Abstract
To reveal the effects of cadmium exposure on the endoplasmic reticulum (ER) stress response, we examined the expression and function of 78-kDa glucose-regulated protein (Grp78), an ER-resident molecular chaperone, in LLC-PK1 cells. In cells treated with 10 μM cadmium chloride, Grp78 protein levels increased after 6 hr and remained elevated at 24 hr. When cells were incubated with 1–20 μM CdCl2 for 6 hr, Grp78 increased in a dose-dependent manner. In addition, Grp78 mRNA levels were elevated in response to CdCl2 exposure. After exposure to 10 μM CdCl2, the levels of activating transcription factor 4 (ATF4) were increased at 2 hr, with a further enhancement after that; this accumulation followed the transient but marked phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α) on serine 51. Although ATF4 mRNA levels increased mildly by CdCl2 exposure, treatment with actinomycin D did not suppress CdCl2-induced accumulation of ATF4 protein, suggesting the involvement of posttranscriptional and, in part, transcriptional mechanisms. Compared with other heavy-metal compounds such as manganese chloride, zinc chloride, mercuric chloride, and lead chloride, CdCl2 could increase the levels of Grp78, ATF4, and the phosphorylated form of eIF2α more markedly without definite cellular damage. The silencing of Grp78 expression using short-interference RNA enhanced CdCl2-induced cellular damage. These results show that cadmium induces the expression of Grp78 probably via phosphorylation of eIF2α and resultant translation of ATF4, and this ER stress response plays a role in protection against cadmium cytotoxicity in this renal epithelial cell.
Keywords: ATF4, cadmium, eIF2α, endoplasmic reticulum stress, Grp78, heavy metal, LLC-PK1 cells, siRNA
Cadmium is an important occupational and environmental pollutant that causes damage to various organs, especially renal proximal tubular cells (Goering et al. 1995). Cadmium has been reported to induce apoptotic cell death in proximal tubules of experimental animals (Hamada et al. 1997). However, the molecular mechanisms responsible for cadmium-induced damage and subsequent regeneration of tubular epithelium have not been fully clarified. It is important to determine the subcellular compartments that respond to cellular stress induced by cadmium exposure and affect diverse areas of cellular function such as signal transduction, gene expression, cell survival, and death.
The endoplasmic reticulum (ER), an essential intracellular organelle, is responsible for the synthesis, posttranslational modification, and delivery of biologically active proteins to their proper target sites within the cell and the extracellular milieu, as well as for Ca2+ homeostasis (Brostrom and Brostrom 2003; Schröder and Kaufman 2005). The accumulation of unfolded proteins in the lumen of the ER causes ER stress and induces a coordinated adaptive program called the unfolded protein response (UPR). The UPR alleviates stress by up-regulating protein folding and degradation pathways in the ER and by inhibiting protein synthesis (Mori 2000; Rutkowski and Kaufman 2004). The UPR induces the expression of 78-kDa glucose-regulated protein [Grp78; also known as immunoglobulin heavy-chain–binding protein (BiP)], an ER-resident molecular chaperone that prevents the aggregation of unfolded or misfolded proteins so that they can be properly refolded (Brostrom and Brostrom 2003; Lee 2001; Schröder and Kaufman 2005). Grp78 is also the member of heat shock protein 70 (HSP70) family of cytoplasmic chaperones (Brostrom and Brostrom 2003). The Grp78 gene is induced by the various perturbations of ER function such as glucose starvation, expression of misfolded or underglycosylated proteins, treatment with reducing agents, and depletion of Ca2+ stores in the ER (Lee 2001).
With respect to heavy metals, cadmium has been reported to induce the expression of Grp78 in mIMCD3 murine inner medullary collecting duct cells (Santos et al. 1998; Zhang et al. 1999), NIH3T3 mouse fibroblasts (Sugisawa et al. 2004), A549 human pulmonary epithelial type II cells (Croute et al. 2005), RLE rat lung epithelial cells (Timblin et al. 1998), and HeLa cells (Cigliano et al. 1996), but not in HepG2 human hepatoma cells (Mumtaz et al. 2002; Tchounwou et al. 2001; Tully et al. 2000) or platyfish culture cells (Yamashita et al. 2004). These findings suggest that the expression of Grp78 depends on the type of cell exposed to cadmium. However, alterations of Grp78 expression in the proximal tubular cells, which are one of the major targets damaged by cadmium exposure, have not been studied. Furthermore, neither the mechanism nor the biologic significance of cadmium-induced Grp78 expression is known. We therefore examined whether treatment with cadmium chloride and other heavy-metal compounds can induce the expression of Grp78 in LLC-PK1 cells, an established porcine renal epithelial cell line with characteristics of the proximal tubule (Gstraunthaler 1988). Because activating transcription factor 4 (ATF4) and ATF6 have been reported to be responsible for transcription of the Grp78 gene (Luo et al. 2003; Roybal et al. 2004; Rutkowski and Kaufman 2004), we determined the expression of ATF4 and the phosphorylation of eukaryotic translation initiation factor 2 on serine 51 of its α subunit (eIF2α), an upstream regulator of ATF4 expression (Harding et al. 2000). Using short-interference RNA (siRNA) against the porcine Grp78 gene, effects of Grp78 knockdown on the cytotoxicity of CdCl2 were also examined in this renal epithelial cell.
