Abstract
T cell antigen receptor (TCR) triggering determines the fate of immature thymocytes. The affinity of the TCR for its endogenous peptide/MHC ligands serves as a signal for positive or negative selection through mechanisms that are still little understood. We have used a conformation-specific antibody to demonstrate that recognition of TCR ligands that lead to negative selection induces a conformational change in the TCR in situ. In contrast, this conformational change is elicited in only a small percentage of immature thymocytes during positive selection. Using a TUNEL assay, we demonstrate that the conformational change in the TCR is strongly linked to activation of programmed cell death in conditions leading to negative selection. Furthermore, the few conformational change-positive thymocytes detected in conditions that preferably lead to positive selection are also TUNEL-positive. Thus, the conformational change in the TCR may underlie the discrimination of ligands leading to positive and negative selection.
Keywords: signal transduction, thymic differentiation, negative selection
The T cell antigen receptor (TCR) complex is composed in most T cells of the ligand-binding TCRα and TCRβ subunits and the signal-transducing CD3γ, CD3δ, CD3ε, and CD3ζ (CD247) (1, 2). The differentiation of T cells is controlled by the transduction of pre-TCR and TCR signals. Differentiation of immature CD4+CD8+ double-positive (DP) to mature CD4+ or CD8+ single-positive thymocytes is initiated when the TCR-αβ heterodimer engages the peptide/MHC (pMHC) (2). A unique feature of the TCR is its ability to scan structurally similar pMHC ligands and transmit distinct biochemical signals depending on the strength of the ligand recognized (3, 4). In developing thymocytes, weak TCR ligands induce positive selection, and stronger ligands induce negative selection (5). However, mature T cells are tolerant to weak TCR ligands in the periphery, and they respond to strong ligands by activating a program of cell proliferation and cell differentiation. The differential responsiveness of immature and mature T cells to weak ligands constitutes the cornerstone of self-tolerance that is acquired in the thymus.
Many studies have focused on how the TCRαβ heterodimer communicates ligand engagement to the CD3 subunits, how the CD3 subunits translate this information into a specific cellular response, and how the TCR complex discriminates between strong and weak ligands. The engagement of the TCR with a weak ligand in mature T cells results in a distinct pattern of intracellular signaling compared with that induced by a full-agonist peptide. For example, stimulation with a weak ligand induces only partial phosphorylation of the CD3ζ and CD3ε chains and the recruitment, but not the phosphorylation, of the ζ-associated protein of 70 kDa, ZAP70 (6, 7). Hence, weak ligands activate only a subset of the downstream signaling pathways that are activated by an agonist peptide (8). Weak ligands bind to the TCR with a lower affinity and a higher dissociation rate than full agonists (9). These kinetic parameters are consistent with a model in which shorter occupancy of the TCR may provoke partial signaling. However, in addition to their different kinetics, occupancy of the TCR by weak ligands may produce structural rearrangement of the TCR that differs from that produced by nominal peptide ligands.
Whereas a conformational change in the TCR may be the earliest event that occurs upon TCR engagement (10), crystallographic analysis of complexes formed by the ectodomains of pMHC and TCRα/β reveal ligand-induced conformational changes only in the complementarity-determining regions (CDR) of the TCR-variable domains (11–13). However, these structural changes were thought to accommodate pMHC binding and, with one exception (14), are not accompanied by a corresponding conformational change in the constant domains of the TCR. Furthermore, the changes in the conformation of the CDR when TCRs bound variant pMHC ligands were only minor when compared with those provoked by nominal peptide antigens (11, 13). These crystallographic studies argue that conformational changes in the CDR loops may not be communicated to the distal domains of the TCR–CD3 complex.
Using a biochemical approach, we found that the TCR undergoes a conformational change in mature T cells when engaged by a strong, but not by a weak, stimulatory mAb (10). This conformational change uncovered a cryptic epitope in the cytoplasmic tail of CD3ε, revealing a polyproline sequence that serves as a binding site for the SH3.1 domain of the cytosolic adapter protein, Nck. Using this biochemical approach, a pull-down assay with GST fused to the SH3.1 domain, we recently showed that this conformational change is induced by positive- and negative-selecting pMHC ligands in vitro and that MHC is required (15). However, this biochemical assay is limited, because it does not discriminate between thymocyte subsets or identify which individual cells are responding to the different stimuli. Most importantly, the biochemical assay does not determine whether the conformational change is really taking place in vivo. However, we identified a mAb that recognizes the polyproline sequence in CD3ε (mAb APA1/1) and that can be used to detect the conformational change in peripheral lymphoid tissues both in vitro and in vivo (16). Here, we have used APA1/1 to characterize the spatial distribution of thymocytes undergoing the conformational change in the TCR during differentiation. We have examined their phenotype, the proximity of the cells to the pMHC ligand-presenting stromal cells, and their frequency in the context of positive and negative selection. Our results show that only a few, or none, of the TCRs of DP thymocytes undergo a conformational change in vivo to pMHC ligands when eliciting positive selection, but many do so in the presence of negative-selecting pMHC, suggesting that the conformational change in the TCR is elicited by strong stimuli in the thymus and could help to activate the negative-selection program.
