Abstract
Objective
To review the current medical literature on robotic prostatectomy (RP) and report clinical outcomes of this newly developed technique.
Data Source
A MEDLINE search was performed using the following headings: prostate cancer, radical prostatectomy, robotics, robot assisted, laparoscopy, telesurgery. In addition, recently published abstracts on RP were reviewed.
Study Selection
Studies that reported clinical and pathological variables of patients undergoing RP were included in this meta-analysis.
Data Extraction
Data were extracted from published articles and abstracts.
Data Synthesis
Robotic systems enhance surgeons' technical abilities and offer the potential of precise surgical technique. Short-term follow-up studies demonstrate that RP is at least comparable in efficacy to open and laparoscopic prostatectomy, including clinical and pathologic parameters. RP has benefits of minimal invasiveness, decreased blood loss, and quicker recovery compared with open surgery. Functional and cancer control results are still immature, but most studies reported favorable outcomes.
Conclusions
RP is a promising minimally invasive surgical approach for men with prostate cancer. Short-term clinical and pathological results are comparable to those with open and laparoscopic prostatectomy.
Introduction
Robotic prostatectomy (RP) represents the latest advancement in surgical treatment of prostate cancer. It relies on minimally invasive surgical approach of laparoscopic prostatectomy augmented by robotic technology. Schuessler and colleagues[1] first performed laparoscopic radical prostatectomy in 1991, but they failed to demonstrate advantages vs open prostatectomy, and the procedure was abandoned. It was not until Guillonneau and Vallancien[2] and Abou and colleagues[3] described and validated their techniques of laparoscopic prostatectomy that interest resurged in minimally invasive surgical treatment of prostate cancer.
Several centers in Europe, and later in the United States, have since reported their short and mid-term results on functional and oncologic outcomes of laparoscopic prostatectomy, which compare favorably with open prostatectomy.[4] Despite the large interest in this approach, laparoscopic prostatectomy has not been widely adopted by the urology community mainly because of difficulty in learning and the related technical challenges.[5]
Feasibility of RP was first demonstrated in 2000 by 2 French centers with more expertise in laparoscopic prostatectomy than many other centers.[6,7] This early experience was met with skepticism. However, shortly thereafter, pioneering work from the Vattikuti Urology Institute in Detroit, Michigan, paved the way for a new era in RP.[8,9] A growing number of institutions in the United States and Europe have since embraced this technique.
The da Vinci surgical system (Intuitive Surgical; Moutainview, California) (Figures 1 and 2) incorporates 3 multijoint robotic arms with 1 arm controlling the binocular endoscope and the other 2 controlling small-wristed instruments (EndoWrist technology). This system is a "master-slave" robot that is controlled by the surgeon who is comfortably seated on the operative console (master), only a few feet away from the patient. Two finger-controlled handles housed in a mobile console control the 2 robotic arms and camera. The stereoscopic view of the operative field provides excellent 3-dimensional visualization with 10-fold magnification.
Figure 1.

Robotic set-up showing da Vinci robotic system.
Figure 2.
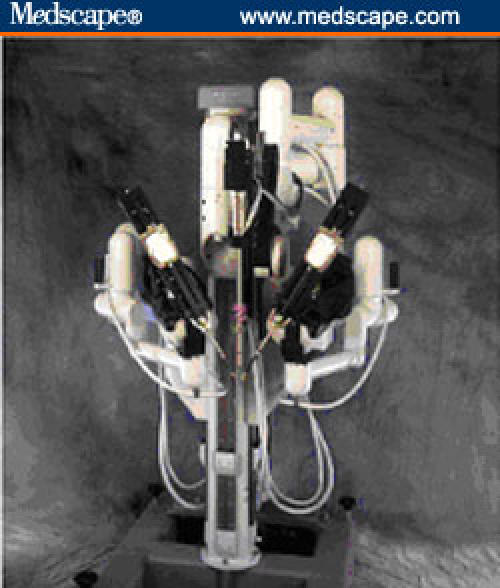
da Vinci robotic arms.
Manipulation of the masters is transmitted to a computer that filters, scales, and relays the surgeon's movements to the robotic arms and instruments. Hand movements can scale to 1:1, 3:1, or 5:1 movement at the tip of the instruments. This scaling allows for finer and precise execution of certain steps of the operation. Physiologic tremor is completely eliminated. There is no measurable delay between the movement of the handles on the console and the movement of the instruments within the patient. The instruments allow 7° of liberty (degrees of excursion) in their movement, simulating the human hand.
With its 3-D view, the da Vinci surgical system aids the surgeon in more easily identifying delicate structures, such as nerves and blood vessels surrounding the prostate gland. The EndoWrist instruments provide the surgeon with the dexterity not available using conventional laparoscopic instruments.
Rationale
Among US men, prostate cancer is the most common cancer and is the second leading cause of cancer-related death.[10] Individuals diagnosed with prostate cancer often must choose whether to undergo treatment that will likely influence the quality of their lives. Patients often select less morbid and minimally invasive treatments if they are comparable in efficacy. Each treatment differs significantly in its side effects and impact on health-related quality of life, such as sexual function and urinary control.[11-15]
Radical prostatectomy is a curative treatment for localized prostate cancer, and it remains the mainstay option in the United States with which to compare other treatment modalities.[16] However, radical prostatectomy is not without long-term adverse events. Full continence, defined as no need for protection, ranges between 80% and 97% after open radical prostatectomy.[17] The return of erectile function ranges between 13% and 68% for unilateral nerve sparing and 32% to 86% for bilateral nerve sparing.[17] These results are also affected by the method of data collection; patient interviews tend to overestimate results, whereas patient-filled validated questionnaires provide more accurate figures.