Materials and Methods
Cell culture
LLC-PK1 cells were obtained from Health Science Research Resources Bank (Japan Health Sciences Foundation, Osaka, Japan) and grown in medium 199 supplemented with 3% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (GIBCO; Invitrogen Corp., Carlsband, CA, USA) in a humidified atmosphere of 5% CO2, 95% air at 37°C. For each experiment, exponentially growing LLC-PK1 cells were plated at 5 × 105 or 2.5 × 105 cells/well in 6-well culture plates, 6 × 104 cells/well in 24-well culture plates, or 1 × 104 cells/well in 96-well culture plates, and cultured for 1 day before the experiments.
siRNA transfection
Duplexed stealth siRNA targeted against the porcine Grp78 gene (GenBank accession no. X92446; GenBank, National Center for Biotechnology Information, Bethesda, MD, USA) was synthesized by Invitrogen. The sequence of 25-mer siRNA was 5′-GGGAAAGAAGGU-UACUCAUGCAGUU-3′. siRNA was transfected into LLC-PK1 cells grown in 6-, 24-, or 96-well culture plates (50% confluence) using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. After incubating for 12 hr, cells were washed with medium 199 and used for the experiments. Transfection efficiency was evaluated using a fluorescent oligonucleotide and estimated to be > 80%.
Treatment with metals
CdCl2, zinc chloride, mercuric chloride, and lead chloride were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and manganese chloride was from Sigma Chemical Co. (St. Louis, MO, USA). The stock solutions were prepared by dissolving each metal compound in water and sterilizing the solution by filtration. LLC-PK1 cells (90% confluence) were incubated with serum-free medium containing the appropriate concentration of CdCl2 or other metals for 6 hr at 37°C. In the time-course study, cells were incubated with 10 μM CdCl2 for 1–24 hr. Untreated control cells were incubated with serum-free medium alone and treated identically to the cells exposed to metals. Actinomycin D, cycloheximide, and thapsigargin (Sigma) were dissolved in dimethyl sulfoxide (DMSO). LLC-PK1 cells were incubated with serum-free medium containing each chemical or DMSO at the same concentration used (0.1, 0.05, or 0.03%).
Western immunoblotting
At the end of the incubation, cells were washed with phosphate-buffered saline and lysed with sodium dodecyl sulfate (SDS)–polyacrylamide gel Laemmli sample buffer. Cell lysates were collected, sonicated, and boiled for 5 min. Protein concentration was determined with the RC DC Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of protein (10 or 20 μg) were subjected to SDS-PAGE on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane (Hybond-ECL; Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). The membrane was blocked with 5% nonfat milk or bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 for 1 hr at room temperature. The antibodies used were Grp78 (76-E6), cAMP-responsive element (CRE) binding protein 2 CREB2 (also known as ATF4; C-20), and actin (I-19) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); 94-kDa glucose-regulated protein (Grp94) and HSP70 (both from Stressgen Bioreagents, Victoria, British Columbia, Canada); and phosphorylated eIF2α (phospho-eIF2α; Ser51) and total eIF2α (both from Cell Signaling Technology, Inc., Beverly, MA, USA). The membrane was incubated overnight at 4°C with the primary antibody, and protein was detected with a Phototope-HRP Western blot detection kit (Cell Signaling Technology) or a SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce Chemical Co., Rockford, IL, USA). After immunodetection, some blots were incubated with Restore Western Blot Stripping Buffer (Pierce) for 30 min at room temperature and reprobed with actin antibody. The bands on the developed film were quantified with NIH Image (version 1.62; National Institutes of Health, Bethesda, MD, USA). The density of each band was normalized to that of actin.
RT-PCR
The reverse-transcription polymerase chain reaction (RT-PCR) analysis for the semiquantification of mRNA was carried out as described previously (Matsuoka and Igisu 1996). Total RNA was isolated using Trizol reagent (Invitrogen), and 0.5 μg of total RNA from each sample was used for cDNA synthesis using the first-strand cDNA synthesis kit (Roche Applied Science, Penzberg, Germany). Equal volumes (1 μL) of the resulting cDNA served as templates for subsequent PCR reactions using DNA polymerase KOD Dash (Toyobo, Osaka, Japan). The primers for Grp78 were designed from the porcine Grp78 mRNA sequence to yield an expected product of 656 bp. The sense primer sequence was 5′-GCACCACCTAC TCGTGCGTT-3′ (bases 245–264), and the antisense primer was 5′-ACCCAGGTGAGT ATCTCCGTTAG-3′ (bases 878–900). The sequences of ATF4 primers (Kato et al. 1999) were 5′-CCAGGTTGCCCCCTTTACGTTCTTG-3′ (sense) and 5′-GTTCTGCTCCATCTTCTTCAGCTTC-3′ (antisense), which yielded a 412-bp product corresponding to nucleotides 678–1,089 on the porcine ATF4 gene. The sequences of β-actin primers (Yano et al. 2004) were 5′-TGAGACCTT CAACACGCCG-3′ (sense) and 5′-ATG GTGATGACCTGCCCGTC-3′ (antisense), which yielded a 378-bp product corresponding to nucleotides 6–383 on the porcine β-actin gene. An aliquot of PCR products (10 μL) was run on a 2% agarose gel containing 0.5 μg/mL ethidium bromide. The densities of each band were recorded with an Image Saver HR (AE-6905H; Atto, Tokyo, Japan) and quantified using NIH Image software. For mRNA analysis, density of each product was normalized to that of β-actin.
