Abstract
Behavioral studies show that the GABAergic system in the central amygdala (CeA) nucleus has a complex role in the reinforcing effects effects of ethanol and the anxiogenic response to ethanol withdrawal. Opioid peptides and nociceptin/orphanin FQ (nociceptin) within the CeA are implicated also in regulating voluntary ethanol consumption and ethanol relapse. Recently, we reported that basal GABAergic transmission was increased in ethanol-dependent rats, and that acute ethanol increases GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in CeA neurons from both naïve and ethanol-dependent rats to the same extent, suggesting lack of tolerance for the acute effect of ethanol. Here, we investigated the effect of nociceptin on IPSCs in CeA neurons and its interaction with ethanol effects on these GABA synapses. We found that nociceptin moderately decreased IPSC amplitudes, acting mostly presynaptically as it increased paired-pulse facilitation ratio of IPSCs and decreased miniature IPSC frequencies (but not amplitudes). Nociceptin also prevented the ethanol-induced augmentation of IPSCs in CeA of naïve rats. Interestingly, in CeA of ethanol-dependent rats, the nociceptin-induced inhibition of IPSCs was increased, indicating an enhanced sensitivity to nociceptin. Nociceptin also blocked the ethanol-induced augmentation of IPSCs in ethanol-dependent rats. Our data suggest that nociceptin has a role in regulating the GABAergic system and opposing the effect elicited by ethanol. Thus, nociceptin may represent a therapeutic target for alleviating alcohol dependence.
Keywords: alcohol, electrophysiology, ethanol dependence, miniature inhibitory postsynaptic currents, paired-pulse facilitation
The heptadecapeptide nociceptin/orphanin FQ (referred to here as nociceptin) is the endogenous ligand of the nociceptin/orphanin FQ peptide receptor (NOP) that was previously called opiate receptor-like 1 (ORL1). Nociceptin has a high structural homology with opioid peptides (1, 2) but does not bind to opioid receptors, and opioid peptides have no actions on NOP (2–4). Nociceptin and NOP are widely expressed in the brain, and their anatomical distribution differs from that of opioid peptides and their receptors (5–8). Still, at the cellular level the actions of nociceptin often appear to be similar to the actions of opioid peptides (9). Nociceptin induces an inwardly rectifying (IR) K+ conductance mediated by G-protein coupled NOP in amygdala (10, 11), hippocampal (12–16), and thalamic (17, 18) neurons, resulting in depressed cell excitability. Nociceptin also modulates other voltage-dependent K+ currents, including the M current (14) and Ca2+ currents (19, 20), and it reduces amplitudes of both non-NMDA receptor-mediated excitatory postsynaptic currents and GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in rat lateral amygdala (17).
At the behavioral level, the general pharmacological profile of nociceptin is different from, and in many cases opposite to, that of the opioids. For example, central administration of nociceptin blocks the behavioral stress response and can reverse opioid-mediated antinociception (21–23), and the systemic administration of a synthetic NOP agonist (Ro 64-6198) produces a marked anxiolytic-like effect (24). Recent studies (25, 26) also suggest a role for the nociceptin-NOP system in alcoholism in mediating relapse associated with ethanol exposure and stressful events. Also, nociceptin attenuates ethanol intake and ethanol-induced conditioned place preference in alcohol preferring rats, and this effect is blocked by administration of selective NOP receptor antagonists (23, 26, 27) but not by naloxone (28). Repeated systemic ethanol administration markedly alters basal nociceptin levels in several brain regions, including the amygdala (29), and these levels clearly differ in rodent lines with different alcohol preferences (30).
Behavioral studies in experimental animals indicate that the acute reinforcing effect of alcohol in nondependent animals is blocked by the administration of GABA and opiate receptor antagonists into the central amygdala (CeA) (31–33). Recently, we demonstrated that acute and chronic ethanol increases the size of IPSCs in rat CeA in part by increasing presynaptic GABA release (34, 35). Also, chronic ethanol-treated rats showed enhanced basal GABA transmission. Like ethanol, corticotropin-releasing factor (CRF) also enhances IPSCs, and the ethanol augmentation of IPSCs in mouse CeA appears to involve presynaptic CRF1 receptors (36). These peptide–GABAergic interactions may underlie the reinforcing effect of ethanol.
Based on this cellular and behavioral evidence, we hypothesized that endogenous nociceptin may function as a “brake” to limit the enhancement of GABAergic transmission in the neural circuitry involved in the reinforcing actions of ethanol. Therefore, we investigated the action of nociceptin on basal GABAergic transmission in CeA and on the ethanol-induced effects at GABAergic synapses by using electrophysiological techniques in an in vitro slice preparation. Our results indicate that nociceptin diminishes basal IPSCs and blocks the acute ethanol-induced augmentation of IPSCs in both control and ethanol-dependent rats by inhibiting the release of GABA. The nociceptin-induced inhibition of IPSCs was increased in neurons of ethanol-dependent rats, indicating increased sensitivity to nociceptin.
Results
Nociceptin Inhibition of GABAergic Synaptic Transmission in CeA Neurons.
Nociceptin (0.01–1 μM) did not significantly alter resting membrane potential, input resistance, or spike amplitudes in CeA neurons (n = 18; data not shown). By using current–voltage (I–V) analyses of direct nociceptin effects in the presence of tetrodotoxin (TTX), nociceptin had no effect on membrane potential or conductance at rest in the I–V relationships of most (75%) neurons (data not shown). However, nociceptin induced an outward current and increased conductance in 25% of tested CeA neurons, as described by Meis and Pape (10).