Functional Outcomes and Cancer Control
Since the description by Walsh[18] of the anatomic approach of radical retropubic prostatectomy it became apparent that surgical technique does matter in the preservation of both sexual and sphincteric functions. Additionally, surgical technique influences cancer control. It has been shown that the surgical technique is an independent predictor of surgical margin status,[19] which is positive on average in 28% of patients after open radical prostatectomy (range, 0-71).[20] Epstein and colleagues[21] reported a 10-year progression-free survival of 79.4% in men with negative margins vs 54.9% in those with positive margins (P < .00001).
Since the robotic system enhances surgeons' technical abilities it may offer the potential of precise surgical technique, and thus more precise removal of the cancer (negative margins in organ-confined disease), and better preservation of sexual function and urinary control. Robotic surgery also has benefits of minimal pain, little blood loss, better cosmetics, and quicker recovery compared with open surgery.[22]
Technique
The technique of RP was described and standardized by the Detroit group.[9,23] The patient is placed in lithotomy position with enough space between the thighs for the robotic system to be wheeled in position. All pressure points are padded carefully. Pneumoperitoneum is created and a total of 5-6 ports are placed. A 12-mm port is placed at the umbilicus for the binocular scope, and two 8-mm ports are used for the robot instrument arms and are placed approximately 10 cm from the midline, slightly below the umbilical line. The remaining ports are placed laterally for assistance. At least 1, but optimally 2, assistants are needed. The following steps are performed sequentially:
Development of the extraperitoneal space;
Lymph node dissection, when clinically indicated;
Incision of the endopelvic fascia and exposure of the prostatic apex;
Dorsal vein stitch;
Retroapical dissection and release of the neurovascular bundles;
Bladder neck transection;
Control of the lateral pedicles and preservation of the neurovascular bundles;
Posterior dissection;
Incision of the dorsal venous complex and urethra;
Specimen entrapment; and
Urethrovesical anastomosis.
Most surgeons use a transperitoneal approach, but an extraperitoneal RP technique has been described.[24] The advantage of this technique is that it does not breach the peritoneal cavity, and any anastomotic leak is contained in the extraperitoneal space. However, due to this limited space, the surgery is made more difficult and the assistant job becomes more challenging.
Results
The cumulative experience in RP published worldwide is summarized in Table 1 through 6.[24-35] To date, the Henry Ford group under the leadership of Dr. Menon has reported on the largest series of patients undergoing RP.[36] Since the development of this robotic program in March 2001, over 1000 cases have been successfully performed. Visitors who have assisted the operations have appreciated the quality of the surgical field, the precision of the surgical steps, and the anatomical details allowed by this technique. For the first 200 cases, the mean operative time was 160 minutes, with a mean estimated blood loss of 153 cc and 0% transfusion rate. A total of 93% of the patients were discharged home within 24 hours.[32]
Table 1.
Baseline Parameters
| Series | Year | Number | FU Duration | Age (Years) | BMI | PSA (ng/mL) | Prostate Volume (mL) | G 2-5 | G 6 | G 7 | G 8-10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbou[25] | 2001 | 1 | NA | 63 | NA | 7 | NA | 0 | 1 (100%) | 0 | 0 |
| Pasticier[26] | 2001 | 5 | NA | 58 (55-63) | NA | 12.4 (8.7-23.6) | 37.4 (30-50) | 0 | 3 (60%) | 1 (20%) | 1 (20%) |
| Rassweiler[27] | 2001 | 6 | NA | 64.2 (57-71) | NA | 8.4 (2.4-12) | 31.3 (19-40) | 0 | 4 (67%) | 1 (16%) | 1 (16%) |
| Bentas[28] | 2003 | 40 | 15 (11-23) | 61.3 (45-72) | 25.5 (20.7-31.4) | 11.5 (0.5-53) | 34.4 (15-100) | NA | NA | NA | NA |
| Gettman[24] | 2003 | 4 | NA | 62 (61-70) | NA | 11.3 (3.6-24) | NA | 0 | 2 (50%) | 2 (50%) | 0 |
| Kaouk[29] | 2003 | 1 | 7 | 48 | NA | 12.5 | 40 | 0 | 0 | 1 (100%) | 0 |
| Ahlering[30] | 2003 | 45 | Up to 9 months | 61.4 (46-71) | 28.1 (20.2-39.4) | 7.3 (1.1-24) | 52.5 (12.5-163) | G 6 or less--20 (44%) | 16 (36%) | 7 (16%) | |
| Ahlering[31] | 2004 | 60 | 3 | 62.9 (43-78) | 26.3 (20.6-33.6) | 8.1 (0.1-62) | 52.5 (18-135) | G 6 or less--37 (62%) | 14 (23%) | 9 (15%) | |
| Tewari and Menon[32] | 2003 | 200 | 7.9 | 59.9 (40-72) | 27.7 (19-38) | 6.4 (0.6-41) | 58.8 (18-140) | 1 (0.5%) | 86 (43%) | 80 (40%) | 21 (10.5%) |
| Samadi[35] | 2002 | 11 | NA | 67 (58-71) | NA | 8.9 (3.9-32) | 67.6 (37-120) | NA | NA | NA | NA |
| Weighted Means | 373 | 61 | 27.3 | 7.6 | 53.8 | G 6 or less 47.51% | 35.71% | 12.15% | |||
FU = follow-up; BMI = body mass index (kg/m2); PSA = prostate-specific antigen; G = Gleason
The initial Frankfurt experience was first reported by Binder and Kramer[37] on 10 patients using a combination of the Walsh retrograde and Campbell antegrade approaches. They updated their series with 40 consecutive patients with 1-year follow-up (see Table 1).[28] In this series, their estimated blood loss was 570 mL (100-2500 mL), with a transfusion rate of 32.5% that they attributed to the technique used. After this initial experience this group adopted the Montsouris technique with minor modifications, and to date has performed more than 100 cases.[38]
The RP program was started in May 2002 at the University of California, Irvine Medical Center. A fellowship-trained urologic oncologist with no laparoscopic experience, assisted at the patient side by an experienced laparoscopist, was able to perform his first RP in 6.5 hours with an estimated blood loss of 300 mL,[39] demonstrating successful transfer of open surgical skills to a laparoscopic environment using a robotic interface. The learning curve to a 4-hour proficiency was 12 patients, and the mean operating time subsequently was 3.45 hours (range, 2.5-5.1 hours) for the initial cohort of 45 patients (see Table 1).[30] To date, Ahlering and colleagues[40] have performed more than 100 cases with a single assistant.