Results
The TCR of only a Small Percentage of DP Thymocytes Is Undergoing a Conformational Change in Vivo.
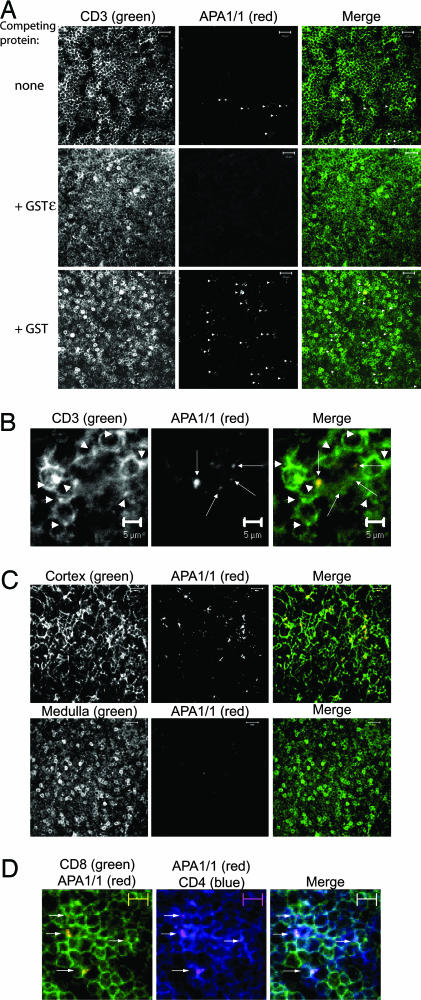
In contrast to the GST-Nck pull-down assay, immunostaining with mAb APA1/1 identifies the cells in which the TCR undergoes a conformational change upon engagement in situ. Immunohistochemistry on sections of the thymus of C57BL/6 mice revealed that most thymocytes were stained with the conventional anti-CD3 antibody 500A2, but only a few were stained with APA1/1 (Fig. 1A). APA1/1 staining was not distributed uniformly in the labeled thymocytes, but was, rather, concentrated in a region of the plasma membrane in which the TCR accumulates (Fig. 1B, arrows). However, we did frequently observe an accumulation of the TCR at one pole of the thymocyte without the concomitant exposure of the APA1/1 epitope (Fig. 1B, arrowheads). This pattern of staining offers evidence that APA1/1 staining is not simply a direct translation of TCR clustering. Furthermore, the specificity of the APA1/1 antibody was demonstrated by the absence of staining when thymus sections were incubated in the presence of an excess of GST protein fused to the cytoplasmic tail of CD3ε (GSTε), which contains the APA1/1 epitope (Fig. 1A). In similar conditions, APA1/1 staining was unaffected, as was the staining with the anti-CD3 500A2 in the presence of GST alone. These results indicate that the APA1/1 antibody specifically recognizes the polyproline epitope of the TCR in tissue sections of the thymus.
Fig. 1.
Binding to the APA1/1 epitope in the nontransgenic murine thymus. (A) APA1/1 is available in a small number of CD3+ thymocytes. Thymus sections (10 μm) from 6-week-old C57BL/6 mice were simultaneously stained with 500A2-Alexa Fluor 488 (anti-CD3) and APA1/1 antibodies, without competing protein, or in the presence of either 4 μg/ml GST or GSTε. The secondary antibody used was anti-mouse Ig Alexa Fluor 594 (red). (Scale bars, 20 μm.) APA1/1 labeling of thymocytes is indicated by arrowheads. (B) The APA1/1 epitope is distributed in a polarized manner in thymocytes. Thymus slices were double-stained as in A with a conventional anti-CD3 antibody and APA1/1. The arrows point to regions in the cells where the APA1/1 epitope is concentrated, whereas the arrowheads show concentrations of CD3 where APA1/1 does not bind. (Scale bars, 5 μm.) (C) APA1/1 antibody binds to the TCR in the cortex of the thymus but not in the medulla. Thymus sections from C57BL/6 mice were stained as in A but with a pancytokeratin antibody as a marker for the cortex and an anti-CD5 antibody as a marker for the medulla. (D) APA1/1 binds to CD4+CD8+ thymocytes. Thymus sections were triple-stained with APA1/1 and with antibodies against CD4 and CD8. Arrows indicate the presence of APA1/1-labeled cells. (Scale bars, 5 μm.) In A–C, the single-colored micrographs are shown in grayscale to present a wider range of staining.