To determine whether nociceptin modulates GABAergic synaptic transmission, we evoked IPSCs by stimulating locally within the CeA. Nociceptin (500 nM) significantly (P < 0.05) reduced (to 86 ± 5% of control; n = 8) the amplitudes of evoked IPSCs (Fig. 1A) over all stimulus strengths, with recovery (104 ± 6% of control) on washout. However, because this synaptic effect could derive from either presynaptic or postsynaptic site of actions, we pursued multiple tests to determine whether nociceptin alters presynaptic transmitter release. In a first set of neurons, we examined paired-pulse facilitation (PPF) of IPSCs. Nociceptin significantly (P < 0.05) increased the PPF ratio of IPSCs (n = 5; Fig. 1B), suggesting decreased GABA release because changes in PPF ratio are inversely related to transmitter release (34, 37).
Fig. 1.
Nociceptin decreases basal GABAergic transmission in CeA. (A) Nociceptin (Noc) decreases the amplitude of evoked IPSCs in CeA neurons. (Upper) Current records of evoked IPSCs in a CeA neuron from a naïve rat. (Lower) In CeA neurons, nociceptin significantly (P < 0.05; n = 8) decreases the mean amplitudes of evoked IPSCs by 15% over all of the used stimulus strengths, with recovery on washout. (B) Application of nociceptin increases the PPF ratio of IPSCs in CeA neurons. (Upper) Current records of IPSCs in response to two stimuli 50 ms apart in a CeA neuron. (Lower) Pooled data of PPF ratios expressed as the second IPSC amplitude over the first. Nociceptin significantly (∗, P < 0.05) increases the mean PPF ratio by 56%, suggesting a presynaptic effect of nociceptin.
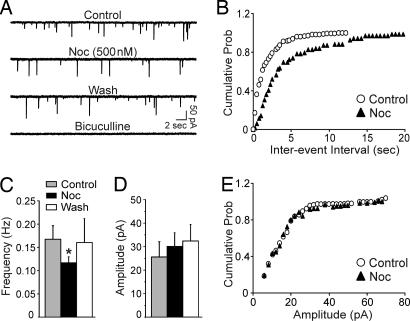
In a second set of experiments, we recorded miniature IPSCs (mIPSCs) by using whole-cell patch–clamp in the presence of TTX. Nociceptin significantly (P < 0.05) decreased the frequency of mIPSCs to 70 ± 7% of control (means: control, 0.17 ± 0.03 Hz; nociceptin, 0.12 ± 0.01 Hz; n = 7) (Fig. 2A and C) and significantly shifted the cumulative frequency distribution to longer interevent intervals (Fig. 2B), suggesting that nociceptin reduces the vesicular release of GABA. Nociceptin did not significantly (P > 0.05) alter the amplitude (Fig. 2 A and D), decay or rise time (data not shown) of mIPSCs.
Fig. 2.
Nociceptin decreases the frequency but not the amplitude of mIPSCs in CeA neurons. (A) Current traces from a representative CeA neuron from a naïve rat. Nociceptin decreases the frequency of the mIPSCs. (B) Cumulative frequency histogram for the neuron of A showing a shift to the right (lower frequencies) during the application of nociceptin. Data are means from a 2-min recording. (C) Mean (±SEM) frequency of mIPSCs (∗, P < 0.05; n = 7). Nociceptin significantly decreases the mean frequency of mIPSCs. (D) The same group of neurons shows that nociceptin does not alter the mean amplitude of mIPSCs. (E) Cumulative amplitude histogram from the neuron of A showing no changes in the distribution of mIPSC amplitudes.
Nociceptin Inhibits the Ethanol-Induced Increase in GABAergic Transmission.
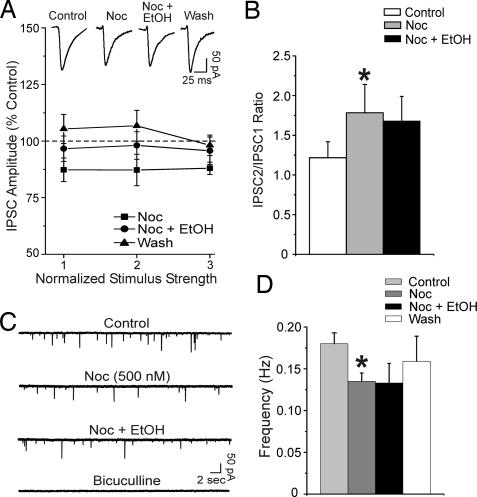
Recently, we reported that in CeA, acute ethanol increased the amplitude of evoked IPSCs, decreased their PPF ratio, and increased the frequency of mIPSCs, indicating an ethanol-induced increase in GABA release at CeA synapses (34, 35). Based on behavioral evidence (38) that nociceptin reduces the rewarding effects of ethanol, we hypothesized that nociceptin limits the ethanol enhancement of GABAergic transmission in the CeA. To test this hypothesis, we first pretreated CeA slices with 500 nM nociceptin for 10 min and then added 44 mM ethanol, a maximally effective concentration (34, 35). As described above (see also Fig. 1), nociceptin reduced the amplitude of evoked IPSCs to ≈88% of control. Interestingly, nociceptin prevented the ethanol-induced enhancement of IPSCs (Fig. 3A). Also, ethanol in the presence of nociceptin did not decrease the PPF ratio of IPSCs (Fig. 3B); rather, the nociceptin-induced increase of PPF ratio persisted. Also, in CeA neurons pretreated with nociceptin, ethanol applied during the neuropeptide did not increase the mIPSC frequencies, suggesting that nociceptin opposes the ethanol effect of releasing GABA (Fig. 3 C and D).
