Abstract
Infective stages of the protozoan parasite Leishmania spp. accumulate a class of β-1,2-mannan oligosaccharides as their major carbohydrate reserve material. Here, we describe the biosynthesis of Leishmania mannan. Mannan precursors were identified by metabolic labeling of Leishmania mexicana promastigotes with [3H]mannose. Label was initially incorporated into a phosphomannose primer and short phosphorylated β-1,2-mannan oligomers that were two to five residues long. Analysis of the mannan primer by Fourier transform ion-cyclotron resonance MS and various enzymatic and chemical treatments and comparison with authentic mannose (Man) phosphates indicated the presence of Man-α-1,4-cyclic phosphate. This primer was synthesized from Man-6-phosphate by means of Man-1-phosphate in a cell-free system. Short mannan chains containing the primer were subsequently dephosphorylated and then further elongated by GDP-Man-dependent transferases in vivo and in the cell-free system. The synthesis of this glycan primer likely constitutes a key regulatory step in mannan biosynthesis and is a potential target for antileishmanial drugs.
Keywords: biosynthesis, Fourier transform ion-cyclotron resonance MS, phosphoglycan, polysaccharide, virulence factor
Leishmania spp. are sandfly-transmitted protozoan parasites that cause a spectrum of diseases in >12 million people worldwide, ranging from self-limiting cutaneous lesions to lethal visceral infections. Although some progress has been made in developing treatments for leishmaniasis, severe problems remain with existing drugs in terms of cost, efficacy, toxicity, and the emergence of drug-resistant parasite strains (1, 2). Infection of the mammalian host occurs when nondividing, metacyclic promastigotes are injected into the skin of the mammalian host by the sandfly vector. Metacyclic promastigotes are internalized by a variety of phagocytic cells but primarily develop within macrophages after differentiating into the obligate intracellular amastigote stage in the mature phagolysosome compartment. Comparatively little is known about the metabolism of the amastigote stages that perpetuate disease in the mammalian host, although recent studies have highlighted the importance of carbohydrate metabolism and specific glycosylation pathways (3, 4).
Leishmania lack classic carbohydrate reserves, such as glycogen or starch, but synthesize a unique class of oligosaccharides composed of linear chains of β-1,2-linked mannose (Man) (5–8). Leishmania β-1,2-mannan clearly functions as a dynamic reserve material and accumulates to millimolar concentrations in infective promastigote and intracellular amastigote stages (8). Mannan also accumulates in parasites subjected to physiological stresses encountered in the macrophage (8), suggesting that it might be important for intracellular survival. Consistent with this notion, loss of expression of all Man-containing molecules in Leishmania mexicana by targeted deletion of the genes involved in GDP-Man biosynthesis resulted in complete loss of virulence in macrophages and susceptible mice (4, 8). In contrast, L. mexicana mutants that had defects in assembly of some or all of their Man-rich surface glycocalyx but that retained the capacity to synthesize mannan remained virulent in macrophages (9, 10). As a source of hexose equivalents for the glycolytic and pentose phosphate pathways, mannan may play a key role in maintaining the energy and redox balances of Leishmania, thereby enhancing parasite virulence in both the sandfly vector and macrophage.
Mannan is rapidly synthesized in vivo under conditions of hexose excess, although none of the steps in this pathway have been defined. The de novo synthesis of intracellular oligo- and polysaccharides generally requires a primer on which a nascent glycan chain is synthesized. Reserve polysaccharides, such as glycogen and starch, are assembled on a specific protein primer (11) or on the polysaccharide synthase itself (12). In contrast, disaccharides and short oligosaccharides are often assembled on sugar phosphates that are common intermediates in central carbon metabolism (13–16). For example, the major pathways of synthesis of sucrose and trehalose involve the transfer of glucose from UDP-glucose to either fructose-6-phosphate or glucose-6-phosphate, respectively, and the dephosphorylation of the corresponding phosphosaccharide (13, 17). Here, we have elucidated the pathway of de novo mannan biosynthesis and elongation in L. mexicana and reconstituted several key biosynthetic steps in a cell-free system. These studies show that mannan is assembled on a unique hexose-phosphate primer and that the unexpected complexity in mannan biosynthesis might be exploited in developing anti-Leishmania therapies.
Results
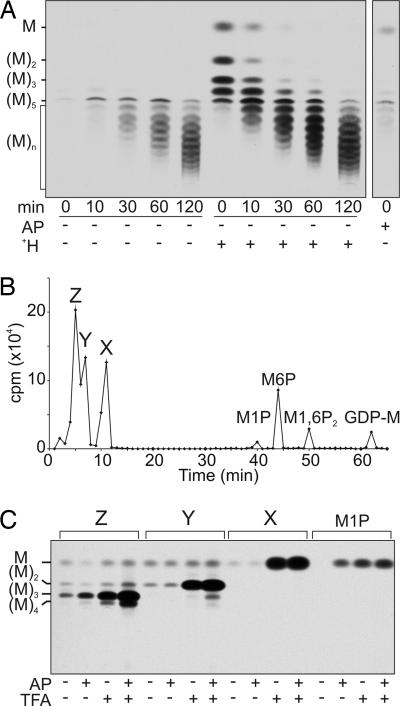
Mannan Is Assembled on an Acid-Labile Primer in Vivo.