To study the distribution of thymocytes in which the TCR has undergone a conformational change, sections were labeled with both APA1/1 and either a pancytokeratin antibody as a marker for the thymus cortex or anti-CD5 as a marker of the medulla (17). The majority of APA1/1-positive cells were concentrated in either the cortex or at the corticomedullar junction (>99%; Fig. 1C and data not shown). The medulla itself remained practically devoid of APA1/1 staining. In agreement with this cortical distribution, virtually all cells recognized by APA1/1 also expressed CD4 and CD8 (>99%, Fig. 1D; and see Table 1, which is published as supporting information on the PNAS web site, for details on quantitation). Only a few APA1/1-labeled cells were situated in the subcapsular area, and these were not recognized by antibodies against CD4 and CD8 (data not shown), suggesting that the APA1/1 epitope might be exposed at some point during the pre-T cell stage. These results indicate that most thymocytes in which the TCR polyproline epitope is exposed correspond to a small population of thymocytes that express CD4 and CD8 in the cortex and at the corticomedullar junction.
The TCR Undergoes a Conformational Change Under Conditions Leading to Negative Selection.
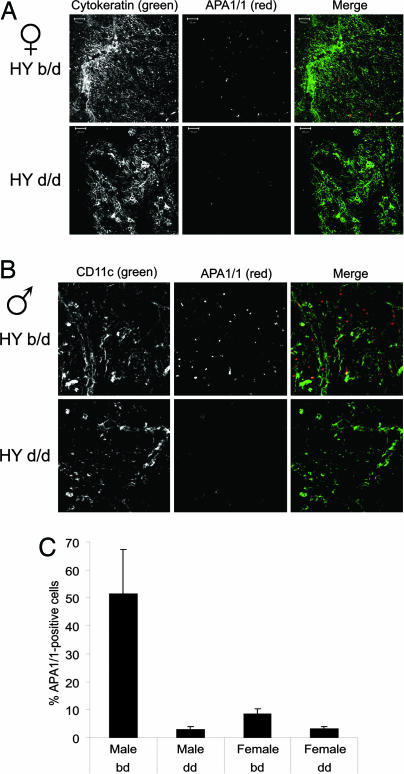
We previously demonstrated that exposure of the APA1/1 epitope in peripheral mature T cells occurred in response to strong, but not to weak, ligands (16). Because, in developing thymocytes, weak TCR ligands induce positive selection and stronger ligands induce negative selection (5), we next aimed to determine whether exposure of the APA1/1 epitope correlated with negative selection. To this end, we took advantage of the HY TCR transgenic mouse model. HY TCR-expressing thymocytes are positively selected in females with the H2-Db background, whereas these thymocytes are negatively selected in males of the same background (18). We detected a slight increase in the number of thymocytes recognized by APA1/1 in females with a selecting background (b/d) compared with females with nonselecting background (d/d, Fig. 2A and C). In males, the difference was much more striking, and there was a 20-fold increase in the number of thymocytes labeled by APA1/1 in the negative-selecting (b/d) vs. the nonselecting (d/d) background. As in nontransgenic mice (Fig. 1D), most APA1/1-positive thymocytes in male HY TCR transgenics were DP (see Fig. 5, which is published as supporting information on the PNAS web site). Indeed, up to 50% of the DP thymocytes were recognized by APA1/1 in the b/d background (Fig. 2 B and C). These results indicate that the conformational change in the TCR could contribute to activation of the negative-selection program.
Fig. 2.
The APA1/1 antibody is bound more strongly during negative selection, but not during positive selection, in HY TCR transgenic mice. (A) Thymus sections of female HY TCR transgenic mice on a positive-selecting background (b/d) or not (d/d). APA1/1-stained sections were counterstained with the pancytokeratin marker that labels the area where most thymocytes recognized by APA1/1 concentrate. (B) Thymus sections of male HY TCR transgenic mice that do or do not express the negatively selecting b background. Sections were counterstained with anti-CD11c, which labels the areas richest in thymocytes recognized by APA1/1. (C) Quantification of APA1/1 binding as a percentage of the APA1/1-labeled cells with respect to the total number of cells (quantified after ToPro staining). Values represent the mean and standard deviation of data generated in sextuplicate (three mice per experimental point, two fields per sample). In A and B, the single-colored micrographs are shown in grayscale to present a wider range of staining.
In both female and male HY TCR transgenics, most thymocytes exposing the APA1/1 epitope were in close contact with epithelial and thymic dendritic cells (Fig. 2 A and B). Furthermore, the APA1/1 staining was polarized toward the antigen-presenting cell (APC). These results were also obtained in nontransgenic mice (data not shown) and suggest that the conformational change in the TCR takes place in thymocytes that contact APCs in the thymus and that the conformational change occurs only at immune synapses.