We have now compiled a total of 10 series with 373 patients (Table 1). None of these series had long-term follow-up, and much of the cancer control and functional data are still immature. However, early results demonstrate favorable operative and pathologic outcomes. The mean operative time was 222 minutes, in addition to 32 minutes of installation time. With experience the set-up time is expected to decrease to 15 minutes or less. The overall estimated blood loss was 231 mL with no transfusions in most reported series. These figures are well below the blood losses reported during open retropubic prostatectomy.
Bentas and coworkers[28] had an exceptionally high transfusion rate of 32.5%, which skewed the weighted mean transfusion rate to 3.9% (Table 2). The mean hospital stay, excluding Bentas' series,[28] was 1.4 days. The mean hospital stay in that study was 17.1 days because patients were discharged home after catheter removal. The pathologic distribution was similar to contemporary series of radical prostatectomy, with the majority of patients having pathologic stage T2a and T2b (Table 3).
Table 2.
Operative Parameters
| Series | Installation Time (min) | Operative Time (min) | Total OR Time (min) | EBL (mL) | Blood Transfusion | Conversion/ Abortion | Hosp Stay (Days) | Catheter Duration (days) |
|---|---|---|---|---|---|---|---|---|
| Abbou[25] | 30 | 420 | 465 | 300 | 0 | 0 | 4 | 3 |
| Pasticier[26] | 93 (76-149) | 222 (150-381) | NA | 800 (700-1600) | 0 | 0 | 5.5 (4-7) | 6.5 (5-9) |
| Rassweiler[27] | 47 (40-50) | 315 (242-480) | NA | NA | 1 (16%) | 0 | NA | 7.3 (5-14) |
| Bentas[28] | NA | 500 (246-780) | 594 (288-930) | 570 (100-2500) | 13 (32.5%) | 2 (5%) | 17.1 (6-32) | 16.7 (5-49) |
| Gettman[24] | NA | 274 (1424-360) | NA | 1013 (550-1500) | NA | 0 | 5.3 (3-9) | 2.7 (2-3) |
| Kaouk[29] | NA | 285 | NA | 300 | 0 | 0 | 2 | 21 |
| Ahlering[30] | 23 (15-40) | 209 (150-600) | 228 (NA) | 145 (25-350) | 0 | 0 | 1.6 (1-7) | 7 |
| Ahlering[31] | NA | NA | 231 (160-340) | 103 (25-400) | 0 | 0 | 1.1 (0.75-4) | 7 |
| Tewari and Menon[32] | NA | 160 (71-315) | NA | 153 (25-750) | 0 | 2 (1%) | 1.2 (< 1-5) | 7 (1-18) |
| Samadi[35] | NA | 300 (200-4200) | NA | 900 (400-1600) | NA | 0 | NA | NA (2-5) |
| Weighted Means | 32 | 222 | 331 | 231 | 0.3% (3.9%)* | 1.1% | 1.4 (3.1)** | 8.1 |
OR = operating room time (includes patient prep) ; EBL = estimated blood loss
*The weighted mean transfusion rate is 3.9% for all reported series, and 0.3% if Bentas' series is excluded.
**The weighted mean of hospital stay is 3.1 days for all reported series, and 1.4 days if Bentas' series is excluded. Patients in this study were discharged home after catheter removal.
Table 3.
Pathologic Parameters
| Series | pT2a | pT2b | pT3a | pT3b | pT4 | LN+ | SM+ |
|---|---|---|---|---|---|---|---|
| Abbou[25] | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 |
| Pasticier[26] | 2 (40%) | 3 (60%) | 0 | 0 | 0 | NA | 1 (20%) |
| Rassweiler[27] | 1 (16%) | 1 (16%) | 4 (67%) | 0 | 0 | 0 | 0 |
| Bentas[28] | 7 (18%) | 18 (45%) | 9 (22%) | 6 (15%) | 0 | NA | 12 (30%) |
| Gettman[24] | 1 (25%) | 2 (50%) | 1 (25%) | 0 | 0 | 0 | 1 (25%) |
| Kaouk[29] | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) |
| Ahlering[30] | 6 (13%) | 21 (47%) | 11 (24%) | 5 (11%) | 1 (2%) | NA | 16 (35%) |
| Ahlering[31] | 16 (27%) | 29 (48%) | 10 (17%) | 4 (7%) | 1 (1%) | NA | 10 (17%) |
| Tewari and Menon[32] | 30 (15%) | 144 (72%) | 14 (7%) | 12 (6%) | 0 | 2 (1%) | 12 (6%)* |
| Samadi[35] | 5 (45%) | 3 (27%) | 2 (18%) | 1 (9%) | 0 | 1 (9%) | 3 (27%) |
| Weighted Means | 18% | 61% | 14% | 7.5% | 0.5% | 0.8% | 15% |
PT = pathological stage; LN+ = positive lymph nodes; SM+ = positive surgical margins
The positive surgical margin rate was very acceptable with a mean of 15%, including both focal and extensive margins (Table 3). The overall complication rate for all reported series was low, with 4.55% minor and 3.75% major complications. There was no preoperative mortality (Table 4 ).
Table 4.