Fig. 3.
Pretreatment with nociceptin prevents the ethanol-induced enhancement of GABAergic transmission in CeA neurons. (A) (Upper) Representative recordings of evoked IPSCs in a CeA neuron. (Lower) Superfusion of nociceptin alone significantly (P < 0.05, n = 6) decreases the mean IPSC amplitudes to 88% of control (as in Fig. 1A) and prevents the enhancement of IPSCs induced by subsequent ethanol (compare with Fig. 4 and ref. 35) in CeA neurons. (B) Nociceptin also significantly (∗, P < 0.05; n = 6) increases the PPF ratio of IPSCs (as in Fig. 1B) and blocks the usual ethanol-induced decrease of PPF ratio (see Fig. 4B and ref. 35). (C) Nociceptin decreases the frequency of mIPSCs. Subsequent addition of ethanol does not increase the mIPSC frequency as normally seen in controls. (D) Mean (± SEM) frequency of mIPSCs for CeA neurons from naïve rats (∗, P < 0.05; n = 3). As in Fig. 2, nociceptin alone significantly decreased the mean mIPSC frequency to 75 ± 10% of control. The subsequent coapplication of ethanol with nociceptin did not alter the frequency of mIPSCs (77 ± 4% of control).
We also applied ethanol first and then superfused the neuropeptide. As reported in ref. 35, superfusion of ethanol alone significantly (P < 0.001) increased the mean amplitude of evoked IPSCs (here to 150 ± 8% of control; Fig. 4A) and significantly (P < 0.05) decreased the mean PPF ratio of IPSCs by 30 ± 8% (Fig. 4B) in CeA neurons. Nociceptin added to the artificial cerebrospinal fluid (ACSF) containing ethanol totally blocked the ethanol-induced enhancement of evoked IPSCs and also reversed the PPF ratio to preethanol levels (103 ± 7% of control; Fig. 4B).
Fig. 4.
Pretreatment with acute ethanol increases GABAergic transmission in CeA neurons, and subsequent nociceptin blocks this effect. (A) (Upper) Representative evoked IPSCs in a CeA neuron from a naïve rat. (Lower) Ethanol significantly (P < 0.05; n = 7) increases (to 150% of control) the mean IPSC amplitudes in CeA neurons. Nociceptin in the presence of ethanol abolishes the ethanol-induced facilitation of evoked IPSC amplitude. (B) (Upper) Current traces of paired IPSCs in a CeA neuron from a naïve rat. (Lower) Ethanol significantly (∗, P < 0.05) reduces by 30% the mean PPF ratio of IPSCs. Nociceptin completely blocks this ethanol effect on PPF. (C) Superfusion of ethanol significantly (∗, P < 0.05) increases mean mIPSC frequencies. Nociceptin that was added during the superfusion of ethanol totally blocks this ethanol effect.
We also repeated the same protocol for mIPSC studies. Ethanol significantly (P < 0.05) increased the mean mIPSC frequency to 178 ± 8% of control (means: control, 0.14 ± 0.03 Hz; ethanol, 0.25 ± 0.05 Hz; P < 0.05; n = 3; Fig. 4C), thus supporting the PPF data (Fig. 4B) and indicating an increased presynaptic release of GABA by acute ethanol (as in refs. 34 and 35). Nociceptin totally blocked this ethanol-induced increase of mIPSC frequencies (mean: 0.15 ± 0.06 Hz; Fig. 4C).
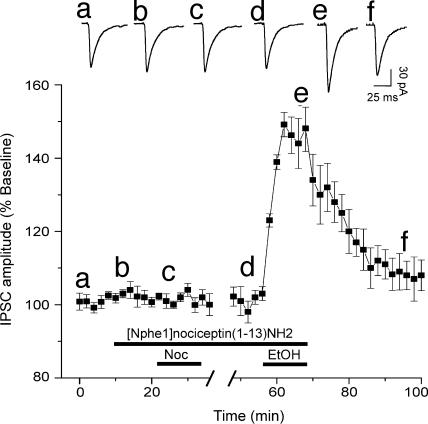
The expression of mRNA for NOP, as well as NOP-binding sites, are highly enriched in the amygdala (4, 8, 39, 40), indicating a functional role of the nociceptin/NOP system in the amygdala. Therefore, to determine whether NOPs regulate baseline evoked and spontaneous GABAergic transmission and to test their involvement in nociceptin effects, we tested a putative selective NOP antagonist ([Nphe1]nociceptin(1–13)NH2) (26, 41, 42) (Fig. 5). This antagonist (1 μM) alone had no effects (103% of control; P > 0.05; n = 5; Fig. 6E) on basal evoked IPSCs in CeA, suggesting lack of constitutive activation of NOP or a tonic activity of the endogenous nociceptin transmission in CeA of naïve rats. However, pretreatment of CeA neurons with the NOP antagonist totally blocked the nociceptin-induced inhibition of IPSC amplitudes but did not alter the ethanol-induced augmentation of IPSCs (Fig. 5). These data suggest that nociceptin exerts its effect through NOPs and that these receptors may not tonically regulate the ethanol-induced IPSC enhancement.
Fig. 5.
NOPs mediate nociceptin effects on CeA GABA synapses. (Upper) Representative evoked IPSCs recorded at different (a–f) treatment times, as shown in the time-course chart below. (Lower) Time-course of the mean effects of the sequential application of NOP antagonist together with nociceptin and then with ethanol (n = 5; stimulus intensity equal to half-maximal IPSC amplitude). The NOP antagonist blocks the nociceptin-induced inhibition of IPSC amplitudes but does not prevent the ethanol-induced enhancement of IPSCs. Lowercase letters match the time to recordings shown in Upper.