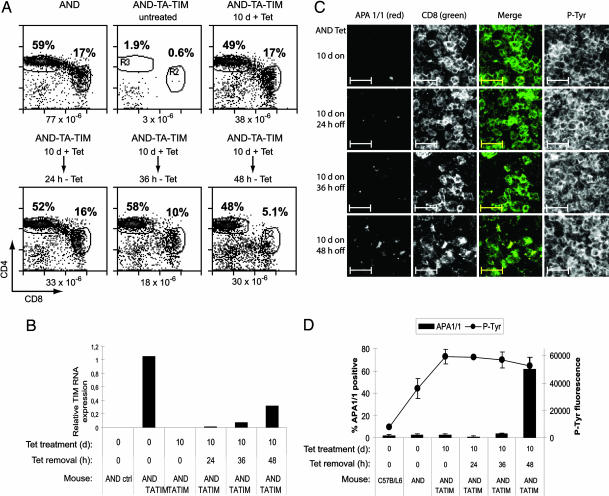
The HY TCR is MHC class I-restricted, and its cognate antigen is constitutively expressed in male mice. In this model, it is therefore not possible to time the expression of a strong TCR ligand and induction of the conformational change. To study the induction of the conformational change in a class II-restricted TCR and, most important, to do a timely study of both induction of the conformational change and negative selection, we analyzed APA1/1 binding in AND TCR transgenic mice that express an agonist peptide under control of a tetracycline-responsive promoter (19). In brief, these mice express a MHC class II-driven transactivator (TA) that is negatively regulated by tetracycline (Tet-off) and which directs the expression of the strong agonist MCCp embedded in the invariant chain (TIM). In the absence of tetracycline, the TIM-encoded MCCp can be presented by endogenous I-Ek and promotes the deletion of thymocytes bearing the AND TCR (Fig. 3A). However, when tetracycline is administered for 8–10 days, the distribution of thymic populations and the size of the thymus is similar to that of control AND TCR transgenic mice, wherein most thymocytes are being positively selected. If tetracycline administration is interrupted, TIM is reexpressed, and the DP population gradually disappears over the next 48 h (Fig. 3A). The expression of the negatively selecting TIM construct induced by the removal of tetracycline was verified by real-time PCR (Fig. 3B). To study the exposure of the APA1/1 epitope during positive and negative selection, tetracycline was given to RAG1-deficient AND-TA-TIM mice for 10 days and then removed for different time periods (Fig. 3C). In this way, more or less synchronized populations of thymocytes undergoing positive and negative selection should be generated. Thymus sections were stained with APA1/1 and anti-CD8 antibodies to evaluate the conformational change within the DP population. As a general marker for cell activation, thymus sections were stained with the anti-phosphotyrosine antibody 4G10. Removal of tetracycline and the concomitant expression of TIM (Fig. 3B) resulted in an increase in the number of CD8+ cells (predominantly DP thymocytes) recognized by APA1/1 (Fig. 3C). However, total tyrosine phosphorylation did not change upon tetracycline removal, suggesting that the negative-selecting ligand specifically induced the conformational change in the TCR and that this was not a result of generally increased signaling by the TCR. This effect was most clearly seen when APA1/1 binding was quantified in the DP cell population. When compared with nontransgenic mice, the expression of the anti-phosphotyrosine marker increased 4-fold in thymuses of AND transgenic and 7-fold in AND-TA-TIM mice maintained on tetracycline for 10 days. Nevertheless, the total tyrosine phosphorylation did not increase upon removal of tetracycline and expression of the TIM negative-selecting ligand (Fig. 3C). However, removal of tetracycline did cause an increase (24-fold) in APA1/1 labeling 48 h later. Furthermore, maximal TIM expression (Fig. 3B) correlated with maximal deletion of DP thymocytes (Fig. 3A) and maximal expression of APA1/1 (Fig. 3C). These results expand those obtained in the HY TCR transgenic system and suggest that the conformational change in the TCR is promoted by ligands that lead to negative, but not to positive, selection.
Fig. 3.
The APA1/1 epitope is strongly exposed in conditions leading to negative selection in AND TCR DP thymocytes. (A) Flow-cytometry analysis of thymic populations from AND TCR transgenic mice with or without the TA (tet-off) and TIM constructs. AND-TA-TIM triple-transgenic mice were given tetracycline in their drinking water for 10 days to repress the expression of the negative-selecting TIM construct (predominant positive-selection conditions). After the tetracycline treatment (10 days), some of the mice were deprived of tetracycline for the number of hours indicated to allow reexpression of the TIM construct. One lobule of each thymus was used for flow cytometry and PCR analysis and the other for immunohistochemistry (see C below) The percentage of DP and CD4+ SP thymocytes produced by each regime is indicated. (B) Expression of TIM mRNA measured by real-time PCR. (C) Sections from the intact lobules of the mice analyzed in A and B were triple-stained with APA1/1 and with antibodies for CD8 and anti-phosphotyrosine (4G10). The merged image shows the staining of APA1/1 within the CD8+ population. The single-colored micrographs are shown in grayscale to present a wider range of staining. (D) Quantification of APA1/1 binding and phosphotyrosine expression. As shown in C, sections were triple-stained with anti-CD4, anti-CD8, and APA1/1 to estimate the percentage of thymocytes within the DP population stained by APA1/1. Values are represented as bars and indicate the mean and standard deviation of data taken in triplicate (one mouse per experimental point, three fields per point). The 4G10 expression was measured as total fluorescence intensity by using the imagej program and expressed in arbitrary units.