Morbidity and Mortality
| Series | Minor Complications | Major Complications | Mortality |
|---|---|---|---|
| Abbou[25] | 0 | 0 | 0 |
| Pasticier[26] | 1 (20%) 1 superficial bowel injury |
0 | 0 |
| Rassweiler[27] | 0 | 0 | 0 |
| Bentas[28] | 8 (20%) 2 venous bleeding 4 anastomotic leak 2 UTI |
5 (12.5%) 2 PE 1 DVT 1 obturator nerve injury |
0 |
| Gettman[24] | 0 | 0 | 0 |
| Kaouk[29] | 0 | 0 | 0 |
| Ahlering[30] | 3 (7%) 2 anastomotic leaks 1 port site bleeding |
3 (7%) 1 DVT 1 anastomotic disruption 1 leg neuromuscular injury |
0 |
| Ahlering[31] | 2 (3%) 1 anastomotic leak 1 ileus |
2 (3%) 1 PE 1 delayed anastomotic bleed |
0 |
| Tewari and Menon[32] | 3 (1.5%) 3 ileus |
4 (2%) 2 wound dehiscence/hernia 1 DVT 1 post-op bleeding |
0 |
| Samadi[35] | 0 | 0 | 0 |
| Weighted Means | 4.55% | 3.75% | 0 |
UTI = urinary tract infection; PE = pulmonary embolism; DVT = deep vein thrombosis
Continence
Table 5 summarizes the postoperative results of return of continence in the 4 largest series with a minimum follow-up of 3 months. The mean follow-up was 6.1 months and all continence data were based on patient-filled questionnaires. Despite the short follow-up, 87% of patients were completely continent and used no pads or just a liner for protection.
Table 5.
Postoperative Continence
| Series | Total Number | Number Evaluated | Age (Years) | Follow-up (Months) | Evaluation Method | Continent (0-1 Pad) | 0 Pads | 1 Pad | 2 or More Pads |
|---|---|---|---|---|---|---|---|---|---|
| Bentas[28] | 40 | 38 | 61.3 (45-72) | 15 (11-23) | Questionnaire | 32 (84%) | 26 (68%) | 6 (16%) | 6 (16%) |
| Ahlering[30] | 45 | NA | 61.4 (46-71) | 3 | Questionnaire | NA (95%) | NA (81%) | NA (14%) | NA (5%) |
| Ahlering[31] | 60 | NA | 62.9 (43-78) | 3 | Questionnaire | NA | NA (76%) | NA | NA |
| Menon and Tewari[33] | 200 | NA | 59.9 (42-76) | 6 | Questionnaire | NA (96%) | NA (96%) [0 pad or 1 liner] | NA (4%) [1 or more pads] | NA |
| Weighted Means | 245 | NA | 60.8 | 6.1 | Questionnaire | 94% | 87.5% | 7% | NA |
Bentas and associates[28] reported postoperative continence status for 38 of 40 patients, 11-23 months after surgery (mean, 15 months). A total of 26 patients (68%) were completely continent without pads after a median of 2 months (range, 0-7 months). Twelve patients (32%) still used pads. Of these patients, 6 used 1 pad a day for safety reasons. Three patients used 1 or 2 pads for mild stress incontinence; 3 patients needed 3 or 4 pads for severe stress incontinence.[28]
In their early experience, Ahlering and colleagues[31] showed that at 1 week, 33% of the patients were pad-free. At 1 month, 63% of the patients were pad-free and 25% used a security pad. At 3 months, 81% of patients used no pads and 14% used a security pad or 1 pad daily, while 5% used 1-3 pads daily. Menon and colleagues[33] reported that at 6 months, 96% of patients were either free of having to wear pads or were using a liner for security reasons, and 4% were using 1 or more pads. Patients who were dry or using a liner were "mostly satisfied" to "delighted" according to patient-filled questionnaires, whereas those wearing pads were "mostly dissatisfied" or "unhappy" with the quality of life. In addition, 4% of patients had a 4-point increase in the International Prostate Symptom Score (IPSS), and 33% had a 4-point decrease in IPSS.[33]
Potency
Table 6 summarizes postoperative sexual function in patients who underwent RP. The data are not mature in most series, and therefore definitive conclusions cannot be drawn at the moment. In the 4 series that reported on potency,[28,30,32,34] 49.5% of patients had intercourse and 79% had return of erections, with or without medical assistance, at a mean weighted follow-up of only 7.7 months.
Table 6.
Postoperative Sexual Function
| Series | Total Number | Number Evaluated | Number Potent Preop | Age | UNS | BNS | FU (months) | Evaluation Method | Potent | Intercourse | Erection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bentas[28] | 40 | 38 | 37 | 61.3 (45-72) | NA | NA | 15 (11-23) | Questionnaire | 8 (22%) | ||
| Ahlering[30] | 45 | 9 | 3 | 57.3 (54-59) | 1 (33%) | 2 (66%) | up to 9 | Questionnaire | 1 (33%) | ||
| Menon and Tewari[32] | 200 | Only preop potent patients | NA | < 60 | NA | NA | 6 | Questionnaire | NA (64%) | NA (64%) | NA (82%) |
| > 60 | NA | NA | 6 | Questionnaire | NA (38%) | NA (38%) | NA (75%) | ||||
| Chien[34] | 16 | 16 | 16 | 59 (NA) | 7 (44%) | 9 (56%) | NA (1-10) | NA | 8 (50%) | 5 (31%) | 14 (87.5%) |
| Weighted Means | 301 | 59.6 | 7.7 | 46.5% | 49.5% | 79% |
UNS = unilateral nerve sparing; BNS = bilateral nerve sparing; FU = follow-up
Menon and colleagues[33] noted that at 6 months, 82% of men who were younger than 60 years of age had "return of sexual activity" and 64% had sexual intercourse. "Return of sexual activity" was defined as definite return of erections and participation in a wide variety of sexual activities, excluding intercourse. Seventy-five percent of men over 60 years of age had return of sexual activity at 6 months and 38% had sexual intercourse.[33] At the time of analysis, 42% of all patients were using sildenafil.[32] Bentas and colleagues[28] noted that at 1-year follow-up, all 37 patients who were potent before radical prostatectomy had a reduction in erectile function. Eight patients had regained sexual activity, but required assistance.
