Abstract
The TraJ protein is a central activator of F-like plasmid conjugal transfer. In a search for regulators of traJ expression, we studied the possible regulatory role of the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex in traJ transcription using a traJ-lacZ reporter system. A comparison of the enzyme activities in the wild-type Escherichia coli strain MC4100 with those in cya and crp mutants indicated that disruption of the formation of the cAMP-CRP complex negatively influenced the activity of the traJ promoter of the F-like plasmid pRK100. The defect in the cya mutant was partially restored by addition of exogenous cAMP. Competitive reverse transcription-PCR performed with RNA isolated from the wild-type and mutant strains showed that the cAMP-CRP complex exerted its effect at the level of transcription. Electrophoretic mobility shift assays with purified CRP demonstrated that there was direct binding of CRP to the traJ promoter region. DNase I footprint experiments mapped the CRP binding site around position −67.5 upstream of the putative traJ promoter. Targeted mutagenesis of the traJ promoter region confirmed the location of the CRP binding site. Consistent with the demonstrated regulation of TraJ by the cAMP-CRP complex, mutants with defects in cya or crp exhibited reduced conjugal transfer from pRK100.
Conjugation leads to the transfer of genetic material from one bacterium to another and is directed by conjugative plasmids. One family of conjugative plasmids is the F-like plasmids present in Escherichia coli and related species. F-like plasmids carry a ∼33-kb transfer (tra) region that harbors approximately 40 genes responsible for conjugal transfer (Fig. 1A). The expression of the tra genes is tightly regulated by both plasmid-encoded and chromosomally encoded proteins (8), although subtle differences exist among the various F-like plasmids (5, 25). The main plasmid-encoded positive regulator of conjugation is the 27-kDa protein TraJ, which is required for initiation of high levels of transcription from the major tra promoter, PtraY. Full activation of PtraY of plasmids F and R1 also requires the chromosomally encoded ArcA protein, which is part of the ArcA-ArcB two-component system that responds to oxygen (30). In plasmids F and R100, the TraY protein further stimulates its own promoter, and this autoactivation enhances the synthesis of proteins that form the scaffolding of the conjugation machinery. TraY also induces DNA bending and stimulates nicking at the origin of transfer in cooperation with IHF (22). This leads to expression of the traM gene, which is essential for DNA transfer of F and R1. Eventually, TraM autorepression limits the activity of the tra operon. In plasmids R1 and R100 but not in plasmid F, traJ expression has been shown to be linked to traM (5, 25).
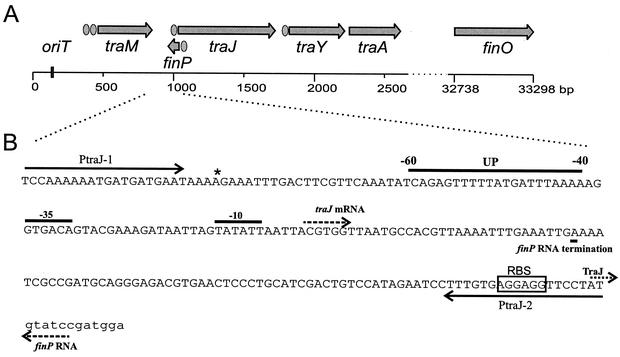
FIG. 1.
Schematic diagram of the F-like plasmid transfer region. (A) F-plasmid tra operon: schematic diagram of the first 2,700 bp and the last 600 bp of the 33.3-kb tra operon (11). The positions of the origin of transfer (oriT), the traM, traJ, traY, traA, and finO genes, and the finP antisense RNA are indicated. Promoters are indicated by ellipses. (B) Genetic organization of the traJ promoter region of the F-like plasmid pRK100. The positions of the −35 and −10 promoter regions, transcription initiation (traJ mRNA and finP RNA) and termination (finP RNA termination), the ribosomal binding site (RBS), and initiation of TraJ translation (TraJ) are indicated. The location of upstream element (UP) from position −40 to position −60 is also shown. The binding sites of oligonucleotide primers PtraJ-1 and PtraJ-2 are indicated by solid arrows. The sequence of the pRK100 plasmid differs at one base (indicated by an asterisk) from the published sequence of the same region of the F plasmid (GenBank accession number U01159).
Even though TraJ is a central positive regulator of the transfer region, our knowledge concerning the mechanisms that regulate expression of the traJ gene itself is limited. TraJ expression is regulated at the translational level through the fertility inhibition FinOP system (7, 10). Fertility inhibition is imposed by the action of two tra gene products, FinP, which is the antisense RNA molecule complementary to the 5′ untranslated region of traJ mRNA, and FinO, which increases the concentration of FinP and thereby promotes the formation of the traJ-FinP duplex. The traJ-FinP duplex is degraded by RNase III, thereby decreasing the amount of the TraJ protein (15). The combined actions of FinO and FinP repress plasmid F transfer by 100- to 1,000-fold, while FinP, by itself, represses F transfer by only 6-fold (18). Furthermore, it has been demonstrated for plasmid F that in cpx mutant strains the TraJ protein level is reduced (29).