Fig. 6.
The nociceptin-induced decrease of evoked and spontaneous GABAergic transmission in CeA is larger in ethanol-dependent compared with naïve rats. (A) (Upper) Representative evoked IPSCs in a CeA neuron from an ethanol-dependent rat. (Lower) Nociceptin markedly decreases the mean IPSC amplitudes, whereas subsequent ethanol has no effect (nociceptin and ethanol curves are superimposable; n = 7), with recovery on washout. (B) (Upper) Representative evoked IPSCs in a CeA neuron from an ethanol-dependent rat. (Lower) Ethanol increases (to 146% of control; n = 6) the mean IPSC amplitudes. Subsequent nociceptin blocks this ethanol effect and further depresses the mean amplitude of IPSCs to 70% of control. (C) Pooled data of PPF ratios of IPSCs in CeA of ethanol-dependent rats. Nociceptin significantly (∗, P < 0.001; n = 6) increases the mean PPF ratio. Ethanol added to nociceptin does not decrease the PPF ratio. (D) In CeA neurons from ethanol-dependent rats, ethanol significantly (∗, P < 0.05; n = 6) increases the mIPSC frequency. Nociceptin not only blocks this ethanol effect on mIPSCs but also significantly (#, P < 0.05, compared with ethanol effect) decreases (to 75% of preethanol condition) the mean mIPSC frequency. (E) The NOP antagonist [Nphe1]nociceptin(1–13)NH2 does not significantly (P < 0.05; n = 5) alter the basal evoked IPSC amplitudes (stimulus intensity equal to half-maximal IPSC amplitude) in naïve rats, but significantly (P < 0.05; n = 7) increases their amplitudes in CeA neurons of ethanol-dependent rats. (E) In the same group of neurons as shown in D, nociceptin does not alter the mean amplitude of mIPSCs (n = 6).
Nociceptin Inhibition of IPSCs Is Greater After Chronic Ethanol.
There is abundant experimental evidence (in humans and animals) that implicates the endogenous opioid system in ethanol reinforcement (43). Note that nociceptin blocks the rewarding effects of ethanol (44). Also, activation of GABAA receptors in the CeA alters ethanol self administration only in ethanol-dependent rats (32, 33), suggesting that GABAergic transmission in this brain region is altered by chronic ethanol consumption (see also ref. 35). Recently, we reported increased baseline GABAergic transmission and a persistent acute ethanol-induced increase of GABA release in CeA neurons of ethanol-dependent rats, suggesting a lack of tolerance to the acute ethanol effects (35). Therefore, in this study, we tested the effects of nociceptin on basal GABAergic transmission after chronic ethanol exposure. The depressant effect of 500 nM nociceptin on basal evoked IPSC amplitudes was significantly (P < 0.05) stronger in CeA neurons from ethanol-dependent rats (to 65% of control, n = 7; Fig. 6A) than in those from naïve rats (to 86%; Fig. 1A). In CeA of ethanol-dependent rats, the nociceptin-induced increase in the mean PPF ratio of IPSCs was also significantly (P < 005) greater (to 199%; Fig. 6C) compared with that of naïve rats (to 156%; n = 6). As for naïve rats, in CeA from ethanol-dependent rats, acute ethanol added to nociceptin in the superfusate did not increase the evoked IPSC amplitudes and did not alter the PPF ratio of IPSCs (Fig. 6 A and C).
Note that the NOP antagonist [Nphe1]nociceptin(1–13)NH2 significantly (P < 0.05) increased (to 115%; n = 7; Fig. 6E) basal evoked IPSC amplitudes in CeA neurons of ethanol-dependent rats, suggesting constitutive activation of NOPs associated with ethanol-dependence that may account for the increased sensitivity of the CeA GABA system to nociceptin.
In CeA neurons of ethanol-dependent rats, 44 mM ethanol significantly increased (to 146%; n = 6) evoked IPSC amplitudes (Fig. 6B) to an extent that was equivalent to that in neurons from naïve rats (to 150%, see Fig. 4), suggesting lack of tolerance of GABAergic transmission to acute ethanol (35). Adding nociceptin to the solution containing ethanol not only totally blocked the ethanol augmentation of the evoked IPSCs but also inhibited the mean IPSC amplitudes to 70% of control. These combined findings suggest that the inhibitory effect of nociceptin is enhanced during ethanol dependence and may reflect an up-regulation of nociceptin system (e.g., increased expression of NOPs).
Also, we analyzed mIPSCs in CeA neurons from ethanol-dependent rats. Note that, as reported in ref. 35, the mean baseline frequency of mIPSCs was significantly (P < 0.001) greater in CeA neurons of ethanol-dependent rats (0.49 ± 0.05 Hz; n = 6; Fig. 6D) than in those of naïve rats (0.14 ± 0.03 Hz; Fig. 4), suggesting an increased vesicular GABA release after chronic ethanol. Also, we confirmed in this study the effect of acute ethanol on mIPSCs in CeA from ethanol-dependent rats (35); superfusion of ethanol enhanced the mean frequency of mIPSCs to 165 ± 16% (0.71 ± 0.08 Hz; n = 6) of control, supporting the PPF data that indicate a lack of tolerance for the increased presynaptic release of GABA by acute ethanol. In CeA neurons of ethanol-dependent rats, nociceptin not only totally blocked the ethanol-induced increase in frequency of mIPSCs but also significantly (P < 0.05) decreased the mean mIPSC frequency to 75 ± 8% of control (0.42 ± 0.08 Hz; Fig. 6D). The mean amplitude of mIPSCs was not altered significantly by ethanol and/or nociceptin (Fig. 6F).