Robot-assisted laparoscopic sural nerve grafting during radical prostatectomy was reported by Kaouk and coworkers[41] in 3 patients. Two patients had bilateral resection of the neurovascular bundles and 1 patient had a unilateral resection with grafting. Prostatectomy was performed robotically in 1 case and in a standard laparoscopic fashion in the 2 remaining cases. During a mean follow-up of 4.3 months, the patient with unilateral nerve preservation was potent without any medication and 1 patient with bilateral nerve grafts reported penile engorgement with sildenafil that was not sufficient for penetration.
Robotic vs Open Radical Prostatectomy
There are 2 published prospective nonrandomized studies comparing open radical retropubic prostatectomy (ORRP) and RP. Tewari and colleagues[32] reported on 100 ORRPs by multiple surgeons and 200 contemporaneous RPs by a single surgeon at the same institution. The baseline characteristics were comparable between both groups (Figure 3).
Figure 3.
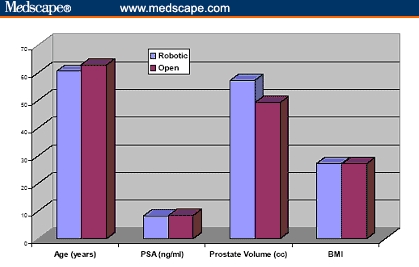
Baseline parameters of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32] (PSA = prostate-specific antigen; BMI = body mass index [kg/m2])
The operative duration was not different (163 vs 160 min). The blood loss was 910 and 150 mL for ORRP and RP, respectively, and transfusion rate was greater after ORRP (67% vs none). It is to be noted, however, that in the ORRP group, only 11% of patients received banked blood transfusions and the remaining 56% received autologous blood. There were 4 times as many complications after ORRP (20% vs 5%), and the hospital stay was longer (3.5 vs 1.2 days). Ninety-three percent of the RP and none of the ORRP patients were discharged within 24 hours. Positive margins were more frequent after ORRP (23% vs 9%) (Figures 4-10).
Figure 4.
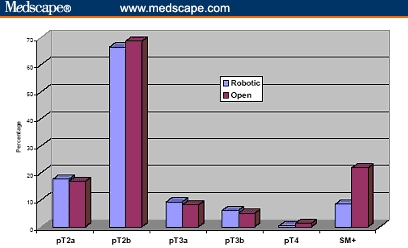
Pathological stages and positive surgical margins rate of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32] (pT = pathological stage; SM+ = positive surgical margin)
Figure 10.
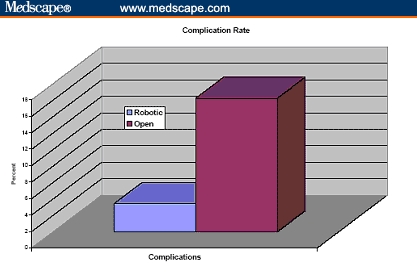
Complication rate of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
The most striking finding was that patients who underwent RP achieved continence more quickly than after ORRP, and the 50% return of continence occurred at 44 and 160 days, respectively. Sexual function was also evaluated in patients who classified themselves as having normal preoperative erections and sexual intercourse, and those who had a bilateral nerve-sparing procedure (number unknown). Patients after RP had a more rapid return of erection (50% return at a mean follow-up of 180 days vs 440 days after ORRP). The return of intercourse was also quicker after RP, with half of the patients achieving intercourse at a mean follow-up of 340 days. At the time of analysis, half of the ORRP patients had not yet achieved return of intercourse at 700 days. Of the RP and ORRP patients, 42% and 65%, respectively, used sildenafil.[32]
Ahlering and coworkers[31] internally compared a single surgeon's ORRP (n = 60) and RP (n = 60) after the robotic learning curve had adequately matured. Both groups had similar clinical characteristics. No statistically significant differences were found between groups for pathologic stage, Gleason score, or margin status (16.7% vs 20%). Operative times (skin to skin) were also similar (231 vs 214 minutes). The RP group had a statistically significant advantage for estimated blood loss (103 vs 418 mL), postoperative hemoglobin change (1.6 vs 3.3 mg/dL), and hospital stay (1.02 vs 2.2 days). The rate of postoperative complications was similar for the ORPP and RP groups (6.7% vs 10%). Complete continence (0 pads) at 3 months of follow-up was not statistically different (76% vs 75%).[31] Figures 1 through 7 show the differences in baseline and operative and postoperative parameters between RP and ORRP. Data represent the weighted means of the 2 aforementioned prospective series.[31,32]
A prospective comparison of postoperative pain between patients undergoing ORRP (n = 40) and RP (n = 40) by a single surgeon found no significant differences in pain scores or narcotic use, which were low in both approaches.[42] In contrast, Tewari and colleagues[32] reported a significant difference in postoperative pain scores in their prospective comparison, favoring RP over ORRP (mean pain score, 3 [range 1-7] vs 7 [range 4-10]).
In conclusion, RP seems to be at least comparable to ORRP in terms of clinical outcomes, including operative time, margin status, catheterization interval, blood transfusion, postoperative complications, and continence rates. RP offers the benefits of minimally invasive surgery, and does not compromise clinical or pathologic outcomes.