Trypan blue exclusion assay
Culture medium was aspirated and reserved. After trypsinization, cells were suspended in medium 199, and the culture medium was returned. The mixture was centrifuged to concentrate the cells. Cellular suspension and 0.4% trypan blue in Hank’s balanced salt solution were mixed, and the number of viable cells was counted using a hemacytometer. The percentage of viable cells (cell viability) was calculated as 100 × [(unstained cells)/(stained + unstained cells)].
LDH assay
The activity of lactate dehydrogenase (LDH) in the supernatant of cells was determined using a cytotoxicity detection kit (LDH; Roche) according to the manufacturer’s instructions. The results were expressed as the percentage of the maximum amount of LDH released from samples that had been treated with 1% Triton X-100 (percentage release).
Statistical analysis
Results were expressed as the mean ± SD. Statistical significance was determined by one-way analysis of variance followed by the Dunnett multiple comparison test. When two groups were compared, Student’s t-test or Welch’s t-test was used; p < 0.05 was considered statistically significant.
Results
Accumulation of Grp78 by CdCl2
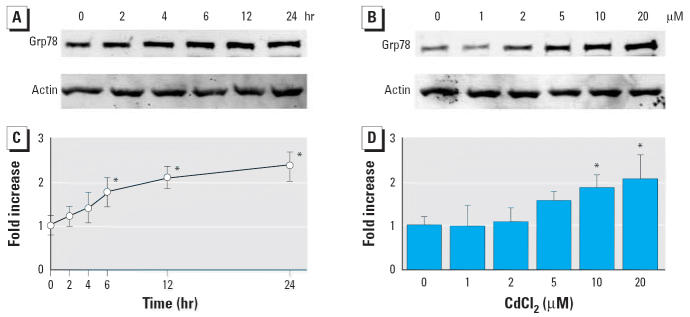
In LLC-PK1 cells treated with 10 μM CdCl2, the level of Grp78 increased significantly after 6 hr and remained elevated at 24 hr, whereas the actin level was not changed after 2- to 24-hr exposures (Figure 1A). When cells were incubated with 1–20 μM CdCl2 for 6 hr, the Grp78 level increased in a dose-dependent manner (Figure 1B). In contrast, the level of Grp94, which is also abundant in the ER lumen (Lee 2001), was not changed clearly in response to CdCl2 exposure (data not shown). After incubation with CdCl2 for 6 hr, the cell viability assayed with trypan blue exclusion was not changed at concentrations < 10 μM and was reduced by 36% at 20 μM CdCl2. Hereafter, cells were exposed to CdCl2 at a concentration of 10 μM.
Figure 1.
Time course (A,C) and dose effects (B,D) of CdCl2-induced accumulation of Grp78 protein in LLC-PK1 cells incubated with 10 μM CdCl2 for 2–24 hr (A,C) or with 0, 1, 2, 5, 10, or 20 μM CdCl2 for 6 hr (B,D). (A,B) Cell lysates subjected to Western immunoblotting using Grp78 and actin antibodies. (C,D) Densitometric analyses of (A and B, respectively). Results shown are from representative analyses. Control values (0 hr or 0 μM) were set to 1; values are mean ± SD of four experiments.
*p < 0.05 compared with control.
Induction of Grp78 gene expression by CdCl2
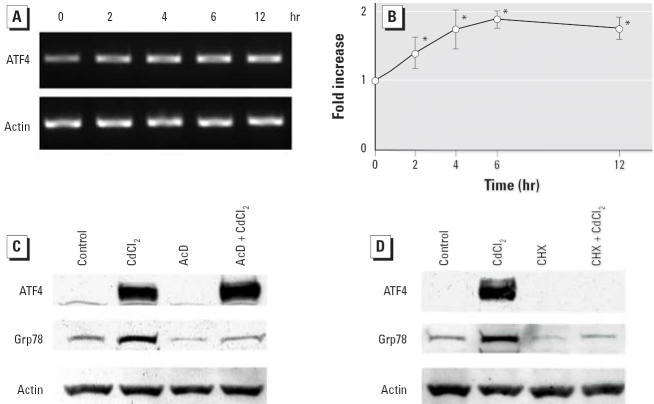
PCR amplification with Grp78 primers showed single bands of the predicted size (656 bp) on an agarose gel stained with ethidium bromide (Figure 2). Consistent with the increase of Grp78, Grp78 mRNA in LLC-PK1 cells treated with 10 μM CdCl2 began to increase significantly after 4 hr and peaked at 6 hr (Figure 2). The expression of β-actin did not change after treatment with CdCl2.
Figure 2.
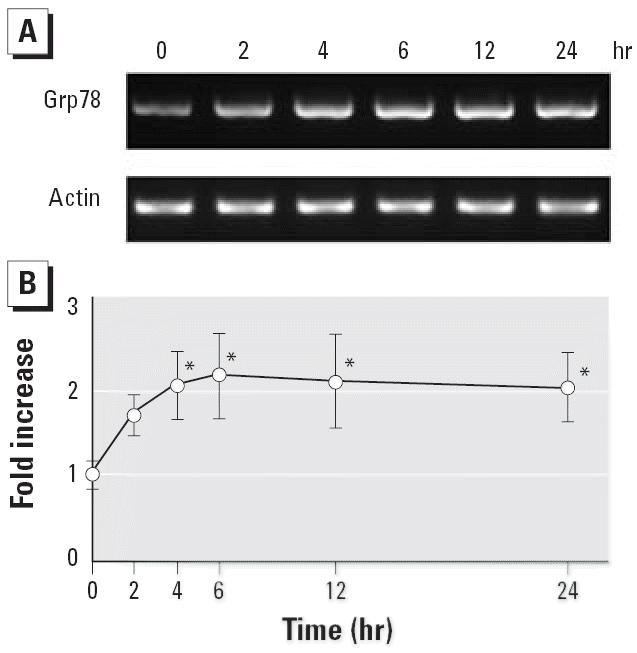
Induction of Grp78 gene expression in LLC-PK1 cells incubated with 10 μM CdCl2 for 2–24 hr; total RNA was isolated and subjected to RT-PCR analysis using Grp78 and β-actin primers. (A) Agarose gel stained with ethidium bromide. (B) Grp78 gene expression shown by densitometric analysis of the gel in (A). The control value (0 hr) was set to 1. Values are mean ± SD of three experiments.