Discussion
Several lines of research suggest a role for opioid systems in the control of ethanol-related behaviors. For example, there is evidence that opiate receptor antagonists decrease the primary reinforcing effects of ethanol in animal models (43, 45–48) and reduce relapse rates effectively in animals (31, 44) and in abstinent human alcoholics in treatment (49–51). However, whereas several studies (21–24) have evaluated the effects of nociceptin on ethanol-related phenomena, to our knowledge there have been no physiological data on the possible interactions of nociceptin and ethanol-induced effects on CeA or other synapses. By using electrophysiological techniques in an in vitro model, this study demonstrates an inhibitory action of nociceptin on basal CeA GABAergic transmission that is mainly due to presynaptic reduction of vesicular GABA release. We also observed an opposing action of nociceptin versus ethanol effects at these synapses, as well as an increased sensitivity of the CeA GABA system to nociceptin in ethanol-dependent rats compared with naïve controls.
Both postsynaptic and presynaptic effects of nociceptin have been amply characterized in slice preparations of rat hippocampus (14, 16, 52), thalamus (17, 53), lateral amygdala (10, 11), and suprachiasmatic nucleus (54). Nociceptin acts on presynaptic NOPs to decrease glutamate and GABA release by various mechanisms, including inhibition of N-P/Q-type Ca2+ channels, reduction in the presynaptic baseline Ca2+ concentration, and inhibition of the vesicle-release machinery (11, 54). Meis and Pape (10) also studied the basic properties of nociceptin-induced postsynaptic currents in detail in CeA neurons, but these studies did not address actions on CeA synaptic transmission. Therefore, we investigated the action of nociceptin on basal GABAergic transmission in CeA. We found that nociceptin inhibited evoked IPSCs, increased IPSC PPF ratio, and decreased mIPSC frequency, consistent with a presynaptic inhibition of vesicular GABA release, as reported for the suprachiasmatic nucleus (54) and lateral amygdala (11). The mechanisms (probably Ca2+-dependent) that underlie these presynaptic effects have yet to be determined.
Behaviorally, nociceptin markedly blunted the rewarding effects of ethanol and prevented reinstatement of ethanol-seeking behavior elicited by environmentally conditioned stimuli in alcohol-preferring rats (26). Nociceptin also abolished the conditioned place preference induced by ethanol, further supporting the idea that nociceptin can abolish the rewarding properties of ethanol (27). The selective NOP antagonist [Nphe1]nociceptin(1–13)NH2 blocked the effect of nociceptin on ethanol drinking (55). Our previous in vitro and in vivo findings support the idea that ethanol reinforcement may occur in part by increased GABAergic transmission in the CeA, which is partially due to enhanced GABA release (34, 35). Interestingly, we found that pretreatment with nociceptin totally prevented the ethanol-induced increase of evoked IPSC amplitudes and mIPSC frequencies, as well as the decrease in PPF ratios of IPSCs. Even the addition of nociceptin after the establishment of ethanol effects abolished these effects. These results suggest that nociceptin is preemptive in regulating GABA release in CeA and opposing the ethanol effects.
Also, this interaction may be more than a mere summation of opposing effects, because a relatively small (12%) nociceptin inhibition of IPSC amplitudes translates into complete block of the large (40–50%) increase in amplitudes elicited by ethanol alone. Our data with the NOP antagonist suggest that these receptors are involved in the nociceptin actions but not in the acute ethanol effects, at least in the in vitro slice preparation. We speculate that the inhibitory modulation of GABA release by nociceptin occurs at either a different (e.g., more proximal) site from that for ethanol and/or they oppose each other’s action at the same site (e.g., CRF release, Ca2+ levels, and cAMP/PKA). The ethanol-GABA effect is likely to involve intermediary substrates such as presynaptic CRF1 and perhaps other receptors/effectors rather than a direct ethanol action (36).
We also speculate that the opposing effect of nociceptin on ethanol effects at the CeA GABAergic transmission may account in part for its behavioral effects on ethanol intake (55). Therefore, we investigated the nociceptin and ethanol actions on basal GABA transmission in CeA neurons from ethanol-dependent rats. We have found (35) an increased basal GABA transmission and GABA “tone” and a lack of tolerance to acute ethanol effects on CeA synapses after chronic ethanol treatment. Interestingly, the nociceptin-induced inhibition of IPSCs was increased and the NOP antagonist was directly active in neurons of ethanol-dependent rats, indicating an increased sensitivity to nociceptin. Also, when CeA neurons of ethanol-dependent rats were preexposed to acute ethanol, nociceptin not only blocked the ethanol-induced enhancement as in naïve rats but also more markedly depressed baseline IPSC amplitudes. These data support the work of others showing changes in basal levels of nociceptin and NOPs after repeated ethanol administration (29, 30). Also, different basal levels of nociceptin and its receptors in rodent lines (C57BL/6J versus DBA/2J mice) contribute to divergent drug-taking behavior (30) and different alcohol preferences (55). We speculate that, during ethanol dependence, neuroadaptations occur in the nociceptin–GABA system and in the interaction of nociceptin with ethanol.