Robotic vs Laparoscopic Prostatectomy
Laparoscopic radical prostatectomy is associated with a steep learning curve. Even in the hands of expert laparoscopists this technique requires approximately 40 cases to master. Wood and Woolf[43] compared 10 robotic prostatectomies with 10 laparoscopic prostatectomies performed by the same surgeon, who is an experienced upper urinary tract laparoscopist. Despite the large laparoscopic experience, the mean operative time for RP (347 min) was less than for laparoscopic prostatectomy (492 min). The estimated blood loss was comparable (< 500 cc), and there were 2 conversions in each group, but no major complications occurred.
Menon and colleagues[8] performed 48 laparoscopic radical prostatectomies and 50 robot-assisted prostatectomies within a 12-month period. The preoperative and intraoperative demographical variables were comparable in both groups, as were the operative times, changes in hemoglobin concentrations, durations of hospitalization, positive margin rates, and overall complication rates. All measured clinical and pathologic parameters were comparable to the best laparoscopic radical prostatectomy results reported in the literature.[8]
Laparoscopic vs Open Radical Prostatectomy
Two studies have compared open radical prostatectomy with standard laparoscopic prostatectomy. One study, by Anastasiadis and associates,[44] prospectively compared the functional results after retropubic (n = 70) and laparoscopic radical (n = 230) prostatectomy, performed by different surgeons. No differences were found between the 2 groups except for the duration of bladder catheterization, which was 7.8 days for the retropubic and 5.8 for the laparoscopic approach. The 2 approaches showed similar outcomes regarding continence and erectile function 1 year after surgery. However, the recovery of nocturnal continence seemed to occur sooner after the laparoscopic approach.
The other study was an internal comparison of 3 surgeons by Rassweiler and associates[45] that compared 219 patients treated with ORRP with 438 patients treated with laparoscopic prostatectomy. Patients treated by laparoscopic prostatectomy were divided into 2 equal chronological groups of 219 each. Mean operating time was significantly shorter after open surgery (196 minutes) compared with the early laparoscopic group (288) but it did not differ significantly from the late laparoscopic group (218). Mean blood loss and transfusion and complication rates favored the laparoscopic groups. The amount of postoperative analgesia was significantly greater after open surgery, and median catheter time was longer, but the continence rates were similar in all 3 groups at 12 months (89.9% vs 90.3% vs 91.7%). The rate of positive margins and biochemical recurrence did not differ significantly between all groups.
Nerve-Sparing Robotic Prostatectomy
Although the data on potency following RP are still scarce, as noted previously (Table 6), good anatomic foundation of the feasibility of robotic nerve-sparing surgery has been shown.[46] Tewari and associates performed dissections of 12 male cadavers using a combination of laparoscopic equipment and open surgical dissection. Dissections mimicked various steps of robotic and laparoscopic prostatectomy, and provided a detailed operative map for nerve preservation.
They described a potential triangular space containing the neurovascular bundles; the inner layer of the periprostatic fascia (also called prostatic fascia) forms the medial vertical wall of this triangle; the outer layer of the periprostatic fascia (also called lateral pelvic fascia) forms the lateral wall, and the posterior wall of this triangle is formed by the anterior layer of the Denonvilliers' fascia.
They noted that the neurovascular bundle was located within this triangle approximately 1.5 mm posterolaterally at the base and about 3 mm at the apex. Cross-connections between branches of the pelvic plexus on each side were also noted.
Learning Curve
The learning curve of a new technique is always a matter of debate, especially during the initial phases of validation. Although robotic surgery uses complex technology, the mechanical interface at the surgeon's console provides quasi-intuitive transfer of open surgical skills. In urology as well as other specialties, there seems to be a consensus that the learning curve is not as steep as for laparoscopic surgery.
In our meta-analysis the cumulative conversion rate is very low (1.1%), which contrasts with high conversion rates reported for laparoscopic prostatectomy, especially during the learning curve. Giulianotti and colleagues[47] showed that for robotic cholecystectomy and Nissen fundoplication, the operative times significantly decreased after 26 and 21 cases, respectively, and that after approximately 20 cases, the operative times were similar to those for traditional laparoscopy.
Ahlering and associates[30] demonstrated successful transfer of open surgical skills in radical prostatectomy to a laparoscopic environment using a robotic interface in a laparoscopy-naive surgeon. The learning curve to 4-hour proficiency was 12 patients and the mean operating time was then 3.45 hours (range, 2.5-5.1 hours) for the initial cohort of 45 patients.
Menon and coworkers[8] estimated that the learning curve of RP was approximately 18 cases in order to reach a comparable level of efficiency as experienced laparoscopic prostatectomy surgeons. They believe that most surgeons with experience in ORRP can become "very good at [RP], usually even better than at open surgery." The success of Menon's team was based on commitment, frequent case schedule, and critical analysis of each case.[48]
The question of whether previous laparoscopic training facilitates the acquisition of robotic skills is not yet fully answered. However, when comparing the initial RP experience of a urologic oncologist and an experienced laparoscopic urologist (10 cases each), Wood and Wolf[43] found that the oncology-trained surgeon had shorter mean operative times (244 vs 347 min). They concluded that open surgical experience in retropubic prostatectomy appeared to be more important than laparoscopic experience when performing an RP.
Costs
The cost issue surrounding robotic surgery is still unresolved. The da Vinci surgical system costs $1.2 million, with a maintenance fee of $100,000/year after the first year. The average cost of disposables is approximately $1500 per case. Guru and colleagues[49] conducted a cost analysis for 30 consecutive patients undergoing RP and 30 contemporaneous ORRP.
Overall costs were divided into 9 categories, of which laboratory and supplies costs were higher for the robotic approach; pharmacy, recovery room, and ward care costs were higher for the open approach; and anesthesia and operative room costs were not statistically different. The overall cost per case, excluding maintenance and depreciation of the robotic system, was similar for both approaches. It was estimated that depreciation over a 5-year period would add $ 1000 per case assuming 300 cases per year.[49] Indeed, only few institutions perform such a high number of cases per annum.