Considering the importance of TraJ in bacterial conjugation promoted by plasmid F and other F-like plasmids, we focused on the discovery of mechanisms of transcriptional regulation of traJ expression. In these studies, we used pRK100, a ∼145-kb natural conjugative F-like plasmid isolated from a uropathogenic E. coli strain, as a model system. This plasmid has been partially characterized (1), and its tra region has been partially sequenced. At the nucleotide level, the sequenced tra genes (traM, finP, traJ, traY, traD, finO) were most similar to genes of plasmid F (4). The regulation of the pRK100 traJ promoter was studied by using a reporter system consisting of a transcriptional fusion of the traJ promoter and the lacZ gene. Expression from this construct was studied in the absence of the FinOP fertility inhibition system to facilitate identification of factors that act at the level of traJ transcription initiation. Our data indicate that expression of the traJ gene varies with the growth cycle and that the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex is a positive regulator of traJ transcription. The regulatory role of this complex was supported by results of gel retardation assays, DNase I footprint experiments, targeted mutagenesis of the CRP binding site, and mating assays.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) medium with aeration at 37°C, unless stated otherwise. When appropriate, bacteria were grown in minimal medium M63 (24) supplemented with 0.2% glucose, 0.1% Casamino Acids, and 1 μg of thiamine per ml. Conditioned medium was prepared by growing strain MC4100 in LB medium for 12 h at 37°C with aeration, removing the bacteria by centrifugation, and filter sterilizing the medium. Conditioned medium was used within 24 h. Starvation for glucose, Casamino Acids, and phosphate was achieved by adding only one-fifth the usual concentrations of these compounds to M63 medium. When appropriate, cAMP (10 mM) was added to LB medium. Ampicillin (100 μg/ml) was added to the growth media as necessary.
TABLE 1.
Bacterial strains and plasmids
| Strains or plasmid | Genotype or properties | Source or reference |
|---|---|---|
| Strains | ||
| MC4100 | araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 ptsF25 deoC1 | 3 |
| RH74 | MC4100 Δcya851 ilv::Tn10 | 20 |
| SBS688 | MC4100 Δcrp39 | 13 |
| DH5α | thi-1 hsdR17 gyrA96 recA1 endA1 glnV44 relA1 φ80dlacZΔM15 phoA8 | NCCB |
| Plasmids | ||
| pRK100 | F-like plasmid | 1 |
| pCB267 | lacZ promoter probe vector | 28 |
| pTJ1 | pCB267; 210-bp traJ promoter insert | This study |
| pGEM-T Easy | T-vector for cloning of PCR products | Promega |
| pUC19 | Multipurpose cloning vector | 33 |
| pINP2 | PGEM-T Easy; 158-bp ΔlacZ insert | This study |
| pYZCRP | CRP encoding | R. H. Ebright |
General DNA manipulation techniques.
Plasmid DNA isolation, ligation, and transformation experiments were performed by using standard methods (27). Restriction endonuclease digestion was carried out as specified by the manufacturer (Promega, Boehringer). DNA fragments were isolated from agarose gels by using a QIAquick gel extraction kit (Qiagen). DNA sequencing was performed by using a dye rhodamine terminator cycling reaction and an ABI PRISM310 genetic analyzer.
Construction of traJ-lacZ fusions.
A 210-bp fragment of pRK100 containing 208 nucleotides upstream of the traJ translation initiation site and the first two nucleotides of the traJ start codon ATG was amplified by PCR by using primers PtraJ-1 (5′-CGGGATCCTCCAAAAAATGATGATGAAT-3′) and PtraJ-2 (5′-GCTCTAGAATAGGAACCTCCTCACAAAG-3′) (Fig. 1B). PtraJ-1 and PtraJ-2 contained BamHI and XbaI restriction sites to facilitate cloning into plasmid pCB267 (28), which generated pTJ1. To generate mutations in the CRP binding site, primers with defined nucleotide substitutions were used in the PCRs. For mutagenesis of the left part of the CRP binding site (TTTGA → GTCGA) primer PtraJ-1-ML (5′-CGGGATCCTCCAAAAAATGATGATGAATAAAAGAAAGTCGACTTCGTTCAAATATCAGAG-3′) was used in combination with PtraJ-2, while for mutagenesis of the right part of the CRP binding site (TCAAA → ATCGA) primer PtraJ-1-MR (5′-CGGGATCCTCCAAAAAATGATGATGAATAAAAGAAATTTGACTTCGTATCGATATCAGAG-3′) was used in combination with PtraJ-2 (the nucleotides that were altered in the mutant sequence are underlined). To enable cloning into pCB267, both primer PtraJ-1-ML and primer PtraJ-1-MR contained a BamHI restriction site. DNA sequencing confirmed that the correct fragments had been cloned.
β-Galactosidase assays.
To measure β-galactosidase activity, relevant strains were grown at 37°C overnight in LB medium, diluted 1/500 into fresh LB or M63 medium, regrown to an optical density at 600 nm of 1, and again diluted 1/500 into fresh LB, M63, or conditioned medium. Samples were periodically removed and assayed for β-galactosidase activity. β-Galactosidase assays were performed essentially as described previously (21) with bacteria treated with sodium dodecyl sulfate-chloroform and washed with Z buffer and o-nitrophenyl-β-d-galactopyranoside as a substrate. Enzyme activity was expressed in Miller units (21).
Generation of ΔlacZ RNA for use as a reverse transcription (RT)-PCR competitive template.