L. mexicana Δpmi promastigotes were grown to stationary phase and metabolically labeled with [3H]Man. The Δpmi mutant is unable to convert [3H]Man-6-phosphate (Man-6-P) to other sugar phosphates, facilitating the labeling of Man-containing glycans. Moreover, Δpmi promastigotes grown in medium lacking exogenous Man lack preexisting pools of mannan, allowing the detection of de novo synthesized mannan intermediates. When mannan-depleted Δpmi promastigotes were pulse–chase labeled with [3H]Man, label accumulated in a series of neutral mannan oligomers containing five or more Man residues over a 2-h chase (Fig. 1A). The absence of smaller oligomers (containing two to four residues) raised the possibility that early intermediates were charged and removed during the desalting step in sample preparation. To investigate this possibility, the polar metabolite fractions were treated with alkaline phosphatase or subjected to mild acid hydrolysis before desalting, conditions that remove all phosphate monoesters or hydrolyze hexose-1-phosphates, respectively (18). Alkaline phosphatase treatment of pulse-labeled glycans did not generate any additional neutral mannan oligomers (Fig. 1A). In contrast, mild acid hydrolysis generated neutral Man and a series of labeled glycans that comigrated with β-1,2-mannan oligomers containing two to four residues on HPTLC (high-performance TLC) (Fig. 1A) and HPAEC (high-pH anion exchange chromatography) (data not shown). Label in these small oligosaccharides was quantitatively chased into species that comigrated with neutral mannan oligomers containing 5–50 residues (Fig. 1A). The labeled glycans could be digested with β-mannosidase, consistent with their assignment as oligomers of β-1,2-mannan (Fig. 2E). These data suggested that mannan is assembled on a charged, acid-labile primer that contains at least one Man residue and that this acid-labile substituent is removed after the mannan chain is extended beyond five residues.
Fig. 1.
An acid-labile phosphomannose residue primes de novo mannan biosynthesis. (A) L. mexicana Δpmi promastigotes were cultivated in medium lacking exogenous Man and pulse–chase labeled with [3H]Man. Polar metabolites were desalted before or after mild acid hydrolysis (H+) (at pH 1.0 for 10 min at 100°C) or alkaline phosphatase digestion (AP). The neutral glycans recovered after these treatments were resolved by HPTLC and detected by fluorography. The migration of β-1,2-mannan standards containing two to five residues are indicated. (B) Polar metabolites from the 10-min [3H]Man labeling were analyzed by HPAEC. The elution positions of Man-1-P (M1P), Man-6-P (M6P), Man-1,6-P2 (M1,6P2), and GDP-Man (GDP-M) are indicated. (C) Analysis of peaks X–Z from B. Aliquots of each peak were desalted before or after mild acid hydrolysis (5 mM TFA at 100°C for 5 min), alkaline phosphatase treatment (AP), or acid hydrolysis followed by alkaline phosphatase digestion. The neutral glycans released by these treatments were analyzed by HPTLC and detected by fluorography.
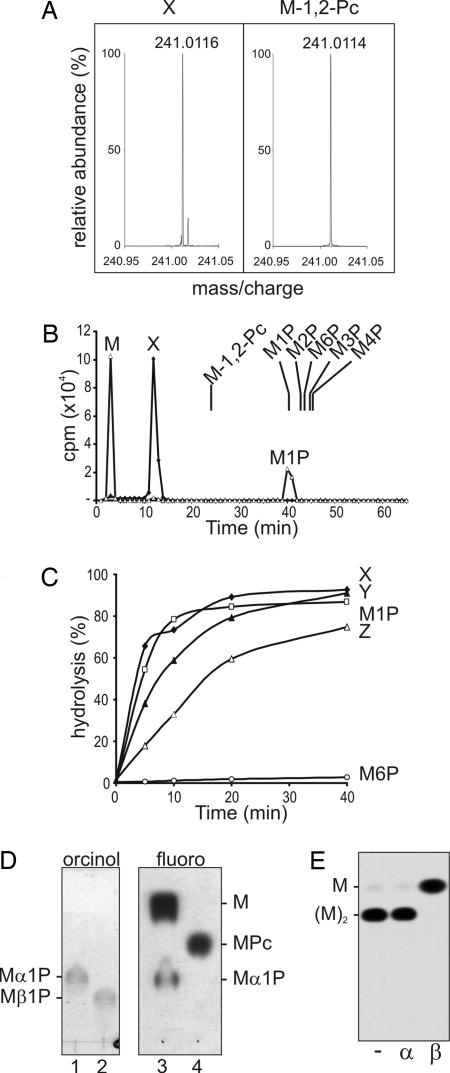
Fig. 2.