The Conformational Change in the TCR Is Strongly Linked to Thymocytes That Are Being Negatively Selected.
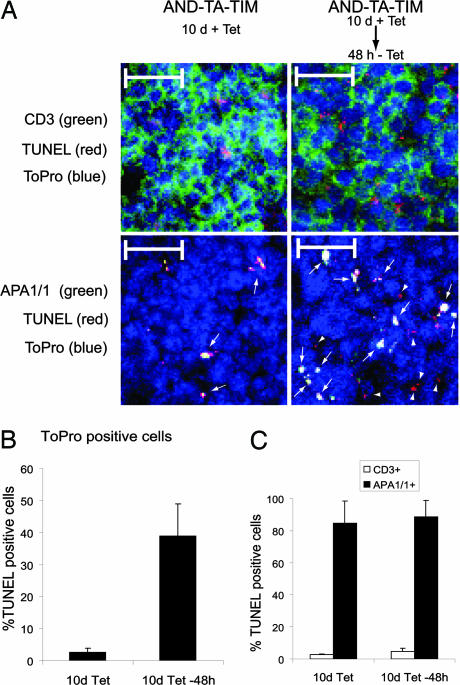
Up to this point, our results suggested a correlation between induction of the conformational change in the TCR and negative selection, because both processes are induced by strong TCR ligands. However, a direct linkage between the two events was missing. We therefore performed a double-staining of thymus sections of AND-TA-TIM mice under conditions of positive and negative selection (Fig. 3A) in search for APA1/1-positive and TUNEL-positive (apoptotic) cells. We found an association between the two markers in conditions that preferably lead to positive selection (10 days of tetracycline treatment) and in conditions that lead to negative selection (48 h after tetracycline removal) (Fig. 4A, arrows). Quantitation of the number of TUNEL-positive cells revealed a 13-fold increase in conditions leading to negative selection vs. conditions leading to positive selection (Fig. 4B), suggesting that expression of the TIM negative-selecting ligand (Fig. 3B) leads to increased apoptosis. The analysis of TUNEL-positive cells revealed a striking association with the APA1/1-positive population, in comparison with the general CD3-positive population (Fig. 4C, after 48-h tetracycline removal). The association between TUNEL and APA1/1 staining was not, however, maintained when dexamethasone, a TCR-independent apoptotic stimulus, was used (see Fig. 6, which is published as supporting information on the PNAS web site). These results indicated that exposure of the APA1/1 epitope was not a consequence of apoptosis and, together with those of Fig. 4, suggest that induction of the conformational change in the TCR is linked to the induction of programmed cell death. Most interesting, the analysis of thymuses in which DP thymocytes preferably undergo positive selection show that 82% of the APA1/1-positive thymocytes are TUNEL-positive, whereas only 3% of the CD3-positive cells are TUNEL-positive (Fig. 4C, 10 days of tetracycline treatment). This result suggests that the small percentage of thymocytes exposing the APA1/1 epitope in conditions that preferably lead to positive selection are indeed undergoing negative selection. The association of APA1/1 staining with apoptosis is not due to an aberrant exposure of the CD3ε epitope The association of APA1/1 staining with apoptosis, therefore, establishes a direct link between the conformational change in the TCR and negative selection.
Fig. 4.
Most APA1/1 epitope-expressing cells are already engaged in programmed cell death. (A) Sections from the intact lobules of AND-TA-TIM triple-transgenic mice shown in Fig. 3 were triple-stained with either APA1/1 or anti-CD3 antibody 500 A2 (green), for TUNEL (red), and with ToPro as a nuclei marker (blue). The merged image is shown and illustrates the presence of TUNEL and APA1/1 DP thymocytes (arrows) and the presence of TUNEL-positive but APA1/1-negative thymocytes (arrowheads). (Scale bars, 20 μm.) (B) Quantification of TUNEL-positive cells. The number of TUNEL-positive cells was counted from micrographs of sections as shown in A and referred to the total number of cells, estimated according to the staining with the nuclei marker ToPro. Three optical fields per mouse were counted with a number of 20–30 TUNEL-positive cells per field for the mouse treated with tetracycline for 10 days, and with a number of 100–200 TUNEL-positive cells per field for the mouse treated with tetracycline for 10 days and subsequently deprived of tetracycline for 48 h. (C) Quantification of the TUNEL-positive cells within the APA1/1-positive population and within the general CD3-positive population. The percentage of TUNEL-positive thymocytes within the APA1/1-positive and CD3-positive populations was estimated as in B.