A 215-bp fragment of the lacZ gene was generated by PCR by using plasmid pTJ1 as the template and primers lacZ-1 (5′-ACGATGCGCCCATCTACACC-3′) and lacZ-2 (5′-ACGACTGTCCTGGCCGTAAC-3′). The DNA fragment generated was digested with MseI, and the restriction fragments were ligated with T4 ligase (Amersham Pharmacia Biotech). PCR amplification of the ligation mixture with primers lacZ-1 and lacZ-2 yielded several products, including the desired 158-bp ΔlacZ product. This fragment was isolated from a 4% NuSieve 3:1 agarose gel (BioWhittaker Molecular Applications) and cloned into the pGEM-T Easy vector (Promega), which generated plasmid pINP2. Sequence analysis confirmed that no base changes other than the deletion had been introduced. pINP2 was cut with restriction endonuclease PstI and used in the Riboprobe in vitro transcription system (Promega) as the template for the ΔlacZ RNA. The amount of in vitro ΔlacZ RNA transcript was determined spectrophotometerically.
RNA isolation.
RNA was isolated from log-phase bacteria that were passaged twice as indicated above and then grown in LB medium for 2 h. For all samples, RNA from the same amount of bacteria was purified with RNAzolB (Campro Scientific). After isopropanol precipitation, the RNA was dissolved in 40 μl of H2O and stored at −70°C. Prior to analysis of the traJ-lacZ mRNA by the competitive RT-PCR (see below), samples were checked for DNA contamination by PCR by using oligonucleotide primers lacZ-1 and lacZ-2. If DNA contamination was detected, samples were treated with RQ1 RNase-free DNase (Promega) until no DNA contamination was detected.
Competitive RT-PCR.
In the competitive RT-PCR (12), 1 μl of a DNA-free RNA sample of the relevant strain and 1 μl of competitive template (either 50, 5, or 0.5 pg of ΔlacZ RNA) were used together with the lacZ-1 and lacZ-2 oligonucleotide primers in the Access RT-PCR system (Promega). The RT-PCR involved 45 min of RT at 48°C, 2 min of avian myeloblastosis virus reverse transcriptase inactivation and RNA-cDNA-oligonucleotide primer denaturation at 94°C, and 40 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C, and extension for 30 s at 72°C, followed by one final extension step of 10 min at 72°C. The RT-PCR products were separated on 2% agarose gels. The competitive RT-PCR was performed twice with two different samples and gave similar results in all tests. The absence of DNA was verified by performing the competitive RT-PCR with RNA as the template but with water added instead of reverse transcriptase.
EMSA.
The electrophoretic mobility shift assay (EMSA) was performed as described previously (2). The 226-bp PCR product obtained with oligonucleotide primers PtraJ-1 and PtraJ-2 (see above), ptraJ, was labeled with α-32P at the BamHI site by using the Klenow enzyme (U.S. Biochemicals). CRP binding reactions were performed essentially as described previously (31). Briefly, 20-ng portions of labeled DNA fragment were mixed with different amounts of purified CRP (generously provided by G. S. Lloyd and S. Busby) in the presence 18 mM cAMP in 20-μl (final volume) reaction mixtures and incubated for 30 min at 37°C. The 214-bp BamHI-PvuII fragment of pUC19 carrying the lacZ promoter was used as a positive control. After the binding reaction, 1 μl of loading buffer (0.1% bromphenol blue and 50% glycerol in water) was added, and samples were loaded onto a 5% polyacrylamide gel electrophoresis gel and electrophoresed at a constant voltage (240 V). The gels were transferred to Whatman 3MM paper, dried, and autoradiographed.
Mobility shift assays with agarose gels were performed as described previously (16), except that the reaction volume was 20 μl and the preparations were incubated for 15 min at 37°C. A 100-bp DNA ladder (MBI Fermentas) was used as the competitor DNA.
DNase I footprint experiments.
DNase I footprint experiments were performed essentially as described previously (32). For these experiments, the 226-bp ptraJ fragment was amplified by using nonlabeled oligonucleotide primer PtraJ-1 and [γ-32P]ATP-end-labeled primer PtraJ-2. Three-microliter portions of the labeled PCR product were used in 20-μl binding reaction mixtures with different amounts of purified CRP protein as described above for the EMSA. After incubation, 0.06 U of DNase I (Amersham Pharmacia) was added to each mixture, and the mixture was incubated for 3 min at room temperature. The reaction was stopped by addition of 1 μl of 60 mM EDTA, followed by 10 min of incubation at 65°C. DNA fragments were purified with a QIAquick nucleotide removal kit (Qiagen) and were eluted in 30 μl of H2O. Six microliters of the final sample was analyzed by denaturing 6% polyacrylamide gel (National Diagnostics) electrophoresis performed at a constant power of 60 W, with a DNA sequence ladder electrophoresed in parallel. The DNA sequence ladder was generated with the appropriate primer by using a Sequenase version 2.0 DNA sequencing kit (U.S. Biochemicals).
Mating assay.
Conjugation experiments were carried out essentially as described previously (9), except that the strains were grown in LB medium, overnight cultures of donor and recipient strains were diluted 100-fold and incubated for 2 h with aeration at 37°C, and each mating mixture consisted of 0.05 ml of the donor, 0.45 ml of the recipient culture, and 0.5 ml of fresh LB medium. When appropriate, the mating mixture was supplemented with cAMP (10 mM). Mating was performed for 2 h at 37°C. Transconjugants were selected on LB media supplemented with the appropriate antibiotics. Conjugal transfer frequencies were expressed as the ratio of transconjugants to recipient or donor cells.
RESULTS
Glucose starvation enhances traJ expression in a CRP-dependent fashion.