*p < 0.05 compared with control.
Accumulation of ATF4 and phospho-eIF2α by CdCl2
ATF4 levels increased clearly after 2 hr, and this elevation became more marked as incubation time increased (Figure 3). In contrast to ATF4 levels, the level of ATF6 detected at 90 kDa was not changed, and its 50-kDa fragment did not appear after 2–24 hr of exposure (data not shown). Phospho-eIF2α levels began to increase after 1 hr, peaked at 2 hr, and then returned to the control level at 12 hr (Figure 3). In contrast, the endogenous level of total eIF2α did not change through the incubation periods examined. Thus, treatment of LLC-PK1 cells with CdCl2 induces transient phosphorylation of eIF2α first, followed by progressive accumulation of ATF4 as time of exposure increases.
Figure 3.
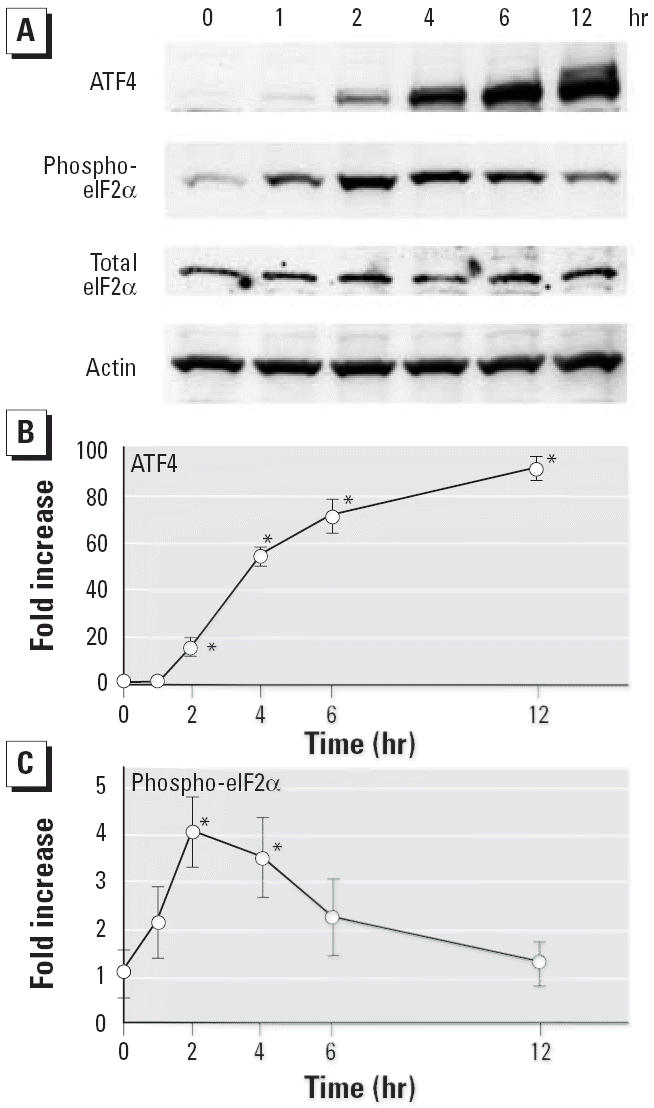
Accumulation of ATF4 and phospho-eIF2α by CdCl2 in LLC-PK1 cells incubated with 10 μM CdCl2 for 1–12 hr; cell lysates were subjected to Western immunoblotting (A) using ATF4, phospho-eIF2α, total eIF2α, and actin antibodies. Densitometric analysis of the Western blot in (A) showing ATF4 (B) and phospho-eIF2α (C). Results shown are representative analyses. The control value (0 hr) was set to 1. Values are mean ± SD of three experiments.
*p < 0.01 compared with control.
Posttranscriptional regulation of ATF4 expression
After exposure to CdCl2, the level of ATF4 mRNA increased after 2 hr, whereas β-actin mRNA levels were not changed after 2–12 hr exposures (Figure 4A). However, the increase of ATF4 mRNA level was < 2-fold and peaked at 6 hr (Figure 4B), suggesting that posttranscriptional mechanisms regulate ATF4 expression in LLC-PK1 cells treated with CdCl2. Therefore, effects of actinomycin D and cycloheximide on CdCl2-induced expression of ATF4 and Grp78 were examined. Treatment of actinomycin D, an inhibitor of transcription, suppressed the accumulation of Grp78 but not of ATF4 (Figure 4C). On the other hand, treatment with cycloheximide, a protein synthesis inhibitor, abolished the expression of both proteins in cells exposed to 10μM CdCl2 for 6 hr (Figure 4D).
Figure 4.