Discussion
In this study, we show that the APA1/1 epitope is displayed in the TCR of only a minority of thymocytes in nontransgenic mice. This result suggests that the TCR in only a fraction of the total thymic population undergoes the conformational rearrangement at any given time. The use of cortical and medullar markers as well as the use of anti-CD4 and anti-CD8 antibodies enabled us to define the population of thymic cells recognized by APA1/1. These cells were predominantly DP thymocytes that are present in the cortex and the corticomedullar junction, in close contact with epithelial and dendritic cells. The phenotype and the location of the APA1/1-labeled thymocytes strongly suggest that the conformational change in the TCR is linked to the processes of positive and/or negative selection. We have recently shown, using the GST-Nck pull-down assay, that both positive- and negative-selecting pMHC ligands induce the conformational change in the TCR in vitro (15). These results indicate that this conformational change distinguishes between null and signaling pMHCs but not between pMHC ligands inducing positive and negative selection. Accordingly, the conformational change in the TCR would not predict the TCR signal strength. Although these data appear to contradict the results obtained with the APA1/1 antibody in mature T cells (16), they could be explained by the observation that the TCR on thymocytes is much more sensitive to pMHC as compared with the TCR of mature T cells (20). The APA1/1 antibody recognizes the proline-rich sequence in the tails of human and murine CD3ε (ref. 21 and data not shown) only after engagement of the TCR (16). We showed that the epitope recognized by APA1/1 is displayed only in immunological synapses when the antigen-presenting cell is loaded with a full, but not a partial, agonist. Because partial agonists promote positive selection in the thymus and full agonists promote negative selection, it was important to determine whether the conformational change in the TCR of thymocytes was correlated with signal strength, as in mature T cells. The association of the conformational change with negative selection was apparent in the HY TCR transgenic model and in an AND TCR triple-transgenic mouse line that expresses the strong MCCp agonist regulated by a tetracycline-responsive promoter. In both class I-restricted (HY) and class II-restricted (AND) TCR transgenic models, we observed a correlation between the expression of the strong negative-selecting agonists and the conformational change in the TCR. The TUNEL and APA1/1 staining does, however, show a direct relation between induction of the conformational change and negative selection in thymuses of AND TCR triple-transgenic mice (Fig. 4). Most interesting, the few APA1/1-positive thymocytes detected in conditions that preferably lead to positive selection are TUNEL-positive, suggesting that these thymocytes are also being negatively selected. Because the AND TCR triple-transgenic mice are in a RAG1-deficient background, all DP thymocytes should bear the AND TCR and could be expected to be positively selected in the absence of the strong MCCp agonist. It is, nevertheless, possible that some leaky expression of MCCp, even in the presence of tetracycline or the natural negatively selecting ligands in the thymus, account for the finding of APA1/1 and TUNEL double-positive cells. Moreover, the existence of some degree of negative selection in AND TCR transgenic mice has been suggested (22, 23).
It has been shown that positively selecting ligands induce a low but sustained activation of extracellular signal-regulated kinase (ERK), whereas negatively selecting ligands induce a robust but brief peak of ERK activity (20, 24). More recently, it has been shown that inhibition of ERK activity, even 24 h after exposure to a positively selecting ligand, blocks maturation of CD8 single-positive thymocytes (25), suggesting that a sustained ERK activation is required for positive selection. Because induction of the conformational change in the TCR is a very early event (10, 16), it was not expected that expression of the conformational change marker (APA1/1) was coincident with expression of a marker for a late event such as apoptosis. The strong linkage between expression of the APA1/1 epitope and TUNEL-positive cells suggests that the TCR undergoes a sustained conformational change in conditions leading to negative selection.
Unlike our earlier study based on the pull-down assay (15), the APA1/1 immunohistochemical data from mature T cells (16) and thymocytes (this study) suggests that the conformational change in the TCR reflects the engagement of strong agonists. The lower rate of dissociation from the TCR is a characteristic that distinguishes strong agonists from weak agonists. According to our results, the kinetic parameters of the TCR–pMHC interaction could be directly translated by the TCR into a conformational change. Thus, the short occupancy of the TCR is correlated with a lower probability of the CD3 tails undergoing a conformational change, whereas occupancy for a longer period results in a sustained rearrangement of the CD3 tails. Therefore, the conformational change in the TCR would be a direct result of sustained agonist binding. A long exposure of the polyproline epitope could be responsible for the activation of a specific set of signaling cascades that might lead to full activation in mature T cells or to apoptosis in DP thymocytes. However, it is still unclear what signaling cascades may be activated as a direct consequence of the exposure of this epitope. So far, the conformational change has been described only for the cytoplasmic tail of CD3ε. However, it seems likely that the tails of other CD3 subunits will also be rearranged upon activation (26), although this result remains to be demonstrated in vivo. If the conformational change influences the tyrosine phosphorylation of the CD3 immunoreceptor tyrosine-based activation motifs, it could constitute an additional mechanism to transduce the strength of interaction with pMHC ligands into different T cell responses. Nevertheless, the only change so far demonstrated is the exposure of the proline-rich sequence in CD3ε. We have identified the association of Nck with this sequence, showing that it binds to CD3ε through its N-terminal SH3.1 domain (10). Whether Nck recruitment to the proline-rich sequence of CD3ε is directly responsible for “sensing” the quality of the ligand and for promoting negative selection remains unknown.