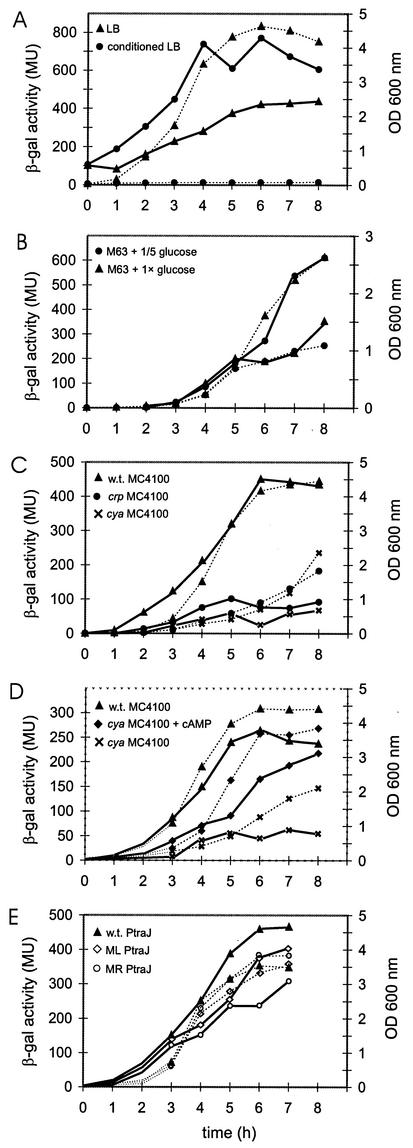
Measurement of the β-galactosidase activity in E. coli strain MC4100 carrying the traJ-lacZ transcriptional fusion at various stages of growth in LB medium demonstrated that there was a gradual increase in enzyme activity with the duration of growth (Fig. 2A). To determine whether the increase in activity was caused by a depletion of nutrients, we assayed β-galactosidase activity after transfer of log-phase bacteria to conditioned LB medium. In this medium, in which the E. coli strain was virtually unable to grow (Fig. 2A), a much stronger increase in enzyme activity was found, which was apparent after 1 h of incubation and which reached its peak after 4 h of incubation (Fig. 2A).
FIG. 2.
β-Galactosidase (β-gal) activity of the traJ-lacZ fusion. At various times (0 to 8 h), the optical densities at 600 nm (OD 600 nm) of the cultures were measured (dotted lines), and aliquots were assayed for β-galactosidase activity (expressed in Miller units [MU])(solid lines). (A) β-Galactosidase activities of the traJ-lacZ fusion in wild-type strain MC4100 grown in LB medium (▴) and in conditioned LB medium (•). (B) β-Galactosidase activities of the traJ-lacZ fusion in wild-type strain MC4100 grown in M63 medium with 1× glucose (•) and with 0.2× glucose (▴). (C) β-Galactosidase activities of the traJ-lacZ fusion in wild-type (w.t.) strain MC4100 (▴) and in the MC4100 cya mutant (×) and crp mutant (•) strains grown in LB medium. (D) β-Galactosidase activities of the traJ-lacZ fusion in wild-type strain MC4100 grown in LB medium (▴) and in the MC4100 cya mutant strain grown in LB medium with (⧫) and without (×) 10 mM cAMP. (E) β-Galactosidase activities in strain MC4100 carrying the wild-type traJ-lacZ fusion (w.t. PtraJ) (▴) or the traJ-lacZ fusion in which the left (ML PtraJ) (◊) or right (MR PtraJ) (○) part of the CRP binding domain was altered. Experiments were carried out three or four times, and representative results are shown.
The factors in the medium responsible for the induction of β-galactosidase activity were investigated by monitoring bacterial enzyme activities during growth in M63 minimal medium containing different amounts of glucose, Casamino Acids, and phosphate. These experiments indicated that traJ-lacZ transcription varied with the availability of glucose in the medium (Fig. 2B), while starvation for Casamino Acids or phosphate had no effect (data not shown).
Since it has been established that one way that bacteria respond to the level of glucose is via the cAMP-CRP complex, we measured traJ-lacZ expression levels in cya and crp mutant backgrounds. As illustrated in Fig. 2C, the β-galactosidase activities in both mutants were considerably lower than that in the parent strain. Complementation experiments demonstrated that there was a strong increase in β-galactosidase activity in the cya mutant when it was grown in the presence of 10 mM cAMP (Fig. 2D), while no significant effects were observed for the parental strain (data not shown). Complementation of the crp mutant via introduction of a plasmid encoding CRP (pYZCRP) was not successful as it led to a loss of the pTJ1 plasmid from the cells. Overall, the results support the notion that the cAMP-CRP complex is required for induction of traJ expression during glucose starvation conditions.
cAMP-CRP complex regulates the level of the traJ transcript.