Posttranscriptional regulation of ATF4 expression. (A) LLC-PK1 cells were incubated with 10 μM CdCl2 for 2–12 hr, and total RNA was isolated and subjected to RT-PCR analysis using ATF4 and β-actin primers. (B) Densitometric analysis of (A). The control value (0 hr) was set to 1; values are mean ± SD of three experiments. (C,D) LLC-PK1 cells were incubated for 6 hr with (C) 10 μM CdCl2, 1 μg/mL actinomycin D (AcD), or 1 μg/mL AcD plus 10 μM CdCl2, or (D) 10 μM CdCl2, 10 μg/mL cycloheximide (CHX), or 10 μg/mL CHX plus 10μM CdCl2. The untreated cells (control) were incubated with serum-free medium containing DMSO at the concentration used in the treated cells (0.05 or 0.1%). Cell lysates were subjected to Western immunoblotting using ATF4, Grp78, and actin antibodies. Results shown are representative of three experiments.
*p < 0.01 compared with control.
Effects of heavy metals on the expression of Grp78, ATF4, and phospho-eIF2α proteins
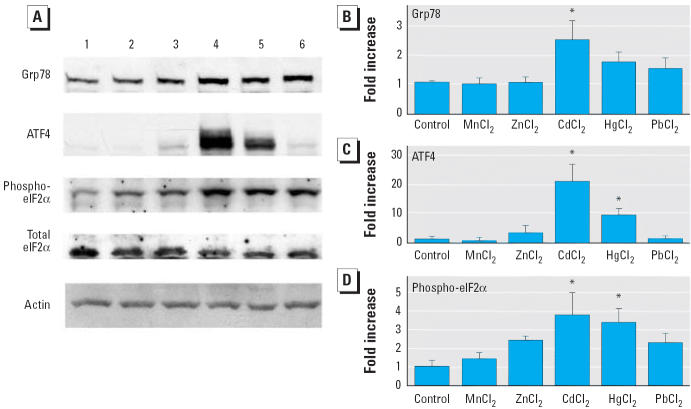
Among the heavy metals examined, only CdCl2 treatment increased the level of Grp78 protein significantly in LLC-PK1 cells (Figure 5A,B). The levels of ATF4 and phospho-eIF2α proteins were elevated in cells exposed to CdCl2 and HgCl2, both of which are nephrotoxic heavy-metal compounds. The increase of ATF4 level was more marked in cells treated with CdCl2 than with HgCl2 (p < 0.05). However, no significant increases were found in cells treated with MnCl2, ZnCl2, or PbCl2 at the same concentration (10 μM) for 6 hr (Figure 5A,B). The cell viability assayed with trypan blue exclusion was 98.7 ± 0.7% for MnCl2, 98.3 ± 0.3% for ZnCl2, 95.6 ± 1.7% for CdCl2, 55.6 ± 7.8% for HgCl2, and 97.3 ± 0.7% for PbCl2 (mean ± SD of three experiments).
Figure 5.
Expression of Grp78, ATF4, and phospho-eIF2α proteins in LLC-PK1 cells incubated with 10 μM of each heavy-metal compound for 6 hr. (A) Representative immunoblot obtained with Grp78, ATF4, phospho-eIF2α, total eIF2α, and actin antibodies. Lane 1, control; lane 2, MnCl2; lane 3, ZnCl2; lane 4, CdCl2; lane 5, HgCl2; lane 6, PbCl2. (B–D) Densitometric analysis showing (B) Grp78, (C) ATF4, and (D) phospho-eIF2α. The control value (without metals) was set to 1; values are mean + SD of three (for ATF4 and phospho-eIF2α) or four (for Grp78) experiments.
*p < 0.05 compared with control.
Effects of Grp78 knockdown on the cytotoxicity of CdCl2
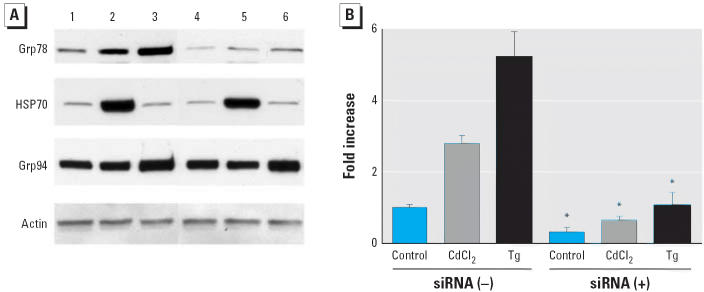
To determine cellular damage, LLC-PK1 cells were incubated with CdCl2 or thapsigargin, an ER stressor that specifically inhibits ER Ca2+-ATPase (Thastrup et al. 1990), for a longer time (12 hr). Treatment with thapsigargin increased the level of Grp78 protein more markedly than did CdCl2 treatment (Figure 6A,B). Transfection with siRNA targeted against the porcine Grp78 gene suppressed the levels of Grp78 protein in control, CdCl2-treated, and thapsigargin-treated cells by 67, 77, and 80%, respectively (Figure 6A,B). In contrast, no significant changes were found in the levels of HSP70 and Grp94 proteins by siRNA transfection, whereas treatment with CdCl2 and thapsigargin induced the expression of HSP70 and Grp94, respectively (Figure 6A). The knockdown of Grp78 expression increased LDH leakage caused by treatment with CdCl2 and thapsigargin by 1.8- and 2.0-fold, respectively (Figure 7). After incubation with 10 or 20 μM CdCl2 for 12 hr, the cell viability assayed with trypan blue exclusion was 73.8 ± 8.6% at 10 μM without siRNA transfection, 60.1 ± 9.3% at 10 μM with transfection, 26.1 ± 5.7% at 20 μM without transfection, and 22.2 ± 4.0% at 20 μM with transfection (mean ± SD of five experiments).