Characterization of the Man-α1,4-cyclic P primer. (A) Fourier transform ion-cyclotron resonance MS of authentic Man-β-1,2-cyclic P and peak X. (B) Mild acid hydrolysis of peak X releases Man-1-P. HPAEC analysis of peak X before (diamonds) and after (triangles) mild acid hydrolysis (50 mM TFA for 10 min at 100°C) under conditions that converted 50% of starting material to free Man (see C). The elution positions of authentic Man phosphates, Man-1-P, Man-2-P, Man-3-P, Man-4-P, Man-6-P, and Man-β-1,2-cyclic P, synthesized herein are indicated. (C) Kinetics of release of Man or mannan oligomers from peaks X–Z after mild acid hydrolysis. Peak X displayed the same acid sensitivity as Man-α-1-P, whereas Man-6-P was resistant to mild acid hydrolysis. (D) Peak X contains an α-linked mannosyl residue. Shown is HPTLC analysis of authentic Man-α-1-P (lane 1) and Man-β-1-P (lane 2) and Peak X after (lane 3) and before (lane 4) mild acid hydrolysis as described in B. Lanes 1 and 2 were stained with orcinol/H2SO4 (orcinol); lanes 3 and 4 were exposed to film (fluoro). (E) Peak Y contains terminal β-Man. Peak Y was acid-dephosphorylated and digested with mock control (−), jack bean α-mannosidase (α), or snail β-mannosidase (β). The products were analyzed by HPTLC and detected by fluorography.
The Mannan Primer Is Man-α-1,4-Cyclic P.
The in vivo-labeled mannan intermediates were analyzed directly by HPAEC using protocols optimized for the resolution of sugar phosphates. Three negatively charged glycans (peaks X, Y, and Z) were weakly retained by HPAEC and clearly resolved from previously characterized Man phosphates: Man-1-P, Man-6-P, Man-1,6-P2, and GDP-Man (Fig. 1B). All three peaks were resistant to alkaline phosphatase, whereas mild acid hydrolysis released neutral glycans that comigrated with Man (from peak X), Man-β-1,2-Man (from peak Y), and β-1,2-mannan oligomers containing three to four residues (from peak Z) (Fig. 1C). In contrast, Man-1-P was completely dephosphorylated by both alkaline phosphatase and acid hydrolysis (Fig. 1C). The elution properties of peaks X–Z on HPAEC and their susceptibility to acid hydrolysis, but not alkaline phosphatase digestion, suggested that they might contain a cyclic phosphate.
The presence of Man-cyclic phosphate was confirmed by high-resolution Fourier transform ion-cyclotron resonance (FTICR) MS of the purified peak X. FTICR MS revealed a quasimolecular ion at a m/z of 241.0116 [M-H]− (Fig. 2A). This mass suggested an elemental formula of C6H10O8P (Δ = +0.2 millimass unit), consistent with hexose-cyclic phosphate. The same mass was observed when authentic Man-β-1,2-cyclic P was analyzed under the same conditions (Fig. 2A). Despite having the same mass as Man-β-1,2-cyclic P, peak X had an elution time that was distinct from this standard on HPAEC (Fig. 2B). Moreover, whereas partial acid hydrolysis of Man-β-1,2-cyclic P generates Man-2-P and Man (ref. 19 and data not shown), partial acid hydrolysis of peak X generated two peaks that comigrated with Man-1-P and Man (Fig. 2B). The putative Man-1-P peak was clearly resolved from all other Man-monophosphates (Fig. 2B) and was converted to Man after extended mild acid hydrolysis, with the same kinetics as Man-α-1-P (Fig. 2C). This peak was also resistant to reduction with NaBH4 (data not shown), indicating that C1 was protected and comigrated with authentic Man-α-1-P rather than Man-β-1-P on HPTLC (Fig. 2D). These results show that peak X contains Man with an α-anomeric linkage to phosphate. Man-α-cyclic phosphates involving C1 and either C2, C3, or C6 are considered highly unlikely because of steric constraints, suggesting that peak X is Man-α-1,4-cyclic P. Attempts to synthesize this unique sugar have been unsuccessful to date. However, the presence of a seven-membered ring structure is consistent with the extreme acid lability of peak X (19).
Peaks Y and Z (containing Man2 and Man3–5, respectively) were also dephosphorylated after mild acid hydrolysis, although at slightly lower rates than peak X (Fig. 2C). Methylation linkage analysis of dephosphorylated peak Y afforded stoichiometric yields of terminal Man (2,3,4,6-tetra-O-methyl-di-O-1,5-acetyl-mannitol) and 2-O-substituted Man (3,4,6-tri-O-methyl-1,2,5-tri-O-acetyl-Man). Dephosphorylated peak Y was resistant to α-mannosidase digestion but was converted to Man after β-mannosidase digestion (Fig. 2E). These analyses show that a second β-Man residue is added to the C2 hydroxyl of the Man-cyclic phosphate primer.
Man-1-P Is the Precursor for the Mannan Primer.