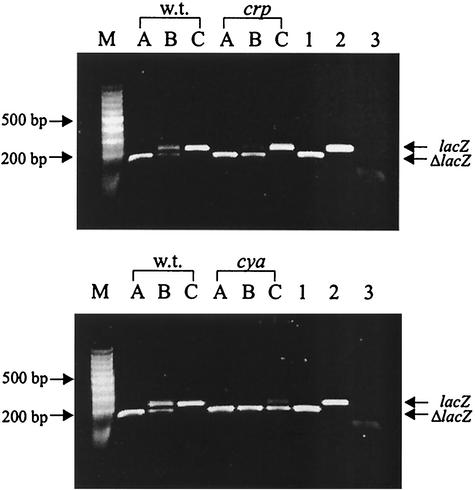
To ascertain that the cAMP-CRP complex influences the level of the traJ transcript, we compared the amounts of traJ-lacZ mRNA in the cya and crp mutants with the amount in the parent strain after 2 h of growth in LB medium using a competitive RT-PCR (12). The strategy used for quantification involved coamplification of a competitive template competing for the same oligonucleotide primers as the target RNA, which, however, after amplification could be distinguished from the target RNA by a difference in size. Since the FinOP system reduced traJ mRNA levels to levels below the level of reliable detection by RT-PCR (data not shown), we used pTJ1-derived traJ-lacZ mRNA as the target and the ΔlacZ RNA as a competitive template. In these experiments semiquantitative results were obtained by comparing the intensities of the DNA bands due to the traJ-lacZ mRNA and the ΔlacZ RNA that were added to the sample (Fig. 3). Application of this approach to the wild-type strain and the cya and crp mutant strains carrying pTJ1 revealed that much less traJ-lacZ mRNA was present in the mutant strains than in the wild-type strain (Fig. 3, upper panel, lane B, and lower panel, lanes B and C). This finding explains the observed reduced β-galactosidase activities in the mutant strains and suggests that the activity is due to cAMP-CRP complex-dependent stimulation of transcription initiation.
FIG. 3.
Semiquantitative determination of traJ-lacZ mRNA in the wild-type strain and crp and cya mutant strains by competitive RT-PCR. RT-PCR was performed with RNA isolated from wild-type strain MC4100 (w.t.) and the crp mutant (crp) (upper panel) and from wild-type strain MC4100 and the cya mutant (cya) (lower panel) in the presence of 50 pg (lanes A), 5 pg (lanes B), or 0.5 pg (lanes C) of competitive ΔlacZ RNA. Plasmid pINP2 carrying ΔlacZ (lane 1) and plasmid pTJ1 carrying the intact lacZ fragment (lane 2) served as positive controls; RT-PCR with water instead of reverse transcriptase (lane 3) served as a negative control. Lane M contained a 100-bp DNA ladder (MBI Fermentas).
Purified CRP binds to the traJ promoter region.
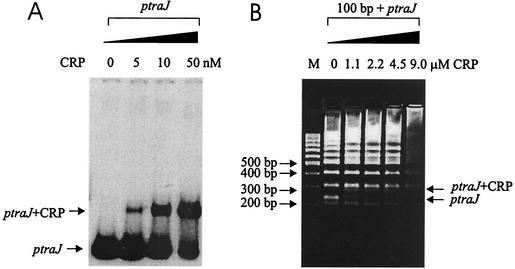
To establish whether CRP regulates the level of the traJ transcript by binding to the traJ promoter region, EMSAs were performed. An EMSA with a 226-bp PCR-amplified and gel-purified ptraJ fragment (containing the traJ promoter) and various concentrations of purified CRP showed that 5 nM CRP was sufficient to cause retardation of migration of the traJ fragment (Fig. 4A). The specificity of the CRP binding was confirmed by a competitive agarose gel shift assay in which the DNA of a 100-bp ladder was used as a control. These experiments indicated that addition of 0.5 μg of CRP (5 nM) was sufficient to cause a shift in the 226-bp ptraJ fragment, while much higher concentrations (1.1 μM) were needed to alter the migration of the random DNA of a 100-bp ladder (Fig. 4B). These results strongly suggest that CRP specifically bound to the traJ promoter region.
FIG. 4.
EMSA demonstrating the binding of purified CRP to the traJ promoter region. (A) Migration of the 32P-end-labeled 226-bp PCR traJ promoter fragment (ptraJ) in the absence and presence of different concentrations of purified CRP as determined by polyacrylamide gel electrophoresis and autoradiography. The arrows indicate the positions of the traJ region and the shifted protein-DNA complex. (B) Migration in an agarose gel of the 226-bp PCR traJ promoter fragment (ptraJ) after addition of different concentrations of CRP in the presence of the 100-bp DNA ladder. Note the specificity of the shift of the ptraJ fragment (arrow) in the presence of CRP. Lane M contained the 100-bp DNA marker.
Mapping of the CRP binding domain by DNase I footprint experiments.
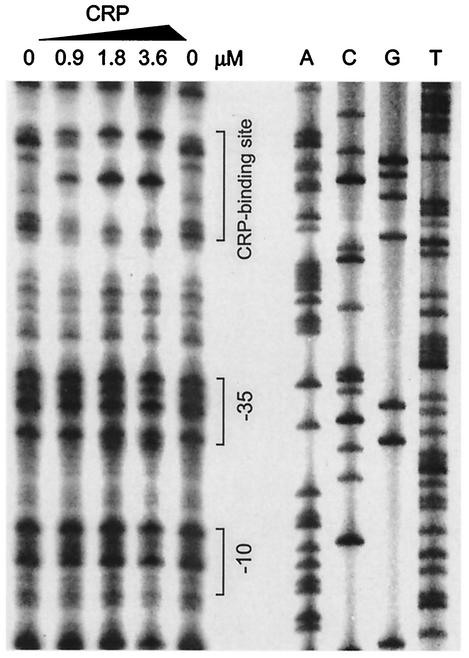
In order to locate the CRP binding site on the traJ promoter region, we performed DNase I protection assays. In these experiments, purified CRP was incubated with a radiolabeled traJ promoter fragment, which was then digested by DNase I and subjected to gel electrophoresis and autoradiography. As shown in Fig. 5, CRP protected a distinct DNA region against DNase I digestion in a dose-dependent fashion. Comparison with the nucleotide sequence of the pRK100 traJ regulatory region examined in parallel indicated that the protected region extended from position −57 to position −78 relative to the transcription site. The corresponding nucleotide sequence (5′-AAATTTGACTTCGTTCAAATAT-3′) strongly resembled the CRP consensus binding site (5′-AAATGTGATCTAGATCACATTT-3′) (31) (Fig. 6).