Figure 6.
siRNA-mediated knockdown of Grp78. (A) Western immunoblotting of cell lysates from LLC-PK1 cells transfected without (lanes 1–3) or with (lanes 4–6) siRNA against the Grp78 gene and incubated with 10μM CdCl2 (lanes 2 and 5) or 1 μM thapsigargin (Tg) (lanes 3 and 6) for 12 hr. Results shown are representative immunoblots obtained with Grp78, HSP70, Grp94, and actin antibodies. (B) Densitometric analysis of Grp78 protein from the immunoblot shown in (A). The control value (untreated cells without siRNA transfection) was set to 1; values are mean + SD of three experiments.
*p < 0.001 compared with corresponding treatment without siRNA transfection.
Figure 7.
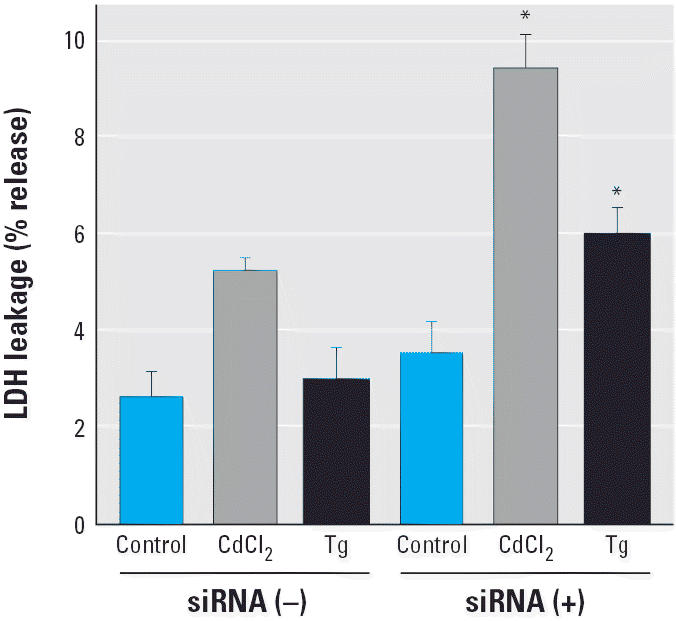
Effects of Grp78 knockdown on the cytotoxicity of CdCl2. LDH leakage was determined in LLC-PK1 cells transfected without or with siRNA against the Grp78 gene and incubated with 10 μM CdCl2 or 1 μM thapsigargin (Tg) for 12 hr. Values are mean + SD of four determinations; results shown are representative of three experiments.
*p < 0.001 compared with corresponding treatment without siRNA transfection.
Discussion
The present study showed that treatment with CdCl2 induced the accumulation of ER chaperone protein Grp78 in a time- and dose-dependent manner in LLC-PK1 cells. The level of Grp78 mRNA was also elevated in response to CdCl2 exposure. Thus, cadmium exposure could cause the induction of Grp78 gene expression, a marker for the ER stress response (UPR) (Lee 2001), in this renal epithelial cell. In addition to cadmium, treatment with thiols, iodoacetamide, tert-butylhydroperoxide, and sulfamethoxazole hydroxylamine has been reported to induce Grp78 expression in LLC-PK1 cells (Halleck et al. 1997; Liu et al. 1997; Ryan et al. 2005), suggesting that ER might be an intracellular sensor of various nephrotoxic chemicals. Cadmium produces reactive oxygen species such as superoxide radical, hydroxyl radical, and nitric oxide (Valko et al. 2005) and reacts with nucleophilic ligands of target molecules (Goering et al. 1995). Cadmium toxicity in yeast has been reported to be mediated through the formation of abnormal proteins that were eliminated by the ubiquitin system (Jungmann et al. 1993). Cadmium has also been reported to mobilize Ca2+ from intracellular stores (Benters et al. 1997; Smith et al. 1989). Taken together, the accumulation of abnormally folded protein and the depletion of Ca2+ stores in the ER might underlie the mechanisms of Grp78 expression in LLC-PK1 cells exposed to CdCl2. Because the level of Grp94, the most abundant glycoprotein in the ER (Lee 2001), was not changed clearly by CdCl2 exposure, the expression of Grp78 and Grp94 might be regulated by distinct mechanisms.
The transcriptional activation of the Grp78 promoter by ER stress depends on site-1 protease– and site-2 protease–mediated proteolytic cleavage of the transcriptional factor ATF6, which specifically targets the ER stress elements (Lee et al. 2002; Ye et al. 2000). On the other hand, another transcriptional factor, ATF4 (also known as CREB2), can bind to an ATF/CRE-like site upstream of the ER stress elements in the mammalian Grp78 promoter (Luo et al. 2003). In the present study, although the levels of the cleaved and the uncleaved forms of ATF6 were not changed (data not shown), ATF4 protein levels increased dramatically with increased time of exposure to CdCl2, as has been reported in mouse Hepa cells (He et al. 2001). These findings suggest that the ATF4 pathway might play a role in CdCl2-induced transcriptional activation of the Grp78 gene at least in LLC-PK1 cells.
