: Darwinian Archaeology and Cultural Evolution. London, U.K.: Thames & Hudson; 2002. [Google Scholar]
- 62.Binford L. R. Constructing Frames of Reference. Berkeley, CA: Univ. of California Press; 2001. [Google Scholar]
- 63.Enard W., Przeworski M., Fisher S. E., Lai C. S. L., Wiebe V., Kitano T., Monaco A. P., Pääbo S. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- 64.Evans P. D., Gilbert S. L., Mekel-Bobrov N., Vallender E. J., Anderson J. R., Vaez-Azizi L. M., Tishkoff S. A., Hudson R. R., Lahn B. T. Science. 2005;309:1717–1720. doi: 10.1126/science.1113722. [DOI] [PubMed] [Google Scholar]
- 65.Balter M. Science. 2005;309:1662–1663. doi: 10.1126/science.309.5741.1662. [DOI] [PubMed] [Google Scholar]
- 66.Lewis-Williams D. The Mind in the Cave. London, U.K.: Thames & Hudson; 2002. [Google Scholar]
- 67.Coolidge F. L., Wynn T. J. Anthropol. Res. 2004;60:55–73. [Google Scholar]
- 68.Henshilwood C., Marean C. Curr. Anthropol. 2003;44:627–651. doi: 10.1086/377665. [DOI] [PubMed] [Google Scholar]
- 69.Lahr M. M., Foley R. Am. J. Phys. Anthropol. 1998;41:127–176. doi: 10.1002/(sici)1096-8644(1998)107:27+<137::aid-ajpa6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Foley R., Lahr M. Cambridge Archaeol. J. 1997;7:3–36. [Google Scholar]
- 71.Bar-Yosef O. In: The Geography of Neandertals and Modern Humans in Europe and the Greater Mediterranean. Bar-Yosef O, Pilbeam D., editors. Cambridge, MA: Peabody Museum, Harvard Univ. Press; 2000. pp. 107–156. [Google Scholar]
- 72.Shea J. J. Evol. Anthropol. 2003;12:173–187. [Google Scholar]
- 73.Vandermeersch B. C. R. Acad. Sci., D, Sci. Nat. 1970;270:298–301. [Google Scholar]
- 74.Defleur A. Les Sépultures Moustériennes. Paris: Centre National de la Recherche Scientifique; 1993. [Google Scholar]
- 75.Belfer-Cohen A., Hovers E. Curr. Anthropol. 1992;34:463–471. [Google Scholar]
- 76.Hovers E., Ilani S., Bar-Yosef O., Vandermeersch B. Curr. Anthropol. 2003;44:491–522. [Google Scholar]
- 77.Inizan M. L., Gaillard J. M. Paléorient. 1978;4:295–306. [Google Scholar]
- 78.Hublin J.-J. In: The Geography of Neandertals and Modern Humans in Europe and the Greater Mediterranean. Bar-Yosef O., Pilbeam D., editors. Cambridge, MA: Peabody Museum, Harvard Univ. Press; 2000. pp. 157–182. [Google Scholar]
- 79.Clark J. D., Beyene Y., WoldeGabriel G., Hart W. K., Renne P. R., Gilbert H., Defleur A., Suwa G., Katoh S., Ludwig K. R., et al. Nature. 2003;423:747–752. doi: 10.1038/nature01670. [DOI] [PubMed] [Google Scholar]
- 80.Mithen S. Prehistory of the Mind. London, U.K.: Thames & Hudson; 1996. [Google Scholar]
- 81.Noble W., Davidson I. Human Evolution, Language and Mind: a Psychological and Archaeological Inquiry. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 82.Mellars P. A. Cambridge Archaeol. J. 1991;1:63–76. [Google Scholar]
- 83.Mellars P. A. The Neanderthal Legacy: An Archaeological Perspective from Western Europe. Princeton, NJ: Princeton Univ. Press; 1996. [Google Scholar]
- 84.Mellars P. A. In: The Origin and Diversification of Language. Jablonski N. G., Aiello L. C., editors. San Francisco, CA: Univ. of California Press; 1998. pp. 89–115. [Google Scholar]
- 85.Chase P. G. In: The Evolution of Culture. Dunbar R., Knight C., Power C., editors. Edinburgh, U.K.: Edinburgh Univ. Press; 1999. pp. 34–49. [Google Scholar]
- 86.Bickerton D. Language & Species. Chicago, IL: Univ. of Chicago Press; 1990. [Google Scholar]
- 87.Bickerton D. In: The Speciation of Modern Homo, Sapiens. Crow T. J., editor. London, U.K.: British Academy; 2002. pp. 103–120. [Google Scholar]
- 88.Cohen A. In: Prehistoric Hunter-Gatherers: The Emergence of Cultural Complexity. Price T. D., Brown J. A., editors. Orlando, FL: Academic; 1985. pp. 99–119. [Google Scholar]
- 89.Crow T. J. In: The Speciation of Modern Homo Sapiens. Crow T. J., editor. London, U.K.: British Academy; 2002. pp. 197–216. [Google Scholar]
- 90.Gopnik M., Dalalakis J., Fukuda S. E., Fukuda S., Kehayia E. In: Evolution of Social Behaviour Patterns in Primates and Man. Runciman W. G., Maynard-Smith J., Dunbar R. I. M., editors. London, U.K.: British Academy; 1996. pp. 223–249. [Google Scholar]
- 91.Renfrew C. In: Modelling the Early Human Mind. Mellars P., Gibson K., editors. Cambridge, U.K.: McDonald Institute for Archaeological Research, Cambridge Univ. Press; 1996. pp. 11–14. [Google Scholar]
- 92.Deacon T. W. The Symbolic Species. London, U.K.: (Penguin; 1997. [Google Scholar]
- 93.Beerli P., Edwards S. V. Evol. Anthropol. 2002;1(Suppl):60–63. [Google Scholar]
- 94.Tchernov E. In: Neandertals and Modern Humans in Western Asia. Akazawa T., Aoki K., Bar-Yosef O., editors. New York: Plenum; 1998. pp. 77–90. [Google Scholar]
- 95.Van Peer P. L’Anthropologie. 2006. in press. [Google Scholar]
- 96.Stringer C. Nature. 2000;405:24–27. doi: 10.1038/35011166. [DOI] [PubMed] [Google Scholar]
- 97.Endicott P., Metspalu M., Kivisild T. In: South Asia at the Crossroads. Petraglia M., Allchin B., editors. Heidelberg: Springer; 2006. in press. [Google Scholar]
- 98.O’Connell J. F., Allen J. J. Archaeol. Sci. 2004;31:835–853. [Google Scholar]
- 99.Bowler J. M., Johnston H., Olley J. M., Prescott J. R., Roberts R. G., Shawecross W., Spooner N. A. Nature. 2003;421:837–840. doi: 10.1038/nature01383. [DOI] [PubMed] [Google Scholar]
- 100.Mulvaney J., Kamminga J. Prehistory of Australia. London: Allen & Unwin; 1999. [Google Scholar]
- 101.Petraglia M. D., Alsharekh A. Antiquity. 2003;77:671–684. [Google Scholar]
- 102.James H. V. A., Petraglia M. D. Curr. Anthropol. 2005;46:S3–S27. [Google Scholar]
- 103.Mellars P. Science. 2006 in press. [Google Scholar]
- 104.Henshilwood C., d’Errico F., Vanhaeren M., van Niekerk K., Jacobs Z. Science. 2004;304:404. doi: 10.1126/science.1095905. [DOI] [PubMed] [Google Scholar]