FIG. 5.
Mapping of the CRP binding site in the traJ promoter region as determined by a DNase I protection assay. A 226-bp PCR 32P-labeled traJ promoter fragment was incubated with different amounts of purified CRP, treated with DNase I, and separated on 6% sequence gels. A nucleotide sequencing reaction mixture was electrophoresed in parallel (lanes A, C, G, and T). The positions of the CRP binding site and the −10 and −35 promoter regions are indicated.
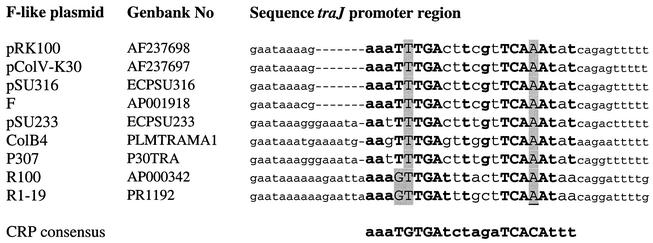
FIG. 6.
Sequence alignment of the traJ promoter regions of different F-like plasmids, indicating the conservation of the identified CRP binding site among F-like plasmids. Nucleotides that are identical to nucleotides in the CRP binding consensus sequence (31) are indicated by boldface type. The more conserved nucleotides are indicated by uppercase letters. Deviations from the CRP consensus sequence are shaded.
Mutagenesis of the CRP binding site identified.
To unequivocally demonstrate that the CRP binding sequence identified acts in regulation of the traJ promoter, we performed site-directed mutagenesis. Mutations in the left (TTTGA → GTCGA) and right (TCAAA → ATCGA) parts of the CRP binding site that was identified (Fig. 6) were introduced by PCR. The mutated traJ-lacZ fusions were cloned onto pCB267, which was then transferred to E. coli strain MC4100. As illustrated in Fig. 2E, mutagenesis of each of the domains of the CRP binding site led to an increase in enzyme activity upon prolonged growth that was less profound than the increase observed for the traJ-lacZ fusion carrying the intact CRP binding site.
Mutations in cya and crp inhibit conjugal transfer of pRK100.
In an attempt to place our findings in a more natural setting, we investigated the apparent regulatory role of the cAMP-CRP complex in traJ transcription by measuring the efficiency of conjugal transfer of pRK100 for the wild-type strain and the cya and crp mutant strains. Conjugation experiments in which E. coli DH5α served as the recipient strain demonstrated that wild-type strain MC4100 mated up to 100-fold more efficiently than the cya and crp mutants (Table 2). Addition of cAMP (10 mM) to the conjugative mixture considerably increased the mating efficiency of the cya mutant (Table 2), which is fully in line with the results obtained with the traJ-lacZ fusion. Although the control of conjugation of F-like plasmids is very complex, these data support the idea that the cAMP-CRP complex influences traJ transcription in the natural pRK100 environment.
TABLE 2.
Conjugal transfer frequencies for plasmid pRK100 from the wild-type strain and crp and cya mutant strains into E. coli strain DH5α (after 2 h of conjugation) in the absence and presence of 10 mM cAMP
| Donor strain | Conjugal frequency (transconjugant/ donor cell)
|
Conjugal frequency (transconjugant/ recipient cell)
|
||
|---|---|---|---|---|
| Without cAMP | With cAMP | Without cAMP | With cAMP | |
| MC4100 (wild type) | 3.0 × 10−5 | 3.6 × 10−5 | 1.1 × 10−3 | 1.3 × 10−3 |
| MC4100 cya | 2.9 × 10−7 | 2.2 × 10−6 | 4.1 × 10−6 | 3.1 × 10−5 |
| MC4100 crp | 7.6 × 10−7 | 7.7 × 10−7 | 5.6 × 10−6 | 5.7 × 10−6 |
DISCUSSION
Conjugation is an important bacterial mechanism for transferring genetic material to other microorganisms. This process is facilitated by a large array of genes that encode the sophisticated conjugation system. Synthesis of the conjugative apparatus represents a heavy metabolic load and, due to the presence of receptors on the surface of conjugative pili, makes a bacterium vulnerable to bacteriophage infection. Conjugation of F-like plasmids is therefore a tightly regulated process, which results in only a few bacteria that synthesize a conjugative apparatus. It has been well documented that the TraJ protein is the central positive regulator of F-like plasmid conjugal transfer. The expression of this protein is normally suppressed by the effects of the FinP antisense RNA and the FinO protein (7, 10). In this paper, we provide direct evidence that the global regulator CRP acts as a positive transcriptional regulator of traJ expression via direct binding to the traJ promoter region.
The first indication that CRP positively influences traJ promoter activity was the increase in β-galactosidase activity in bacteria carrying a traJ-lacZ fusion during a prolonged period of growth and, more specifically, in conditioned medium in the absence of bacterial growth. This indicated that depletion of nutrients is a signal for enhanced traJ-lacZ activity. The finding that glucose was an important factor in regulation led us to hypothesize that CRP may act as a putative positive regulator molecule. Glucose starvation results in rapid stimulation of cAMP synthesis, which in turn leads to high intracellular levels of the cAMP-CRP complex. The significantly lower β-galactosidase activity in cya and crp mutants carrying the traJ-lacZ fusion, the partial restoration of the defect in the cya mutant by addition of exogenous cAMP, and the reduced traJ-lacZ mRNA levels detected by competitive RT-PCR collectively indicated that the cAMP-CRP complex is involved in the regulation of traJ-lacZ activity. The proposed function of CRP may form the basis of the observed association between intracellular levels of cAMP and the expression of transfer-related activities from F-like plasmids (14, 19).