Upon ER stress, the double-stranded RNA-activated protein kinase–like ER kinase (PERK) (also known as pancreatic eIF2α kinase or PEK), an ER-resident trans-membrane protein, oligomerizes and phosphorylates eIF2α at serine 51 (Schröder and Kaufman 2005). The phosphorylation of eIF2α leads to inhibition of translation initiation by preventing the association of mRNA with ribosomal 60S and 40S subunits (Mori 2000). In contrast to most proteins, ATF4 circumvents this translation block because it has upstream open reading frames in its 5′ untranslated region that are bypassed only when eIF2α is phosphorylated (Harding et al. 2000; Rutkowski and Kaufman 2004). In LLC-PK1 cells exposed to CdCl2, the phosphorylation of eIF2α protein on serine 51 was found in advance of the accumulation of ATF4 protein. Although the level of ATF4 mRNA increased mildly in response to CdCl2 exposure, the inhibition of transcription by actinomycin D failed to suppress CdCl2-induced ATF4 expression, suggesting that posttranscriptional and, in part, transcriptional mechanisms are involved. In contrast, the inhibition of protein synthesis by cyclo-heximide suppressed ATF4 expression completely. Therefore, cadmium might induce the expression of Grp78 via phosphorylation of eIF2α and resultant translation of ATF4 in LLC-PK1 cells. In addition to PERK, the heme-regulated inhibitor (HRI), the double-stranded RNA-activated protein kinase, and the general control of amino acid biosynthesis kinase are known to phosphorylate eIF2α in mammalian cells (Kaufman 1999; Rutkowski and Kaufman 2004). After exposure to CdCl2, an HRI-related kinase, Hri2p, induced the phosphorylation of eIF2α in yeast (Zhan et al. 2004). It remains to be determined which kinase(s) is responsible for cadmium-induced eIF2α phosphorylation in LLC-PK1 cells.
When the effects of heavy metals (10 μM) on the levels of Grp78, ATF4, and phospho-eIF2α proteins were compared in LLC-PK1 cells, another nephrotoxic heavy-metal compound, HgCl2, also could increase the levels of these proteins, but less significantly than did CdCl2. In contrast, the viability of cells treated with HgCl2 was more severely reduced than with CdCl2 (p < 0.05). In cells treated with 10 μM MnCl2, ZnCl2, or PbCl2 or 1 μM HgCl2 (data not shown), no significant alteration of proteins expression or cellular damage was observed. These findings suggest that heavy-metal–induced expression of Grp78 protein and its upstream regulators were not caused merely by cellular damage. The different expression of Grp78 in LLC-PK1 cells after treatment with heavy metals might be related to their distinct intracellular accumulation and biochemical properties.
To clarify the biologic significance of cadmium-induced Grp78 expression, LLC-PK1 cells were transfected with siRNA against the porcine Grp78 gene and then exposed to CdCl2 or thapsigargin, an inducer of Grp78 without affecting HSP70 synthesis (Elia et al. 1996). Compared with cells without siRNA transfection, the cellular damage induced by either CdCl2 or thapsigargin was more severe in Grp78 knockdown cells. Although the effects of silencing of Grp78 expression on cadmium cytotoxicity were relatively small (1.8-fold increase in LDH leakage and 18.6% reduction in the trypan blue exclusion assay) in the present study, it has also been reported that LLC-PK1 cells expressing an antisense Grp78 construct were more susceptible to the cellular damage induced by hydrogen peroxide (Hung et al. 2003), iodoacetamide (Liu et al. 1997), and tert-butylhydroperoxide (Liu et al. 1998). These data and our results indicate that the expression of Grp78 plays a role in the protection against nephrotoxic insults including cadmium exposure, at least partially, in LLC-PK1 cells. In addition to its functions as ER molecular chaperone and Ca2+-binding protein (Lee 2001), Grp78 has been suggested to suppress oxyradical accumulation and mitochondrial dysfunction (Yu et al. 1999), repair DNA damage (Zhai et al. 2005), and inhibit caspase-7 and caspase-12 activation (Rao et al. 2002; Reddy et al. 2003). Additional studies, including animal models, are required to further reveal the protective role of cadmium-induced Grp78 expression in the proximal tubular cells.
Footnotes
We thank H. Inamura for technical help.
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (JSPS) and a Grant-in-Aid for JSPS Fellows from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Benters J, Flögel U, Schäfer T, Leibfritz D, Hechtenberg S, Beyersmann D. Study of the interactions of cadmium and zinc ions with cellular calcium homoeostasis using 19F-NMR spectroscopy. Biochem J. 1997;322:793–799. doi: 10.1042/bj3220793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Cigliano S, Remondelli P, Minichiello L, Mellone MC, Martire G, Bonatti S, et al. Analysis of metal-regulated metallo-thionein and heat shock gene expression in HeLa-derived cadmium-resistant cells. Exp Cell Res. 1996;228:173–180. doi: 10.1006/excr.1996.0314. [DOI] [PubMed] [Google Scholar]
- Croute F, Beau B, Murat J-C, Vincent C, Komatsu H, Obata F, et al. Expression of stress-related genes in a cadmium-resistant A549 human cell line. J Toxicol Environ Health A. 2005;68:703–718. doi: 10.1080/15287390590925447. [DOI] [PubMed] [Google Scholar]
- Elia G, De Marco A, Rossi A, Santoro MG. Inhibition of HSP70 expression by calcium ionophore A23187 in human cells. An effect independent of the acquisition of DNA-binding activity by the heat shock transcription factor. J Biol Chem. 1996;271:16111–16118. doi: 10.1074/jbc.271.27.16111. [DOI] [PubMed] [Google Scholar]
- Goering PL, Waalkes MP, Klaassen CD. 1995. Toxicology of cadmium. In: Toxicology of Metals: Biochemical Aspects Handbook of Experimental Pharmacology, Vol 115 (Goyer RA, Cherian MG, eds). New York:Springer-Verlag, 189–214.