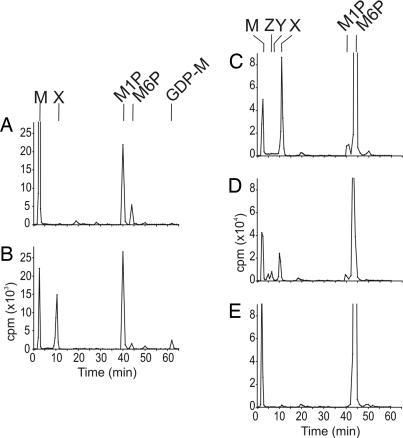
We next established a cell-free system to study the biosynthesis of the mannan-cyclic phosphate primer. We have found that incubation of cytosolic extracts of L. mexicana Δpmm promastigotes (lacking phosphomannomutase) with GDP-[3H]Man resulted in the formation of [3H]Man-1-P by means of the action of GDP-Man pyrophosphorylase (Fig. 3A). Some [3H]Man-6-P and [3H]Man was also generated by the cytosolic extract, indicating the presence of an alternative mutase and phosphatase activity, respectively, in this mutant (Fig. 3A). Although peak X was not synthesized by this cytosolic fraction, addition of equal cell equivalents of the particulate fraction resulted in efficient labeling of peak X (Fig. 3B). Addition of unlabeled Man-α-1-P (1 mM), but not Man-6-P or Man, competitively inhibited formation of [3H] peak X in the combined cytosol/particulate assay (data not shown), suggesting that the labeled Man-α-1-P is the immediate precursor to this species. In support of this conclusion, [3H]Man-6-P was converted to peak X in a phosphomannomutase-dependent manner. Incubation of the L. mexicana Δgmp membrane fraction with [3H]Man-6-P resulted in efficient synthesis of peak X (Fig. 3C), which was further mannosylated to form peak Y (Man2-cyclic P) and peak Z (Man3–4-cyclic P) when GDP-Man was included in the chase (Fig. 3D). In contrast, [3H]Man-6-P was not converted to peak X or higher oligomers when the particulate fraction from the L. mexicana Δpmm mutant was used (Fig. 3E). These data show that Man-6-P must first be converted to Man-1-P before synthesis of peak X can occur. They also preclude the possibility that GDP-Man is the immediate precursor of peak X; this was confirmed by the absence of peak X synthesis in the Δgmp particulate fraction that had been incubated with GDP-[3H]Man (Fig. 6, which is published as supporting information on the PNAS web site). Collectively, these data suggest that the promastigote particulate fraction contains all of the enzyme activities needed to convert Man-6-P to Man-α-1-P and then to peak X.
Fig. 3.
Biosynthesis of the mannan primer in vitro. (A) L. mexicana Δpmm promastigotes were hypotonically lysed, and cytosolic and particulate fractions were obtained by centrifugation. Incubation of the cytosolic fraction with GDP-[3H]Man for 1 h resulted in the formation of [3H]Man and [3H]Man-1-P, but not peak X. (B) The Δpmm cytosolic fraction was incubated with GDP-[3H]Man (30 min) before addition of the particulate fraction, and the reconstituted system was incubated for an additional 45 min. Addition of the particulate fraction stimulated the formation of peak X. (C and D) Incubation of L. mexicana Δgmp particulate fractions with [3H]Man-6-P with (D) or without (C) inclusion of unlabeled GDP-Man. (E) Incubation of L. mexicana Δpmm particulate fraction with [3H]Man-6-P. In all of these experiments, radiolabeled products were recovered after chloroform:methanol:water [1:2:0.8 (vol/vol)] extraction and biphasic partitioning in water:1-butanol and analyzed by HPAEC. The elution positions of peaks X, Y, Z, Man-1-P (M1P), Man-6-P (M6P), and GDP-Man (GDP-M) are indicated.
Mannan Is Elongated by Sequential Addition of Single Man Residues.
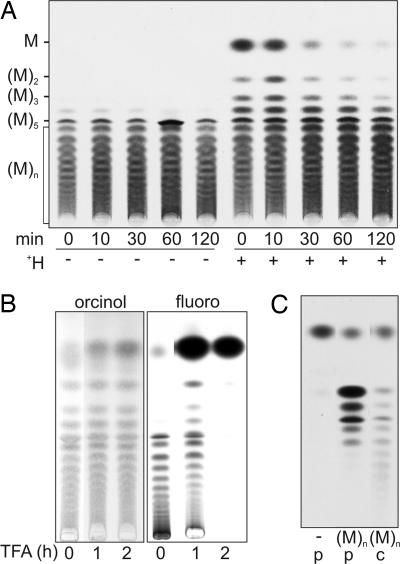
The steady-state pool of mannan is largely unphosphorylated and comprises oligomers that are 5–50 residues long (8). To investigate whether dephosphorylated mannan chains can also be elongated, L. mexicana Δpmi promastigotes were grown in media containing exogenous Man (to allow accumulation of intracellular pools) and then pulse–chase labeled with [3H]Man. HPTLC analysis of untreated and acid-treated extracts demonstrated that the preexisting pools of mannan were labeled to the same extent as de novo synthesized mannan chains (Fig. 4A). The [3H]Man added to nonphosphorylated mannan was quantitatively released with 0.2 M trifluoroacetic acid (TFA) under conditions that result in only partial hydrolysis of the unlabeled mannan pool (Fig. 4B). These results suggest that the elongation of existing mannan chains involves the addition of single (or few) Man residues rather than the processive addition of multiple Man residues. Mannan elongation was reconstituted in vitro by incubating cytosolic or particulate fractions with unlabeled mannan and GDP-[3H]Man (Fig. 4C). Interestingly, the particulate fraction only formed mannan oligomers that were 2–6 residues long, whereas the cytosolic fraction formed oligomers up to 12 residues long (Fig. 4C), possibly indicating the presence of distinct elongating activities in these fractions.