However, from a healthcare perspective, the uncalculated savings gained in quicker patient recovery and return to normal activity and productivity will have to be factored in for future analyses.
Conclusion
The current evidence suggests that RP is at least comparable in efficacy to open and laparoscopic prostatectomy, including clinical and pathologic parameters. Long-term cancer control and functional results are needed to supplement these early observations. RP is a promising approach for the surgical treatment of prostate cancer.
Figure 5.
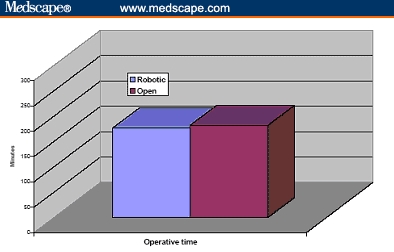
Operative time of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
Figure 6.
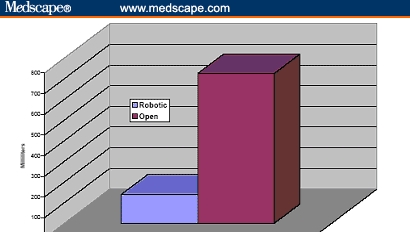
Blood loss of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
Figure 7.
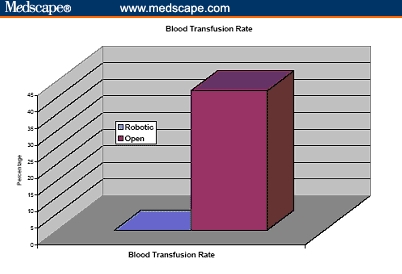
Blood transfusion rate of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
Figure 8.
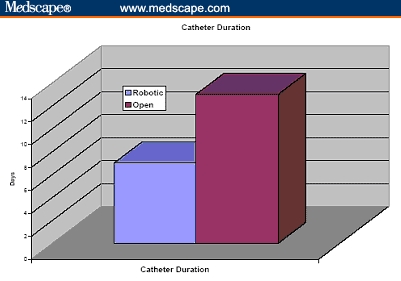
Catheter duration in cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
Figure 9.
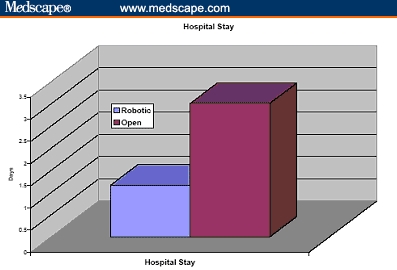
Hospital stay of cumulative 260 patients undergoing robotic prostatectomy compared with 160 patients undergoing open radical retropubic prostatectomy.[31,32]
Contributor Information
Assaad El-Hakim, Fellow in Urology, Cornell Institute of Robotic Surgery, Department of Urology, New York-Presbyterian Hospital, Weill Medical College of Cornell University, New York, NY.
Ashutosh Tewari, irector of Robotic Prostatectomy, Associate Professor, Department of Urology, Cornell Institute of Robotic Surgery, Department of Urology, New York-Presbyterian Hospital, Weill Medical College of Cornell University, New York, NY.
References
- 1.Schuessler WW, Schulam PG, Clayman RV, et al. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854-857. [DOI] [PubMed] [Google Scholar]
- 2.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643-1649. [DOI] [PubMed] [Google Scholar]
- 3.Abbou CC, Salomon L, Hoznek A, et al. Laparoscopic radical prostatectomy: preliminary results. Urology. 2000;55:630-634. [DOI] [PubMed] [Google Scholar]
- 4.Rassweiler J, Schulze M, Teber D, et al. Laparoscopic radical prostatectomy: functional and oncological outcomes. Curr Opin Urol. 2004;14:75-82. [DOI] [PubMed] [Google Scholar]
- 5.Kavoussi LR. Laparoscopic radical prostatectomy: irrational exuberance? Urology. 2001;58:503-505. [DOI] [PubMed] [Google Scholar]
- 6.Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165:1964-1966. [DOI] [PubMed] [Google Scholar]
- 7.Pasticier G, Rietbergen JB, Guillonneau B, et al. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001;40:70-74. [DOI] [PubMed] [Google Scholar]
- 8.Menon M, Shrivastava A, Tewari A, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945-949. [DOI] [PubMed] [Google Scholar]
- 9.Tewari A, Peabody J, Sarle R, et al. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. 2002;60:569-572. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Murray T, Samuels A, et al. Cancer Statistics, 2003. CA Cancer J Clin. 2003;53:5-26. [DOI] [PubMed] [Google Scholar]
- 11.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557-566. [DOI] [PubMed] [Google Scholar]
- 12.Litwin MS, Shpall AI, Dorey F, et al. Quality-of-life outcomes in long-term survivors of advanced prostate cancer. Am J Clin Oncol. 1998;21:27-32. [DOI] [PubMed] [Google Scholar]
- 13.Henderson A, Laing RW, Langley SE. Quality of life following treatment for early prostate cancer: does low dose rate (LDR) brachytherapy offer a better outcome? A review. Eur Urol. 2004;45:134-141. [DOI] [PubMed] [Google Scholar]
- 14.Litwin MS, McGuigan KA, Shpall AI, et al. Recovery of health related quality of life in the year after radical prostatectomy: early experience. J Urol. 1999;161:515-519. [PubMed] [Google Scholar]
- 15.Brucker PS, Cella D. Measuring self-reported sexual function in men with prostate cancer. Urology. 2003;62:596-606. [DOI] [PubMed] [Google Scholar]
- 16.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160:2418-2424. [DOI] [PubMed] [Google Scholar]
- 17.Salomon L, Sebe P, De La Taille A, et al. Open versus laparoscopic radical prostatectomy: Part II. BJU Int. 2004;94:244-250. [DOI] [PubMed] [Google Scholar]
- 18.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473-485. [DOI] [PubMed] [Google Scholar]
- 19.Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292-2295. [DOI] [PubMed] [Google Scholar]
- 20.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299-315. [PubMed] [Google Scholar]
- 21.Epstein JI, Partin AW, Sauvageot J, et al. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol. 1996;20:286-292. [DOI] [PubMed] [Google Scholar]