Direct evidence that CRP regulates traJ promoter activity via specific recognition of a nucleotide sequence in the traJ promoter region was obtained in series of experiments. (i) Mobility shift experiments demonstrated that there was direct binding of CRP to the traJ promoter region; (ii) DNase I footprint experiments showed that binding of purified CRP to a fragment carrying the traJ promoter region provided protection against DNase I activity; and (iii) targeted mutagenesis of the putative CRP binding sites in the traJ promoter region reduced the activation of TraJ under starvation conditions. Binding of CRP to target promoter sequences is known to regulate gene expression. Upon interaction with cAMP, CRP undergoes a conformational change that enables binding of the cAMP-CRP complex to a distinct 22-bp DNA consensus sequence. This binding and the protein-protein interaction between CRP and RNA polymerase enhance the intrinsic promoter activity (17).
Mapping of the CRP binding domain in the traJ promoter region by DNase I footprint analysis and confirmation by targeted mutagenesis revealed that the binding site was centered around position −67.5 upstream of the putative transcriptional start site of traJ and that it had strong similarity to the CRP consensus sequence (31). The location at position −67.5 differs from the location that has been putatively determined for the F-plasmid in the database (GenBank accession number U01159). It has been proposed that this possible CRP site that does not constitute a CRP consensus sequence (31) is located in the first short stem-loop in the traJ mRNA. Comparative sequence analysis indicated that the CRP binding site identified in the traJ promoter region of pRK100 is conserved among other F-like plasmids (Fig. 6).
Promoters activated by CRP have been grouped into three classes according to the location of the bound CRP. In class I CRP-dependent promoters the CRP binding sites are located at sites near position −61.5, −71.5, −81.5, or −91.5 with respect to the transcription start point, whereas in class II CRP-dependent promoters the CRP binding site is centered near position −41.5. In these cases, CRP and RNA polymerase are located at the same face of the DNA, and CRP is thought to exert its effect by direct contact with the RNA polymerase (6, 23). Class III CRP-dependent promoters have the CRP binding site situated further upstream, and they require an additional regulator besides the cAMP-CRP complex. Assuming that the transcription start point in the pRK100 traJ promoter is the same as that in the related F-plasmid, the CRP binding site on pRK100 is located at position −67.5, which implies that CRP and the RNA polymerase bind at different faces of the DNA. This unusual position of a CRP binding site might be of particular interest, since it may indicate that besides the CRP-cAMP complex, an additional regulator binds to the DNA helping CRP to establish contact with RNA polymerase.
The pRK100 traJ promoter has many characteristics of a strong promoter. The putative −35 and −10 sequences exhibit strong similarity to the consensus sequence of E. coli promoters, and the distance between the −35 and −10 hexamers is 17 bp, which is optimal for promoter activity. Furthermore, a putative UP element is present upstream of the −35 region (Fig. 1) (26). This element is known to increase promoter activity. The regulation of the traJ promoter activity by the cAMP-CRP complex demonstrated in the present study suggests that the initiation of transcription due to −35 and −10 sequences and the UP element may not be sufficient to increase traJ mRNA levels above the levels of FinP antisense RNA. The positive effect of the cAMP-CRP complex on traJ transcription may be needed to derepress the transcription of the tra genes and to enable synthesis of the conjugation machinery. This scenario is particularly attractive as it relates conjugation activity to distinct environmental conditions encountered by the bacteria (such as the limited availability of glucose). In combination with the strong negative regulation by the FinOP system, the effect of the cAMP-CRP complex and perhaps additional regulatory intermediates may enable fine tuning of the conjugation process, which is necessary to balance efficient DNA transfer and exposure of the required structural machinery to the environment. However, it should be noted that control of conjugation is extremely complex and that much more work with intact conjugative plasmids is needed to firmly establish the role of the cAMP-CRP complex beyond the level of traJ transcription.
Acknowledgments
We are grateful to Georgina S. Lloyd and Stephen Busby for providing CRP, Vid Mlakar and Damijan Nipiè are acknowledged for help with the mating assays, Matjaž Brinc is acknowledged for assistance with the glucose starvation experiments, and Irena Kuhar is acknowledged for fruitful discussions.
B.J.A.M.J. was a recipient of a fellowship from the Royal Netherlands Academy of Arts and Sciences. M.S.'s stays in Utrecht at the Department of Infectious Diseases and Immunology were partially supported by grants from The Netherlands Organization for International Cooperation in Higher Education (Nuffic) and the European Molecular Biology Organization (EMBO).