Fig. 4.
Elongation of preexisting pools of mannan. (A) L. mexicana Δpmi promastigotes were cultivated in RPMI medium 1640 supplemented with 200 μM Man and pulse–chase labeled with [3H]Man. Promastigotes were sampled at the indicated times during the chase in 200 μM Man, and polar metabolites were desalted before or after mild acid hydrolysis (+H). The labeled products were analyzed by HPTLC and detected by fluorography. (B) The neutral [3H]mannan fraction from A was hydrolyzed in TFA (0.2 M at 100°C) for 0, 1, and 2 h and then analyzed by HPTLC. Lanes 1–3 were stained with orcinol/H2SO4; lanes 4–6 were subjected to fluorography. (C) Incubation of particulate (p) and cytosolic (c) fractions from L. mexicana Δgmp promastigotes with buffer control (−) or unlabeled neutral β-1,2-mannan [(M)n] and GDP-[3H]Man.
Discussion
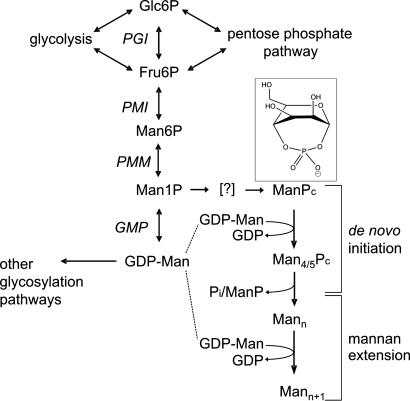
We have elucidated a previously uncharacterized pathway of oligosaccharide biosynthesis in Leishmania. The major features of this pathway are summarized in Fig. 5. Hexose phosphates from the glycolytic and pentose phosphate pathways can be interconverted to Man-6-P and Man-α-1-P. The latter is the precursor for both GDP-Man and the primer Man-α-1,4-cyclic P (ManPc in Fig. 5). The primer is extended with at least three β-1,2-linked Man residues, all donated from GDP-Man, before being dephosphorylated by a phosphatase or endomannosidase activity. De novo synthesized and preexisting neutral mannan chains can be further elongated by sequential additions of Man from GDP-Man, and both processes contribute to mannan accumulation in stationary-phase promastigotes. Remarkably, the de novo biosynthesis of mannan and subsequent elongation reactions appear to be partially segregated between particulate and cytosolic fractions. The final products of these pathways, a mixed population of neutral mannan oligosaccharides that are 5–40 residues long, reach concentrations of 1–10 mM in the cytosol of pathogenic stages (8).
Fig. 5.
Schematic outline of mannan biosynthesis. Hexose phosphates derived from the glycolytic, gluconeogenic, and pentose phosphate pathways are converted to Man-6-P and Man-1-P. Man-α-1-P can be converted to either the mannan chain primer (ManPc) or to GDP-Man, the donor for mannan chain assembly. The conversion of Man-α-1-P to ManPc is predicted to be energetically unfavorable and to involve an as-yet-unidentified activated species (denoted by the brackets). MannPc species containing four to five Man residues are dephosphorylated and further elongated by sequential additions of single Man residues. (Inset) Structure of Man-α-1,4-cyclic P primer.