Our combined findings suggest a nociceptin–ethanol interaction with GABAergic transmission in CeA that markedly adapts during development of ethanol dependence. It is possible that Ca2+ levels are a common site of action for nociceptin and ethanol in controlling the release of GABA. Because nociceptin-induced inhibition of IPSCs was increased in neurons of ethanol-dependent rats, endogenous nociceptin and its receptors may have a prominent role in controlling the inhibitory CeA GABA system and opposing the GABA potentiation induced by acute and chronic ethanol. The increased GABAergic tone after chronic ethanol is likely to be producing a net inhibitory effect on CeA neuronal excitability. Functionally, the CeA may act as an inhibitory gate regulating the flow of information from cortical and thalamic afferents through intraamygdaloid circuits to downstream brain regions (e.g., bed nucleus of stria terminalis) (56). We hypothesize that the overall increased nociceptin sensitivity of CeA GABAergic synapses represents a compensatory mechanism counteracting the alterations following chronic ethanol to maintain allostasis (57).
It may seem to be counterintuitive or paradoxical that a NOP agonist, thought to be anxiolytic, would depress GABA transmission in a brain region known to be involved in stress-related behavior. However, because most medial amygdala CeA neurons that we study are GABAergic inhibitory interneurons with recurrent or feedback connections, and nociceptin depresses an inhibitory function in these neurons, it may inhibit downstream regions (e.g., bed nucleus of stria terminalis) by increasing GABA and CRF release there. However, a better reconciliation of the cellular and behavioral data may require a more detailed understanding and correlation of the amygdalar circuitry involved and the neuron types affected in both the in vivo and in vitro studies. Lastly, as a “preemptive antagonist” of ethanol, nociceptin or agents that target NOP could represent promising treatment candidates for alcoholism, as an alternative or adjunct to existing medications such as naltrexone. Because NOP agonists exert marked anxiolytic and antistress effects (22, 24, 25, 58), they may provide an advantage over naltrexone, which is known to be anxiogenic (59, 60).
Materials and Methods
Slice Preparation.
We prepared CeA slices as described in refs. 34 and 35, from male Sprague–Dawley rats (150–300 g; 4–7 weeks old) that were anesthetized with halothane (3%) and decapitated. We rapidly removed the brains into ice-cold ACSF gassed with 95% O2 and 5% CO2. Transverse slices (400 μm thick) were cut on a Vibratome Series 3000 (Technical Products International, St. Louis), incubated in an interface configuration (30 min), and then completely submerged and continuously superfused (flow rate, 2–4 ml/min) with warm (31°C), gassed ACSF (130 mM NaCl/3.5 mM KCl/1.25 mM NaH2PO4/1.5 mM MgSO4·7H2O/2.0 mM CaCl2/24 mM NaHCO3/10 mM glucose).
Chronic Ethanol Treatment.
We used the standard ethanol-inhalation method of The Scripps Research Institute Alcohol Research Center for developing ethanol-dependent rats (35, 61, 62). In the ethanol-treated group, we exposed rats to continuous ethanol vapors for ≥2 weeks (35, 62). Sham controls were treated similarly but without exposure to ethanol vapor. On experiment days, the chronic ethanol-treated rats were maintained in the ethanol vapor chamber until preparation of the CeA slices. We made recordings in ethanol-free ACSF from slices of ethanol-dependent rats 2–8 h after cutting the slices, as described in ref. 35. We did not detect any observable postsynaptic excitability (35).
Blood Alcohol Level (BAL) and Body Weight.
We determined BALs of the ethanol-dependent animals from tail-blood samples that were taken three times per week. Control animals were also sampled routinely. The mean BAL of all ethanol-dependent animals was 167.2 ± 11 mg/dl (n = 14). The mean body weight of ethanol-dependent animals was 225 ± 22 g (n = 14), compared with 253 ± 16 g (n = 36) for sham control animals. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrophysiology.
We recorded from a total of 115 CeA neurons (mean resting membrane potential, −76 ± 3 mV) with sharp micropipettes (3 M KCl) by using discontinuous voltage- or current-clamp mode (34, 35). We evoked pharmacologically isolated GABAA–IPSCs by stimulating locally within the CeA through a bipolar stimulating electrode while superfusing the glutamate receptor blockers 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) and dl-2-amino-5-phosphonovalerate (30 μM) together with 1 μM CGP 55845A (a GABAB receptor antagonist). To determine the response parameters for each cell, an input–output protocol was performed. We stimulated at a range of currents (typically, 50–250 μA; 0.125 Hz) starting at the threshold current that is required to elicit an IPSC, and the stimulus strength was increased in three steps of 50 μA (rate of one pulse per 8 s) until the maximum amplitude was reached. We normalized the three stimulus intensities as 1–3×. Most neurons were held near their resting membrane potential. We applied hyperpolarizing and depolarizing current steps (increments, 200 pA; duration, 750 ms) to generate I–V curves. The evoked IPSC amplitudes and I–V responses were quantified by clampfit software (Axon Instruments, Union City, CA).
We examined PPF in each neuron by using 50-ms interstimulus intervals (35). The stimulus strength was adjusted such that the amplitude of the first IPSC was 50% of maximal, determined from the I–O relationship. We calculated the PPF ratio as the second IPSC amplitude over that of the first IPSC. We took all measures before nociceptin and/or ethanol superfusion (control), during their superfusion (5–10 min), and after washout (20–30 min). We express all values as mean ± SEM. We subjected data to a between-subjects or within-subject ANOVA with repeated measures and, when appropriate, to the Newman–Keuls post hoc test, with P < 0.05 considered statistically significant. In some cases, we also used Student’s paired or unpaired t test.
Based on previous hippocampal studies in our laboratory (14, 16) and others (10, 11, 17), we initially tested different concentrations of nociceptin (0.01, 0.5, and 1 μM) in CeA neurons (data not shown). Nociceptin 500 nM produced a nearly maximal inhibition that was reversible on washout; therefore, to obtain a full recovery of nociceptin effects in CeA neurons, we carried out all of the experiments that are described here by using 500 nM nociceptin.
