Introduction
Today, the treatment of ulcerative colitis (UC) has improved enormously because of the use of therapeutic tools, such as immunomodulator agents (corticosteroids, azathioprine (AZA), cyclosporine, and antitumor necrosis factor-alpha antibodies).[1]
The immunosuppressive agents predispose the patient to opportunistic infections.[2] Infections, such as disseminated cytomegalovirus, pyogenic liver abscess, pneumonia, septic phlebitis, hepatitis, Q fever, herpes zoster, and herpetic encephalitis, have been reported in patients receiving purine analogs.[2-5] In a review of the literature, we have not found any case of herpetic encephalitis associated with the immunosuppressive treatment of UC. Here, we describe a patient with UC, who developed herpetic encephalitis during immunosuppressive treatment, and its successful management.
Case Report
A 22-year-old woman was admitted to the hospital because of an acute exacerbation of UC and a decreased level of consciousness. She had known UC for 3 years. Her medications included 5-aminosalicylate (Asacol 3 g/day) and prednisolone 20 mg/day.
One year before her recent admission, severe bloody diarrhea 20-25 times per day, abdominal pain, and generalized weakness developed. The work-ups for infectious causes of bloody diarrhea were negative. Because she did not improve on a high-dose intravenous corticosteroid (hydrocortisone 400 mg/day), treatment with intravenous cyclosporine 250 mg/ day was started and her bloody diarrhea quickly improved. Eight days later, intravenous cyclosporine was switched to an oral formula (Neoral 200 mg every 12 hours), and AZA 50 mg/day was initiated. Three months later, cyclosporine was discontinued and prednisolone 10 mg/day and AZA 75 mg/day were continued. She did well for 8 months, but subsequently, bloody diarrhea 10-15 times daily and generalized mild abdominal pain recurred. One month later, she was admitted to the emergency department because of a decreased level of consciousness that had happened 12 hours before admission. Her family said that she had had an intense temporal headache for the past few days, and bloody diarrhea became more severe during this period. There was no history of nasolabial or anogenital herpetic lesions. On physical examination, her temperature was 39°C and her systolic blood pressure was 70 mm Hg. On neurologic examination, she was in a deep comatose state and did not respond to painful stimuli. There was a mydriatic right pupil. Laboratory examinations revealed white blood cell counts of 12,000/mcL (82% polymorphonuclears, 18% lymphocytes), a hemoglobin count of 9 g/dL, and a serum potassium level of 2.8 meq/L. The results of other tests, including serum sodium, blood urea nitrogen, serum creatinine, and serum aminotransferase levels, were normal.
A tracheal tube was inserted and intravenous fluid therapy was initiated. Then, a noncontrast computerized tomographic (CT) scan of the brain was performed, which revealed multiple bilateral frontal lobe hemorrhages (Figure 1).
Figure 1.
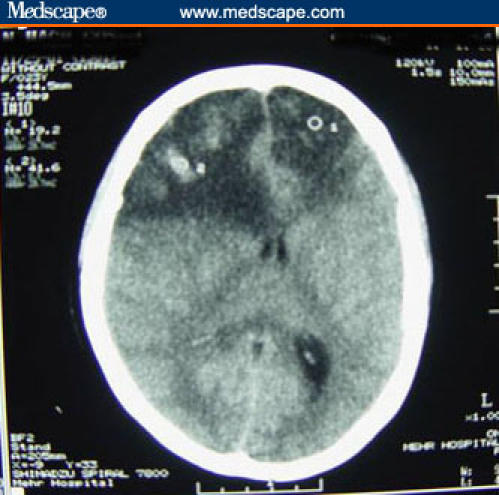
A noncontrast computerized tomographic (CT) scan of the patient at the time of the last admission, showing bilateral frontal lobe extensive hypodense regions (especially in the right side) with a midline shift to the left side, subfalcian herniation, and multiple foci of hemorrhage within the lesion.
Following neurosurgical consultation, a right frontal lobe craniotomy was performed. There were severe necrosis and multiple foci of hemorrhage in the brain tissue. Histopathologic examination of the brain tissue was reported as herpes simplex encephalitis (HSE). Polymerase chain reaction (PCR) of the brain tissue was also positive for the herpes simplex virus (HSV) DNA genome.
Corticosteroid and AZA were discontinued temporarily, and intravenous acyclovir 500 mg/day was started. Her clinical condition gradually improved and her level of consciousness became normal, but her diarrhea continued.
In a noncontrast brain CT scan performed a few days postoperatively, there was a decrease in the size of the frontal lobe lesion with the disappearance of a mass effect (Figure 2).
Figure 2.
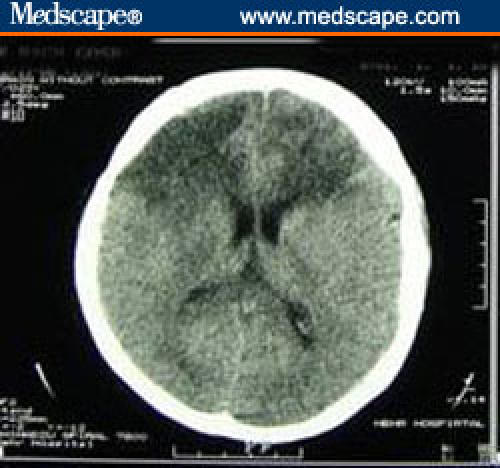
Repeated CT scan 14 days later, showing decrement in the size of the lesion with the disappearance of a mass effect.