Many prokaryotes and eukaryotes synthesize disaccharides and/or short oligosaccharides that variously act as storage materials, signaling molecules, osmoticants, and/or compatible solutes, protecting intracellular proteins from aggregation or denaturation (13, 14, 17). In most cases, di/oligosaccharide synthesis is initiated by transfer of a sugar from a nucleotide-sugar donor to a sugar- or polyol-monophosphate (13, 15, 20). The involvement of a hexose-cyclic phosphate primer has not been reported in other pathways of oligosaccharide biosynthesis. The formation of Man-α-1,4-cyclic P from Man-α1-P is likely to be energetically unfavorable and to involve an activated intermediate (e.g., Man-α-1-pyrophosphate). Regardless of the mechanism of formation, a Man-α-1,4-cyclic P primer is likely to be fixed in a mannopyranose B1,4 boat or 3S1 skew conformation, enhancing the accessibility of the C2 hydroxyl to the initiating mannosyltransferase (Fig. 5 Inset). The formation of a specific primer, distinct from other intermediates in Man metabolism, would also provide an additional level of regulation of de novo mannan biosynthesis and utilization of the essential sugar donor GDP-Man. GDP-Man is the direct or indirect Man donor for a number of other important glycosylation pathways in Leishmania, including N-glycosylation, glycosylphosphatidylinositol biosynthesis, and phosphoglycosylation (4). Direct assembly of mannan chains on either Man-1-P or Man-6-P would be expected to have a direct effect on the downstream biosynthesis of GDP-Man and lead to global changes in glycosylation patterns. In contrast, assembly of mannan chains on the primer Man-α-1,4-cyclic P requires an additional step that effectively uncouples de novo mannan synthesis from GDP-Man biosynthesis and mannan elongation. The requirement for a specific primer may also ensure that de novo synthesis of mannan can be shut down during periods of mannan catabolism. Because mannan catabolism results in the formation of Man-1-P (unpublished data), the requirement for a specific primer would prevent a futile cycle of mannan catabolism and resynthesis under nutrient starvation conditions.
Despite the fact that the major steady-state pools of mannan accumulate in the cytosol (8), our data suggest that all of the enzymes involved in de novo mannan biosynthesis are localized within a particulate fraction. In contrast, enzymes involved in the elongation of preexisting pools of mannan partitioned into both the particulate and cytosolic fractions. The particulate fraction also contained phosphomannomutase activity, as evidenced by the efficient conversion of Man-6-P to Man-1-P and Man-α-1,4-cyclic phosphate. Phosphomannomutase is primarily associated with the cytosol (9), raising the possibility that de novo mannan biosynthesis is catalyzed by a particulate-bound enzyme complex that includes cytoplasmic proteins. Enzymes involved in the synthesis and degradation of glycogen in muscle cells have also recently been localized to non-membrane-bound particulate complexes that are enriched in cytoskeletal proteins (21). Recruitment of glycogen synthase and phosphorylase to these structures was associated with increased activity and was regulated by phosphorylation (21). Whether the enzymes involved in mannan metabolism associate with the cytoskeleton or other intracellular structures, such as glycosomes that harbor many of the enzymes involved in glycolysis, is not yet known.
Mannan is a major energy reserve in Leishmania and accumulates in pathogenic promastigote and intracellular amastigote stages that initiate and perpetuate infection in the mammalian host, respectively (8). L. mexicana mutants that are defective in the biosynthesis of mannan and other Man-containing glycoconjugates are unable to survive in macrophages and display increased sensitivity to heat shock (4, 8, 9), suggesting that mannan accumulation facilitates parasite survival in macrophages. In this study, we show that mannan accumulation can occur by means of the de novo assembly of mannan oligomers on the Man-cyclic phosphate or by extension of preexisting mannan chains. The surprising complexity of mannan biosynthesis in Leishmania is consistent with mannan having a central role in regulating parasite intermediary metabolism. Because the major steps in mannan synthesis can now be reconstituted in vitro, it will be possible to develop inhibitors that target this parasite-specific pathway to further characterize the functions of mannan and the utility of these compounds as anti-Leishmania agents.
Materials and Methods
Parasite Culture.
Promastigote stages of L. mexicana wild-type (MNYC/BZ/62/M379) and mutant strains lacking phosphomannose isomerase (Δpmi), phosphomannomutase (Δpmm), and GDP-Man pyrophosphorylase (Δgmp) (4, 9, 22) were grown in RPMI medium 1640 containing 10% FBS at 27°C. The growth medium for L. mexicana Δpmi promastigotes was routinely supplemented with 200 μM Man unless otherwise stated.
Metabolic Labeling of Mannan.
L. mexicana Δpmi promastigotes were grown to stationary phase, harvested by centrifugation (800 × g for 10 min), and washed twice in glucose-free RPMI medium 1640 containing 1% BSA. Promastigotes were suspended in glucose-free RPMI medium 1640 containing 1% BSA (108 cells per ml) and pulse-labeled with d-[2-3H]Man [50 μCi/ml and 21 Ci/mmol (1 Ci = 37 GBq)] at 27°C. After 5 or 10 min, promastigotes were rapidly pelleted (12,000 × g for 20 s) and resuspended in conditioned medium (2 × 107 cells per ml) containing unlabeled Man (200 μM). Parasites were incubated at 27°C, and aliquots were removed at the indicated times. After centrifugation (12,000 × g for 20 s), cell pellets were washed with ice-cold PBS (pH 7.5) and extracted in 380 μl of chloroform/methanol/water [1:2:0.8 (vol/vol)]. Insoluble material was removed by centrifugation (15,000 × g for 5 min), and the supernatant was dried under nitrogen. Polar metabolites, including mannan oligomers, were recovered in the lower aqueous phase after biphasic partitioning in water:1-butanol [1:2 (vol/vol)].