The importance of the CD3ε proline-rich sequence in thymic development has recently been brought into question (27). Bone marrow chimeras in CD3ε-deficient mice have been used to express a CD3ε mutated in the proline-rich sequence before studying T cell development. No gross abnormalities were observed in the distribution of thymic populations, in accordance with our observation that the conformational change is not elicited during positive selection. Unfortunately this system does not offer information regarding the role of the CD3ε proline-rich sequence in negative selection because it was not studied in transgenic TCR models (27).
Therefore, we propose that the direct recruitment of Nck to the TCR is necessary to activate the negative-selection program. Indeed, misshapen-Nck-interacting (NIK)-related kinase (MINK) is a serine–threonine kinase that binds to Nck (28) and that has been shown to selectively connect the TCR to a signaling pathway leading to negative but not positive selection (29). The results shown in this study linking the conformational change in the TCR with negative selection are in keeping with the proposed role for MINK (29), and they suggest that association of Nck-MINK with the TCR during negative selection depends on a conformational change in the TCR complex.
Methods
Cells and Mice.
TCR transgenic mice bearing a TCR responsive to the male HY antigen presented by H-2Db (HY) or a TCR responsive to MCCp presented by I-Ek (AND) have been described (30, 31). The AND-TA-TIM triple-transgenic mice express the AND TCR together with a TIM construct containing the I-Ek-restricted MCC epitope under regulation of a tetracycline-responsive promoter (Tet-off) (19). These mice were bred onto a RAG1-deficient H-2k-positive background. All mice were bred and maintained free of pathogens at our animal facility.
Antibodies and Other Reagents.
The anti-CD3ε mouse mAb APA1/1 has been described (32) and is commercially available from Upstate Biotechnology (Lake Placid, NY). Hamster anti-mouse CD3 Alexa Fluor 488 (clone 500A2), rat anti-mouse CD8α Alexa Fluor 488 (clone 5H10), and rat anti-mouse CD4 Alexa Fluor 647 (clone RM4–5) mAbs were all purchased from Caltag (South San Francisco, CA). The biotin anti-mouse CD5 was from BD Pharmingen, hamster anti-mouse CD3 145–2C11 hybridoma was obtained from the American Type Culture Collection, the FITC-labeled monoclonal anti-pancytokeratin (clone C11) was from Sigma, and the anti-phosphotyrosine (4G10) was from Upstate Biotechnology. Hamster anti-murine CD11c mAb N418 was a gift from C. Ardavín (Centro Nacional de Biotecnología).
Immunohistochemical Studies.
Thymuses were fixed with 4% paraformaldehyde in PBS, washed with PBS, and incubated overnight in a 30% sucrose solution in PBS at 4°C. The thymuses were embedded in optimal cutting temperature compound (Tissue-Tec; Miles Scientific, Naperville, IL), and, subsequently, 10-μm cryostat sections were obtained by using a 2800 Frigocut N cryostat (Leica). The sections were fixed again with 4% paraformaldehyde in PBS, quenched with 20 mM NH4Cl, and stained for 1 h with antibodies in a solution containing 5% FBS. The secondary antibodies used were goat anti-mouse Alexa Fluor 488, Alexa Fluor 594 or Alexa Fluor 647 (Caltag), and the sections were finally mounted in fluorescent mounting medium (DAKO). The samples were visualized on a confocal LSM510 Meta laser scanning microscope (Zeiss) at ×40 magnification, with the pinhole adjusted to a 1.5-μm section. Detectors were set to obtain the optimal signals below saturation. All samples that are compared in a figure were prepared simultaneously, and the visualization and acquisition of the images were also performed the same day, with the same settings. Images were analyzed with the LSM 5 Image Browser and imagej software. Quantitation was done by a code-blind observer.
TUNEL Assay.
Apoptotic cell death was determined in 10-μm-thick cryostat sections of murine thymuses by using TUNEL staining according to the manufacturer’s instructions (Roche). dUTP labeling of DNA strand breaks was visualized on a confocal LSM519 Meta microscope.
Quantitative PCR.
Expression of the TIM construct was measured by real-time PCR as described (19).
Supplementary Material
Acknowledgments
We thank Carlos Ardavín (Centro Nacional de Biotecnologia, Madrid) Ed Palmer (University of Zurich, Zurich), Diane Mathis, and Christophe Benoist (Joslin Diabetes Center, Boston) for reagents and protocols and Matilde Cañelles, Kristin Hogquist, Mark Sefton, and Marisa Toribio for critical reading of the manuscript. This work was supported by Grant SAF2005-00937 from the Comisión Interministerial de Ciencia y Tecnología (to B.A.), a Formación de Profesorado Universitario Fellowship (to R.M.R.), and grants from the Ramón y Cajal Program (to H.M.v.S.) and Fundación Areces (to the Centro de Biología Molecular Severo Ochoa).