Whole-Cell Patch–Clamp Recording of mIPSCs.
We recorded mIPSCs by using the “blind” method of whole-cell patch–clamp in the presence of 10 μM cyano-7-nitroquinoxaline-2,3-dione, 30 μM dl-2-amino-5-phosphonovalerate, 1 μM CGP 55845A, and 1 μM TTX. Recordings were made with electrodes (input resistance 2–3 MΩ) filled with an internal solution containing 135 mM KCl, 10 mM Hepes, 2 mM MgCl2, 0.5 mM EGTA, 5 mM ATP, and 1 mM GTP (pH 7.2–7.4; osmolarity 275–290 milliosmol) (35). The mIPSCs were analyzed by using mini 5.1 software (Synaptosoft, Leona, NJ). We measured the frequency, amplitude, decay, and rise time of mIPSCs within individual neurons by using cumulative probability analysis, with statistical significance determined by the Kolmogorov–Smirnov nonparametric two-sample test (significance set at P < 0.05).
Drugs.
CGP 55845A was a gift from Novartis Pharma (Basel). We purchased dl-2-amino-5-phosphonovalerate, cyano-7-nitroquinoxaline-2,3-dione, nociceptin, and [Nphe1]nociceptin(1–13)NH2 from Tocris Cookson (Holloway Road, MO); bicuculline from Sigma; TTX from Calbiochem; and ethanol from Remet (La Mirada, CA).
Acknowledgments
We thank Drs. T. Bartfai, F. Bloom, S. Henriksen, G. F. Koob, and M. Tallent for critical comments on the manuscript and Sam Madamba for technical assistance. This work was supported by National Institutes of Health Grants AA013517 [National Institute of Alcohol Abuse and Alcoholism-funded Integrative Neuroscience Initiative on Alcoholism (INIA)], AA015566, DA03665, and AA06420.
Abbreviations
- CeA
central amygdala
- IPSC
inhibitory postsynaptic current
- NOP
nociceptin/orphanin FQ peptide receptor
- I–V
current–voltage
- TTX
tetrodotoxin
- PPF
paired-pulse facilitation
- mIPSC
miniature IPSC
- CRF
corticotropin-releasing factor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Meunier J. C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P., Butour J. L., Guillemot J. C., Ferrara P., Monsarrat B., et al. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 2.Reinscheid R. K., Nothacker H. P., Bourson A., Ardati A., Henningsen R. A., Bunzow J. R., Grandy D. K., Langen H., Monsma F. J., Jr., Civelli O. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 3.Mollereau C., Parmentier M., Mailleux P., Butour J. L., Moisand C., Chalon P., Caput D., Vassart G., Meunier J. C. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 4.Lachowicz J. E., Shen Y., Monsma F. J., Jr., Sibley D. R. J. Neurochem. 1995;64:34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- 5.Anton B., Fein J., To T., Li X., Silberstein L., Evans C. J. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K., Watanabe M., Ichikawa T., Kobayashi T., Yano R., Kumanishi T. J. Comp. Neurol. 1998;399:139–151. doi: 10.1002/(sici)1096-9861(19980914)399:1<139::aid-cne11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Neal C. R., Jr., Mansour A., Reinscheid R., Nothacker H. P., Civelli O., Watson S. J., Jr. J. Comp. Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- 8.Sim L. J., Childers S. R. J. Comp. Neurol. 1997;386:562–572. doi: 10.1002/(sici)1096-9861(19971006)386:4<562::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Standifer K. M., Pasternak G. W. Cell Signal. 1997;9:237–248. doi: 10.1016/s0898-6568(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 10.Meis S., Pape H. C. J. Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meis S., Pape H. C. J. Physiol. 2001;532:701–712. doi: 10.1111/j.1469-7793.2001.0701e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda K., Kobayashi K., Kobayashi T., Ichikawa T., Kumanishi T., Kishida H., Yano R., Manabe T. Brain Res. Mol. Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- 13.Yu T. P., Xie C. W. J. Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- 14.Madamba S. G., Schweitzer P., Siggins G. R. J. Neurophysiol. 1999;82:1776–1785. doi: 10.1152/jn.1999.82.4.1776. [DOI] [PubMed] [Google Scholar]
- 15.Amano T., Matsubayashi H., Tamura Y., Takahashi T. Brain Res. 2000;853:269–274. doi: 10.1016/s0006-8993(99)02245-3. [DOI] [PubMed] [Google Scholar]
- 16.Tallent M. K., Madamba S. G., Siggins G. R. J. Neurosci. 2001;21:6940–6948. doi: 10.1523/JNEUROSCI.21-17-06940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meis S., Munsch T., Pape H. C. J. Neurosci. 2002;22:718–727. doi: 10.1523/JNEUROSCI.22-03-00718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meis S. Neuroscientist. 2003;9:158–168. doi: 10.1177/1073858403252231. [DOI] [PubMed] [Google Scholar]
- 19.Calo G., Bigoni R., Rizzi A., Guerrini R., Salvadori S., Regoli D. Peptides (Tarrytown, NY) 2000;21:935–947. doi: 10.1016/s0196-9781(00)00230-8. [DOI] [PubMed] [Google Scholar]