Then, 5-aminosalicylate, AZA, and intravenous corticosteroid were started, and her diarrhea partially improved. She was discharged from the hospital after 14 days of treatment with acyclovir while she was taking 5-aminosalicylate, AZA, and prednisolone. Three months later, regarding suboptimal response to medical therapy, the patient underwent total proctocolectomy and ileoanal pouch anastomosis. On 6 months of follow-up, the patient is not on any drugs and she is doing well without any neurologic residua.
Discussion
Administration of the immunomodulatory agents in treatment of patients with inflammatory bowel disease targets the pathogenesis of the disease by modifying the mucosal immune system.[1] AZA has an important role in reducing the required dose of corticosteroids in patients who experience complications of corticosteroid therapy or cannot tolerate other medical treatments.[2,3]L
The prevalence of infectious adverse effects of AZA has been 7.4% in 396 patients -- 120 UC and 276 Crohn's disease -- of which 1.8% were severe, including 1 case of herpes zoster encephalitis. Five of the 7 patients who developed severe infections were also taking corticosteroids.[2]
Baringer and colleagues[6] demonstrated that immunosuppression predisposes rabbits to HSE. In this study, methylprednisolone caused a delay in clearance of the virus from the central nervous system (CNS), and cytarabine resulted in failure of normal clearance of the virus from the infected animal's CNS. However, it appears that the severe presentation of herpetic encephalitis in our patient may not only have been the result of AZA-induced immunosuppression, but also the effect of corticosteroid was important.
HSE has an acute life-threatening course[7] and may not be distinguishable clinically from other causes of encephalitis, but certain features, if present, are characteristic, such as medial temporal and inferior frontal lobe involvement, fever as high as 39ºC to 41ºC, and early progression to an altered state of consciousness.[8] Our patient had frontal lobe involvement; her conscious level decreased rapidly to a comatose state; and she was febrile. Also, herpes labialis is present in less than 10% of cases of HSV-1 encephalitis.[8] Our patient didn't have this symptom, too. She had probably acquired HSV-1 infection in the past asymptotically.
PCR of the cerebrospinal fluid is the diagnostic test of choice for diagnosis of HSE. It is rapid and can detect viral DNA within 1 hour.[9] Lakeman and Whitley[10] found a sensitivity of 98% and specificity of 100%.
The "gold standard" for diagnosis of HSE is brain biopsy, with identification of HSV in the tissue by cell culture and immunohistochemical staining (sensitivity, 99%; specificity, 100%), but with the advent of PCR, it is used uncommonly.
Because our patient presented with brain herniation, the craniotomy and drainage of brain hemorrhage and necrotic tissue were mandatory; thus, PCR for HSV DNA was performed on the tissue sample rather than the cerebrospinal fluid.
HSV typically causes life-threatening encephalitis that has a mortality rate of 70% if left untreated, and only 11% of survivors regain normal brain function.[10] Treatment consists of acyclovir (10 mg/kg, for 14 days), which is a synthetic purine nucleoside that inhibits viral DNA synthesis. Although it is believed that treatment with acyclovir should begin before alteration of consciousness and is useless for comatose patients,[8] our patient responded well to acyclovir, despite deep coma at presentation. Thus, acyclovir was used successfully even in a patient with altered consciousness.
As early treatment is crucial, acyclovir should be prescribed on suspicion to HSE, because it lowers the mortality from 70% to 19%.[10]
It is advised that treatment with AZA be discontinued temporarily, and begun again if indicated after the treatment of HSE is completed. The corticosteroid dosage should be lowered and gradually tapered if possible.
In conclusion, HSE and other CNS infections should be considered in any immunosuppressed patient who presents with an altered level of consciousness and fever.
Contributor Information
Seyed Meysam Alimohamadi, Research Fellow, Digestive Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
Seyed Hossein Mirmadjless, Chairman, Department of Gastroenterology, Mehr General Hospital, Tehran, Iran.
Reza Malekzadeh, Chairman; Professor, Digestive Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1. Matsuda K, Watanabe T, Abo Y, et al. Severe complications of ulcerative colitis after high dose prednisolone and azathioprine treatment. J Gastroenterol. 1999;34:390-394. [DOI] [PubMed] [Google Scholar]
- 2.Present Dh, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in treatment of inflammatory bowel disease: short and long-term toxicity. Ann Intern Med. 1989;111:641-649. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol. 1998;33(suppl):92-99. [DOI] [PubMed] [Google Scholar]
- 4.Actis GC, Bresso F, Astegiano M, et al. Safety and efficacy of azathioprine in the maintenance of cyclosporine-induced remission of ulcerative colitis. Aliment Pharmacol Ther. 2001;15:1307-1311. [DOI] [PubMed] [Google Scholar]
- 5.Korelitz BI, Fuller SR, Warman JI, Myron D. Shingles during the course of treatment with 6-mercaptopurine for inflammatory bowel disease. Am J Gastroenterol. 1999;94:424-426. [DOI] [PubMed] [Google Scholar]
- 6.Baringer JR, Klassen T, Grumm F. Experimental herpes simplex virus encephalitis. Effect of corticosteroids and pyrimidine nucleoside. Arch Neurol. 1976;33:442-446. [DOI] [PubMed] [Google Scholar]
- 7.Whitley RJ. Herpes simplex virus infections of the central nervous system. Am J Med. 1988;85(suppl2A):61. [PubMed] [Google Scholar]
- 8.Gluckman SJ. Causes and treatment of viral encephalitis. UpToDate. Version 11.3.
- 9.Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis. 1995;20:414-420. [DOI] [PubMed] [Google Scholar]
- 10.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171:857-863. [DOI] [PubMed] [Google Scholar]