- Gstraunthaler GJA. Epithelial cells in tissue culture. Renal Physiol Biochem. 1988;11:1–42. doi: 10.1159/000173147. [DOI] [PubMed] [Google Scholar]
- Halleck MM, Holbrook NJ, Skinner J, Liu H, Stevens JL. The molecular response to reductive stress in LLC-PK1 renal epithelial cells: coordinate transcriptional regulation of gadd153 and grp78 genes by thiols. Cell Stress Chaperones. 1997;2:31–40. doi: 10.1379/1466-1268(1997)002<0031:tmrtrs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Tanimoto A, Sasaguri Y. Apoptosis induced by cadmium. Apoptosis. 1997;2:359–367. doi: 10.1023/a:1026401506914. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AMK, et al. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278:29317–29326. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- Jungmann J, Reins H-A, Schobert C, Jentsch S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- Kato Y, Koike Y, Tomizawa K, Ogawa S, Hosaka K, Tanaka S, et al. Presence of activating transcription factor 4 (ATF4) in the porcine anterior pituitary. Mol Cell Endocrinol. 1999;154:151–159. doi: 10.1016/s0303-7207(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Bowes RC, III, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Liu H, Miller E, van de Water B, Stevens JL. Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. J Biol Chem. 1998;273:12858–12862. doi: 10.1074/jbc.273.21.12858. [DOI] [PubMed] [Google Scholar]
- Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through an upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem. 2003;278:37375–37385. doi: 10.1074/jbc.M303619200. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Igisu H. Induction of c-fos expression by tributyltin in PC12 cells: involvement of intracellular Ca2+ Environ Toxicol Pharmacol. 1996;2:373–380. doi: 10.1016/s1382-6689(96)00074-9. [DOI] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Mumtaz MM, Tully DB, El-Masri HA, De Rosa CT. Gene induction studies and toxicity of chemical mixtures. Environ Health Perspect. 2002;110(suppl 6):947–956. doi: 10.1289/ehp.02110s6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topo-isomerase inhibitors. Role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Roybal CN, Yang S, Sun C-W, Hurtado D, Vander Jagt DL, Townes TM, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ryan PM, Bedard K, Breining T, Cribb AE. Disruption of the endoplasmic reticulum by cytotoxins in LLC-PK1 cells. Toxicol Lett. 2005;159:154–163. doi: 10.1016/j.toxlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Santos BC, Chevaile A, Kojima R, Gullans SR. Characterization of the Hsp110/SSE gene family response to hyperosmolality and other stresses. Am J Physiol. 1998;274:F1054–F1061. doi: 10.1152/ajprenal.1998.274.6.F1054. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Smith JB, Dwyer SD, Smith L. Cadmium evokes inositol polyphosphate formation and calcium mobilization. Evidence for a cell surface receptor that cadmium stimulates and zinc antagonizes. J Biol Chem. 1989;264:7115–7118. [PubMed] [Google Scholar]
- Sugisawa N, Matsuoka M, Okuno T, Igisu H. Suppression of cadmium-induced JNK/p38 activation and HSP70 family gene expression by LL-Z1640-2 in NIH3T3 cells. Toxicol Appl Pharmacol. 2004;196:206–214. doi: 10.1016/j.taap.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Ishaque AB, Schneider J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. Mol Cell Biochem. 2001;222:21–28. [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timblin CR, Janssen YMW, Goldberg JL, Mossman BT. GRP78, HSP72/73, and cJun stress protein levels in lung epithelial cells exposed to asbestos, cadmium, or H2O2. Free Radic Biol Med. 1998;24:632–642. doi: 10.1016/s0891-5849(97)00325-0. [DOI] [PubMed] [Google Scholar]
- Tully DB, Collins BJ, Overstreet JD, Smith CS, Dinse GE, Mumtaz MM, et al. Effects of arsenic, cadmium, chromium, and lead on gene expression regulated by a battery of 13 different promoters in recombinant HepG2 cells. Toxicol Appl Pharmacol. 2000;168:79–90. doi: 10.1006/taap.2000.9014. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Hirayoshi K, Nagata K. Characterization of multiple members of the HSP70 family in platyfish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene. 2004;336:207–218. doi: 10.1016/j.gene.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Kubota T, Sendo T, Oishi R. A prostacyclin analog beraprost sodium attenuates radiocontrast media-induced LLC-PK1 cells injury. Kidney Int. 2004;65:1654–1663. doi: 10.1111/j.1523-1755.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Zhai L, Kita K, Wano C, Wu Y, Sugaya S, Suzuki N. Decreased cell survival and DNA repair capacity after UVC irradiation in association with down-regulation of GRP78/BiP in human RSa cells. Exp Cell Res. 2005;305:244–252. doi: 10.1016/j.yexcr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Zhan K, Narasimhan J, Wek RC. Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics. 2004;168:1867–1875. doi: 10.1534/genetics.104.031443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang X-Y, Cohen DM. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol. 1999;276:F786–F793. doi: 10.1152/ajprenal.1999.276.5.F786. [DOI] [PubMed] [Google Scholar]