- 20.Abdulla F. A., Smith P. A. J. Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogil J. S., Grisel J. E., Zhangs G., Belknap J. K., Grandy D. K. Neurosci. Lett. 1996;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- 22.Jenck F., Moreau J. L., Martin J. R., Kilpatrick G. J., Reinscheid R. K., Monsma F. J., Jr., Nothacker H. P., Civelli O. Proc. Natl. Acad. Sci. USA. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciccocioppo R., Martin-Fardon R., Weiss F., Massi M. NeuroReport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- 24.Jenck F., Ouagazzal A. M., Pauly-Evers M., Moreau J. L. Mol. Psychiatry. 2000;5:572–574. doi: 10.1038/sj.mp.4000793. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Fardon R., Ciccocioppo R., Massi M., Weiss F. NeuroReport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- 26.Ciccocioppo R., Economidou D., Fedeli A., Angeletti S., Weiss F., Heilig M., Massi M. Psychopharmacology. 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccocioppo R., Panocka I., Polidori C., Regoli D., Massi M. Psychopharmacology. 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- 28.Ciccocioppo R., Polidori C., Antonelli L., Salvadori S., Guerrini R., Massi M. Peptides (Tarrytown, NY) 2002;23:117–125. doi: 10.1016/s0196-9781(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 29.Lindholm S., Ploj K., Franck J., Nylander I. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:303–306. doi: 10.1016/s0278-5846(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 30.Ploj K., Roman E., Gustavsson L., Nylander I. Brain Res. Bull. 2000;53:219–226. doi: 10.1016/s0361-9230(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 31.Heyser C. J., Roberts A. J., Schulteis G., Koob G. F. Alcohol Clin. Exp. Res. 1999;23:1468–1476. [PubMed] [Google Scholar]
- 32.Hyytia P., Koob G. F. Eur. J. Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- 33.Roberts A. J., Cole M., Koob G. F. Alcohol Clin. Exp. Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberto M., Madamba S. G., Moore S. D., Tallent M. K., Siggins G. R. Proc. Natl. Acad. Sci. USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberto M., Madamba S. G., Stouffer D. G., Parsons L. H., Siggins G. R. J. Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie Z., Schweitzer P., Roberts A. J., Madamba S. G., Moore S. D., Siggins G. R. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen M., Hablitz J. J. J. Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- 38.Ciccocioppo R., Angeletti S., Panocka I., Massi M. Peptides (Tarrytown, NY) 2000;21:1071–1080. doi: 10.1016/s0196-9781(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 39.Rodi D., Polidori C., Bregola G., Zucchini S., Simonato M., Massi M. Brain Res. 2002;957:354–361. doi: 10.1016/s0006-8993(02)03678-8. [DOI] [PubMed] [Google Scholar]
- 40.Bunzow J. R., Saez C., Mortrud M., Bouvier C., Williams J. T., Low M., Grandy D. K. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 41.Calo G., Guerrini R., Bigoni R., Rizzi A., Marzola G., Okawa H., Bianchi C., Lambert D. G., Salvadori S., Regoli D. Br. J. Pharmacol. 2000;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu I. S., Grass S., Calo G., Guerrini R., Wiesenfeld-Hallin Z., Xu X. J. Life Sci. 2002;70:1151–1157. doi: 10.1016/s0024-3205(01)01496-5. [DOI] [PubMed] [Google Scholar]
- 43.Gianoulakis C. Curr. Top Med. Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- 44.Kotlinska J., Rafalski P. Postepy Hig. Med. Dosw (Online) 2004;58:209–215. [PubMed] [Google Scholar]
- 45.Gerrits M. A., Lesscher H. B., van Ree J. M. Eur. Neuropsychopharmacol. 2003;13:424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Krystal J. H., Cramer J. A., Krol W. F., Kirk G. F., Rosenheck R. A. N. Engl. J. Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 47.Oswald L. M., Wand G. S. Physiol. Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Wilson M. A., Burghardt P. R., Lugo J. N., Jr., Primeaux S. D., Wilson S. P. Ann. N.Y. Acad. Sci. 2003;985:472–475. doi: 10.1111/j.1749-6632.2003.tb07102.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Malley S. S., Krishnan-Sarin S., Farren C., Sinha R., Kreek M. J. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien C. P. Am. J. Psychiatry. 2004;161:1741–1742. doi: 10.1176/ajp.161.10.1741. [DOI] [PubMed] [Google Scholar]
- 51.Volpicelli J. R., Alterman A. I., Hayashida M., O’Brien C. P. Arch. Gen. Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 52.Xie C. W., Lewis D. V. J. Pharmacol. Exp. Ther. 1991;256:289–296. [PubMed] [Google Scholar]
- 53.Albrecht D., Bluhdorn R., Siegmund H., Berger H., Calo G. Br. J. Pharmacol. 2001;134:333–342. doi: 10.1038/sj.bjp.0704264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gompf H. S., Moldavan M. G., Irwin R. P., Allen C. N. Neuroscience. 2005;132:955–965. doi: 10.1016/j.neuroscience.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 55.Ciccocioppo R., Economidou D., Fedeli A., Massi M. Physiol. Behav. 2003;79:121–128. doi: 10.1016/s0031-9384(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 56.Lang E. J., Pare D. Neuroscience. 1998;83:877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- 57.Koob G. F., Le Moal M. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 58.Ciccocioppo R., Sanna P. P., Weiss F. Proc. Natl. Acad. Sci. USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C., Rodgers R. J. Psychopharmacology. 1990;102:507–513. doi: 10.1007/BF02247133. [DOI] [PubMed] [Google Scholar]
- 60.Le A. D., Poulos C. X., Harding S., Watchus J., Juzytsch W., Shaham Y. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 61.Rogers J., Wiener S. G., Bloom F. E. Behav. Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- 62.Roberto M., Schweitzer P., Madamba S. G., Stouffer D. G., Parsons L. H., Siggins G. R. J. Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]