In Vitro Labeling of Mannan.
Stationary-phase L. mexicana promastigotes were harvested by centrifugation (800 × g for 10 min), washed in PBS, and suspended in ice-cold hypotonic lysis buffer (1 mM Hepes-NaOH, pH 7.4/2 mM EGTA/2 mM DTT/protease inhibitor mixture minus EDTA) (Roche Diagnostics). After 10 min on ice, lysed parasites were centrifuged (2,000 × g for 5 min at 4°C) and the supernatant (cytosol) was retained while the pellets were washed with assay buffer (5 mM Hepes-NaOH, pH 7.4/2 mM EGTA/5 mM MgCl2/1 mM MnCl2/1 mM DTT/protease inhibitor mixture minus EDTA). For experiments shown in Fig. 3 A and B, two aliquots of the cytosol fraction (70 μl; 4 × 107 cell equivalents) of the L. mexicana Δpmm mutant were incubated with GDP-[3H]Man (10 μl; 0.25 μCi) at 27°C. After 30 min, lysed cell pellets (10 μl) corresponding to 4 × 107 cell equivalents were added to one of the cytosol treatments and incubated for a further 45 min. The reactions were terminated by addition of 1-butanol (200 μl), and the aqueous phases were analyzed directly by HPAEC. For experiments shown in Fig. 3 C–E, the membrane fractions of hypotonically lysed L. mexicana Δgmp or Δpmm promastigotes were suspended in assay buffer (70 μl), and the reaction was initiated by addition of [3H]Man-6-P (1 μCi in 10 μl) and, in some cases, 1 mM GDP-Man. After incubation (27°C for 45 min), the reactions were terminated, and labeled polar metabolites were extracted as described above.
HPAEC and TLC.
Phosphosugars were analyzed on a CarboPac PA-1 column (4 × 250 mm preceded by a PA-1 guard column, Dionex) connected to a Dionex GP-50 gradient pump and ED50 integrated pulsed amperometric detector. Data were processed with chromeleon 6.50 software (Dionex). The CarboPac column was eluted with 1 mM sodium hydroxide (E1) and 1 mM sodium hydroxide containing 1 M sodium acetate (E2) by using the gradient T0 = 2% (vol/vol) E2, T15 = 2% (vol/vol) E2, T30 = 10% (vol/vol) E2, T40 = 20% (vol/vol) E2, T55 = 100% (vol/vol) E2, and T65 = 100% (vol/vol) E2 at a flow rate of 1 ml/min. Radioactivity was determined by scintillation counting. Neutral glycans and phosphorylated sugars were further analyzed on Silica Gel 60 aluminum-backed HPTLC or TLC sheets (Merck), respectively. Neutral glycans were resolved in 1-butanol:ethanol:water [4:3:3 (vol/vol)], and phosphorylated sugars were resolved in ethyl acetate:methanol:water [2:2:1 (vol/vol)] and 2% acetic acid. Unlabeled sugars were detected with orcinol/H2SO4, and radiolabeled species were detected with EN3HANCE spray (PerkinElmer Life Sciences) and exposure to Biomax MR film (Kodak).
Chemical and Enzymatic Analysis.
Samples (≈0.01 mM) were dissolved in methanol:water [1:1 (vol/vol)] and infused (4 μl/min) into a Finnigan hybrid linear quadrupole ion trap-Fourier transform (LTQ-FT) mass spectrometer (Thermo Electron, San Jose, CA) equipped with an electrospray ionization source. The ions of interest were first mass selected in the linear ion trap and then mass analyzed in the ion-cyclotron resonance cell of the Fourier transform mass spectrometer for accurate, high-resolution mass measurement. Typical source conditions were as follows: spray voltage of 2.6–3.3 kV, capillary temperature of 250–300°C, nitrogen sheath gas of 5–25 arbitrary units, tube lens voltage of −110 to −80 V, and capillary voltage of −40 to −20 V. Mass isolation was carried out by using standard isolation and excitation procedures of the “advanced scan features” in the LTQ-FT software. Phosphoglycans were digested with bovine intestinal mucosa alkaline phosphatase in 0.1 M NH4HCO3 (pH 8.0) at 37οC for 2 h. Digestions with snail β-mannosidase or jack bean α-mannosidase (Sigma) were performed in 0.1 M citric acid-sodium phosphate buffer (pH 4.5) (0.5 units per 20-μl reaction) or in 0.1 M sodium acetate buffer (pH 5.0) (2 units per 20-μl reaction), respectively. Man-1-phosphate linkages were hydrolyzed for 5 min (100°C) in 5 mM trifluoroacetic acid or in HPAEC eluent adjusted to pH 1.0 with 1 M HCl. Glycosidic linkages were partially hydrolyzed in 0.2 M TFA (1 or 2 h at 100οC). Dephosphorylated peaks X–Z were permethylated with NaOH and iodomethane, and permethylated alditol acetates were analyzed as described in ref. 23. Relative response factors were determined by reference to dimannose standards containing α1–2, α1–3, and α1–6 linkages (Sigma).