- 22.Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 200260:864-868. [DOI] [PubMed] [Google Scholar]
- 23.Menon M, Tewari A, Peabody J; The VIP Team. Vattikuti Institute prostatectomy: technique. J Urol. 2003;169:2289-2292. [DOI] [PubMed] [Google Scholar]
- 24.Gettman MT, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy: description of the extraperitoneal approach using the da Vinci robotic system. J Urol. 2003;170:416-419. [DOI] [PubMed] [Google Scholar]
- 25.Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165:1964-1966. [DOI] [PubMed] [Google Scholar]
- 26.Pasticier G, Rietbergen JB, Guillonneau B, et al. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001;40:70-74. [DOI] [PubMed] [Google Scholar]
- 27.Rassweiler J, Frede T, Seemann O, et al. Telesurgical laparoscopic radical prostatectomy. Initial experience. Eur Urol. 2001;40:75-83. [DOI] [PubMed] [Google Scholar]
- 28.Bentas W, Wolfram M, Jones J, et al. Robotic technology and the translation of open radical prostatectomy to laparoscopy: the early Frankfurt experience with robotic radical prostatectomy and one year follow-up. Eur Urol. 2003;44:175-181. [DOI] [PubMed] [Google Scholar]
- 29.Kaouk JH, Desai MM, Abreu SC, et al. Robotic assisted laparoscopic sural nerve grafting during radical prostatectomy: initial experience. J Urol. 2003;170:909-912. [DOI] [PubMed] [Google Scholar]
- 30.Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738-1741. [DOI] [PubMed] [Google Scholar]
- 31.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology. 2004;63:819-822. [DOI] [PubMed] [Google Scholar]
- 32.Tewari A, Srivasatava A, Menon M, et al. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205-210. [DOI] [PubMed] [Google Scholar]
- 33.Menon M, Tewari A; Vattikuti Institute Prostatectomy Team. Robotic radical prostatectomy and the Vattikuti Urology Institute technique: an interim analysis of results and technical points. Urology. 2003;61(suppl 1):15-20. [DOI] [PubMed] [Google Scholar]
- 34.Chien GW, Orvieto MA, Galocy RM, et al. Antegrade nerve preservation during robotic laparoscopic prostatectomy: the video. J Urol. 2004;171(4 suppl; Abstract V1977): 522. [Google Scholar]
- 35.Samadi DB, Nadu A, Olsson E, et al. Robot assisted laparoscopic radical prostatectomy- Initial experience in eleven patients. J Urol. 2002;167(4 suppl; Abstract 1554):390. [Google Scholar]
- 36.Tewari A, Menon M. Vattikuti Institute prostatectomy: surgical technique and current results. Curr Urol Rep. 2003;4:119-123. [DOI] [PubMed] [Google Scholar]
- 37.Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408-410. [DOI] [PubMed] [Google Scholar]
- 38.Wolfram M, Brautigam R, Engl T, et al. Robotic-assisted laparoscopic radical prostatectomy: the Frankfurt technique. World J Urol. 2003;21:128-132. [DOI] [PubMed] [Google Scholar]
- 39.Perer E, Lee DI, Ahlering T, et al. Robotic revelation: laparoscopic radical prostatectomy by a nonlaparoscopic surgeon. J Am Coll Surg. 2003;197:693-696. [DOI] [PubMed] [Google Scholar]
- 40.Lee DI, Eichel L, Skarecky DW, et al. Robotic laparoscopic radical prostatectomy with a single assistant. Urology. 2004;63:1172-1175. [DOI] [PubMed] [Google Scholar]
- 41.Kaouk JH, Desai MM, Abreu SC, et al. Robotic assisted laparoscopic sural nerve grafting during radical prostatectomy: initial experience. J Urol. 2003;170:909-912. [DOI] [PubMed] [Google Scholar]
- 42.Webster TM, Herrell SD III, Baumgartner RG, et al. Robotic assisted laparoscopic retropubic prostatectomy: assessment of preoperative pain. J Urol. 2004;171(4 suppl; Abstract 165):44.14665840 [Google Scholar]
- 43.Wood DP, Wolf JS. Oncology rather than laparoscopy surgical experience is more important in learning to perform a robot-assisted laparoscopic radical prostatectomy. J Urol. 2004;171(4 suppl; Abstract 813):215.14665879 [Google Scholar]
- 44.Anastasiadis AG, Salomon L, Katz R, et al. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003;62:292-297. [DOI] [PubMed] [Google Scholar]
- 45.Rassweiler J, Seemann O, Schulze M, et al. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol. 2003;169:1689-1693. [DOI] [PubMed] [Google Scholar]
- 46.Tewari A, Peabody JO, Fischer M, et al. An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur Urol. 2003;43:444-454. [DOI] [PubMed] [Google Scholar]
- 47.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [DOI] [PubMed] [Google Scholar]
- 48.Menon M. Robotic radical retropubic prostatectomy. BJU Int. 2003;91:175-176. [DOI] [PubMed] [Google Scholar]
- 49.Guru KA, Bhabdari A, Peabody JO, et al. Cost comparison between robotic-assisted laparoscopic prostatectomy (Vattikuti Institute Prostatectomy) and radical retropubic prostatectomy. J Urol. 2004;171(4 suppl; Abstract 164):43. [Google Scholar]