Abbreviations
- DP
CD4+CD8+double-positive
- pMHC
peptide antigen-loaded MHC
- TCR
T cell antigen receptor
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Alarcon B., Gil D., Delgado P., Schamel W. W. Immunol. Rev. 2003;191:38–46. doi: 10.1034/j.1600-065x.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 2.Terhorst C., Exley M., Franco R., Hall C., Kang J., Mueller B., Sancho J., She J., Wileman T. Year Immunol. 1993;7:1–24. [PubMed] [Google Scholar]
- 3.Werlen G., Hausmann B., Naeher D., Palmer E. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 4.Davis M. M., Krogsgaard M., Huppa J. B., Sumen C., Purbhoo M. A., Irvine D. J., Wu L. C., Ehrlich L. Annu. Rev. Biochem. 2003;72:717–742. doi: 10.1146/annurev.biochem.72.121801.161625. [DOI] [PubMed] [Google Scholar]
- 5.Starr T. K., Jameson S. C., Hogquist K. A. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Sloan-Lancaster J., Shaw A. S., Rothbard J. B., Allen P. M. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 7.Madrenas J., Wange R. L., Wang J. L., Isakov N., Samelson L. E., Germain R. N. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 8.Chau L. A., Bluestone J. A., Madrenas J. J. Exp. Med. 1998;187:1699–1709. doi: 10.1084/jem.187.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alam S. M., Davies G. M., Lin C. M., Zal T., Nasholds W., Jameson S. C., Hogquist K. A., Gascoigne N. R., Travers P. J. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 10.Gil D., Schamel W. W., Montoya M., Sanchez-Madrid F., Alarcon B. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 11.Hennecke J., Wiley D. C. Cell. 2001;104:1–4. doi: 10.1016/s0092-8674(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 12.Reiser J. B., Gregoire C., Darnault C., Mosser T., Guimezanes A., Schmitt-Verhulst A. M., Fontecilla-Camps J. C., Mazza G., Malissen B., Housset D. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph M. G., Wilson I. A. Curr. Opin. Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 14.Kjer-Nielsen L., Clements C. S., Purcell A. W., Brooks A. G., Whisstock J. C., Burrows S. R., McCluskey J., Rossjohn J. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 15.Gil D., Schrum A. G., Alarcon B., Palmer E. J. Exp. Med. 2005;201:517–522. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risueño R. M., Gil D., Fernandez E., Sanchez-Madrid F., Alarcon B. Blood. 2005;106:601–608. doi: 10.1182/blood-2004-12-4763. [DOI] [PubMed] [Google Scholar]
- 17.Canelles M., Park M. L., Schwartz O. M., Fowlkes B. J. Nat. Immunol. 2003;4:756–764. doi: 10.1038/ni953. [DOI] [PubMed] [Google Scholar]
- 18.von Boehmer H., Teh H. S., Kisielow P. Immunol. Today. 1989;10:57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]
- 19.van Santen H. M., Benoist C., Mathis D. J. Exp. Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogquist K. A. Curr. Opin. Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 21.Borroto A., Mallabiabarrena A., Albar J. P., Martinez A. C., Alarcon B. J. Biol. Chem. 1998;273:12807–12816. doi: 10.1074/jbc.273.21.12807. [DOI] [PubMed] [Google Scholar]
- 22.Matechak E. O., Killeen N., Hedrick S. M., Fowlkes B. J. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 23.Yelon D., Berg L. J. J. Immunol. 1997;158:5219–5228. [PubMed] [Google Scholar]
- 24.Werlen G., Hausmann B., Palmer E. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 25.McNeil L. K., Starr T. K., Hogquist K. A. Proc. Natl. Acad. Sci. USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aivazian D., Stern L. J. Nat. Struct. Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 27.Szymczak A. L., Workman C. J., Gil D., Dilioglou S., Vignali K. M., Palmer E., Vignali D. A. J. Immunol. 2005;175:270–275. doi: 10.4049/jimmunol.175.1.270. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y., Leo C., Yu S., Huang B. C., Wang H., Shen M., Luo Y., Daniel-Issakani S., Payan D. G., Xu X. J. Biol. Chem. 2004;279:54387–54397. doi: 10.1074/jbc.M404497200. [DOI] [PubMed] [Google Scholar]
- 29.McCarty N., Paust S., Ikizawa K., Dan I., Li X., Cantor H. Nat. Immunol. 2005;6:65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 30.Bluthmann H., Kisielow P., Uematsu Y., Malissen M., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. Nature. 1988;334:156–159. doi: 10.1038/334156a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaye J., Hsu M. L., Sauron M. E., Jameson S. C., Gascoigne N. R., Hedrick S. M. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon B., Ley S. C., Sanchez-Madrid F., Blumberg R. S., Ju S. T., Fresno M., Terhorst C. EMBO J. 1991;10:903–912. doi: 10.1002/j.1460-2075.1991.tb08023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.