Synthesis of Phosphomannose Standards.
Man-3-P and Man-4-P were synthesized from the corresponding selectively acetylated 3-hydroxy and 4-hydroxy Mans by sequential phosphorylation and deprotection. β-d-Man-1-P was prepared from acetylated β-d-mannosyl diphenyl phosphate. β-d-Man-1,2-cyclic P was synthesized by dicyclohexylcarbodiimide-mediated dehydration of β-d-mannopyranosyl phosphate and d-Man-4-P, respectively. Details of each synthesis are provided in Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Ilg (Intervet Innovation, Schwabenheim, Germany) for generously providing the L. mexicana Man mutants. This work was supported by the Australian National Health and Medical Research Council, the Australian Research Council, and a Howard Hughes International Fellowship (to M.J.M.).
Abbreviations
- Man
mannose
- HPAEC
high-pH anion exchange chromatography
- HPTLC
high-performance TLC
- TFA
trifluoroacetic acid.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Croft S. L., Coombs G. H. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Croft S. L., Sundar S., Fairlamb A. H. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burchmore R. J., Rodriguez-Contreras D., McBride K., Merkel P., Barrett M. P., Modi G., Sacks D., Landfear S. M. Proc. Natl. Acad. Sci. USA. 2003;100:3901–3906. doi: 10.1073/pnas.0630165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garami A., Ilg T. EMBO J. 2001;20:3657–3666. doi: 10.1093/emboj/20.14.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Previato J. O., Mendonca-Previato L., Lewanczuk R. Z., Travassos L. R., Gorin P. A. Exp. Parasitol. 1982;53:170–178. doi: 10.1016/0014-4894(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 6.Previato J. O., Xavier M. T., Brazil R. P., Gorin P. A., Mendonca-Previato L. J. Parasitol. 1984;70:449–450. [PubMed] [Google Scholar]
- 7.Keegan F. P., Blum J. J. Mol. Biochem. Parasitol. 1992;53:193–200. doi: 10.1016/0166-6851(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 8.Ralton J. E., Naderer T., Piraino H. L., Bashtannyk T. A., Callaghan J. M., McConville M. J. J. Biol. Chem. 2003;278:40757–40763. doi: 10.1074/jbc.M307660200. [DOI] [PubMed] [Google Scholar]
- 9.Garami A., Mehlert A., Ilg T. Mol. Cell. Biol. 2001;21:8168–8183. doi: 10.1128/MCB.21.23.8168-8183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilg T., Demar M., Harbecke D. J. Biol. Chem. 2001;276:4988–4997. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 11.Francois J., Parrou J. L. FEMS Microbiol. Rev. 2001;25:125–145. doi: 10.1111/j.1574-6976.2001.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 12.Ugalde J. E., Parodi A. J., Ugalde R. A. Proc. Natl. Acad. Sci. USA. 2003;100:10659–10663. doi: 10.1073/pnas.1534787100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbein A. D., Pan Y. T., Pastuszak I., Carroll D. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 14.da Costa M. S., Santos H., Galinski E. A. Adv. Biochem. Eng. Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- 15.Borges N., Marugg J. D., Empadinhas N., da Costa M. S., Santos H. J. Biol. Chem. 2004;279:9892–9898. doi: 10.1074/jbc.M312186200. [DOI] [PubMed] [Google Scholar]
- 16.Karnezis T., McIntosh M., Wardak A. Z., Stanisich V. A., Stone B. A. Trends Glycosci. Glycotechnol. 2000;12:211–227. [Google Scholar]
- 17.Lunn J. E., MacRae E. Curr. Opin. Plant Biol. 2003;6:208–214. doi: 10.1016/s1369-5266(03)00033-5. [DOI] [PubMed] [Google Scholar]
- 18.Ilg T., Overath P., Ferguson M. A., Rutherford T., Campbell D. G., McConville M. J. J. Biol. Chem. 1994;269:24073–24081. [PubMed] [Google Scholar]
- 19.Khorana H. G., Tener G. M., Wright R. S., Moffatt J. G. J. Am. Chem. Soc. 1957;79:430–436. [Google Scholar]
- 20.Huber S. C., Huber J. L. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:431–444. doi: 10.1146/annurev.arplant.47.1.431. [DOI] [PubMed] [Google Scholar]
- 21.Prats C., Cadefau J. A., Cusso R., Qvortrup K., Nielsen J. N., Wojtaszewki J. F., Hardie D. G., Stewart G., Hansen B. F., Ploug T. J. Biol. Chem. 2005;280:23165–23172. doi: 10.1074/jbc.M502713200. [DOI] [PubMed] [Google Scholar]
- 22.Garami A., Ilg T. J. Biol. Chem. 2001;276:6566–6575. doi: 10.1074/jbc.M009226200. [DOI] [PubMed] [Google Scholar]
- 23.McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. J. Biol. Chem. 1990;265:19611–19623. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.