REFERENCES
- 1.Ambrožič, J., A. Ostroveršnik, M. Starčič, I. Kuhar, M. Grabnar, and D. Žgur-Bertok. 1998. Escherichia coli ColV plasmid pRK100: genetic organization, stability and conjugal transfer. Microbiology 144:343-352. [DOI] [PubMed] [Google Scholar]
- 2.Buratowski, S., and L. A. Chodosh. 1999. Mobility shift DNA-binding assay using gel electrophoresis, p. 12.2.1-12.2.7. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 4.Cavalli, L. L., E. Lederberg, and J. M. Lederberg. 1953. An effective factor controlling sex compatibility in Bacterium coli. J. Gen. Microbiol. 8:89-103. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey, W. B. 1994. traJ sense RNA initiates at two different promoters in R100-1 and forms two stable hybrids with antisense finP RNA. Mol. Microbiol. 13:313-326. [DOI] [PubMed] [Google Scholar]
- 6.Ebright, R. H. 1993. Transcription activation at class I CAP-dependent promoters. Mol. Microbiol. 8:797-802. [DOI] [PubMed] [Google Scholar]
- 7.Finnegan, D. J., and N. S. Willetts. 1973. The site of action of the F transfer inhibitor. Mol. Gen. Genet. 127:307-316. [DOI] [PubMed] [Google Scholar]
- 8.Firth, N., K. Ippen-Ihler, and R. A. Skurray. 1996. Structure and function of the F factor and mechanism of conjugation, p. 2377-2401. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasnik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 9.Franklin, A., and R. Möllby. 1983. Concurrent transfer and recombination between plasmids encoding for heat-stable enterotoxin and drug resistance in porcine enterotoxinogenic Escherichia coli. Med. Microbiol. Immunol. 172:137-147. [DOI] [PubMed] [Google Scholar]
- 10.Frost, L. S., S. H. Lee, N. Yanchar, and W. Paranchych. 1989. finP and fisO mutations in FinP anti-sense RNA suggest a model for FinOP action in the repression of bacterial conjugation by the Flac plasmid JCFL0. Mol. Gen. Genet. 218:152-160. [DOI] [PubMed] [Google Scholar]
- 11.Frost, L. S., K. Ippen-Ihler, and R. A. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Rev. 58:162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilliland, G., S. Perrin, and H. F. Bunn. 1990. Competitive PCR for quantification of mRNA, p. 60-69. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 13.Gomez-Gomez, J. M., F. Baquero, and J. Blazquez. 1996. Cyclic AMP receptor protein positively controls gyrA transcription and alters DNA topology after nutritional upshift in Escherichia coli. J. Bacteriol. 178:3331-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood, C. R., and M. Meynell. 1975. Cyclic AMP and the production of sex pili by E. coli K-12 carrying derepressed sex factors. Nature 254:628-660. [DOI] [PubMed] [Google Scholar]
- 15.Jerome, L. J., T. V. Biesen, and L. S. Frost. 1999. Degradation of FinP antisense RNA from F-like plasmids: the RNA-binding protein, FinO, protects FinP from ribonuclease E. J. Mol. Biol. 285:1457-1473. [DOI] [PubMed] [Google Scholar]
- 16.Jordi, B. J. A. M., A. E. Fielder, C. M. Burns, J. C. D. Hinton, N. Dover, D. W. Ussery, and C. F. Higgins. 1997. DNA binding is not sufficient for H-NS mediated repression of proU expression. J. Biol. Chem. 272:12083-12090. [DOI] [PubMed] [Google Scholar]
- 17.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:744-795. [DOI] [PubMed] [Google Scholar]
- 18.Koraimann, G., K. Teferle, G. Markolin, W. Woger, and G. Högenauer. 1996. The FinOP repressor system of plasmid R1: analysis of the antisense RNA control of traJ expression and conjugative DNA transfer. Mol. Microbiol. 21:811-821. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, S., and S. Srivastava. 1983. Cyclic AMP and its receptor protein are required for expression of transfer genes of conjugative plasmid F in Escherichia coli. Mol. Gen. Genet. 190:27-34. [DOI] [PubMed] [Google Scholar]
- 20.Marschall, C., V. Labrousse, M. Kreimer, D. Weichart, A. Kolb, and R. Hengge-Aronis. 1998. Molecular analysis of the regulation of csiD, a carbon starvation-inducible gene in Escherichia coli that is exclusively dependent on sigma-S and requires activation by cAMP-CRP. J. Mol. Biol. 276:339-353. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 22.Nelson, W. C., M. T. Howard, J. A. Sherman, and S. W. Matson. 1995. The traY gene product and integration host factor stimulate Escherichia coli DNA helicase I-catalyzed nicking at the F plasmid oriT. J. Biol. Chem. 270:28374-28380. [PubMed] [Google Scholar]
- 23.Niu, W., Y. Kim, G. Tau, T. Heyduk, and R. H. Ebright. 1996. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell 87:1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardee, A. B., F. Jacob, and J. Monod. 1959. The genetic control and cytoplasmatic expression of “inducibility” in the synthesis of β-galactosidase by E. coli. J. Mol. Biol. 1:165-178. [Google Scholar]
- 25.Pölzleitner, E., E. L. Zechner, W. Renner, R. Fratte, B. Jauk, G. Högenauer, and G. Koraimann. 1997. TraM of plasmid R1 controls transfer gene expression as an integrated control element in a complex regulatory network. Mol. Microbiol. 25:495-507. [DOI] [PubMed] [Google Scholar]
- 26.Ross, W., S. E. Aiyar, J. Salomon, and R. L. Gourse. 1998. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J. Bacteriol. 180:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Schneider, K., and C. F. Beck. 1986. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene 42:37-48. [DOI] [PubMed] [Google Scholar]
- 29.Silverman, P. M., L. Tran, R. Harris, and H. M. Gaudin. 1993. Accumulation of the F plasmid TraJ protein in cpx mutants of Escherichia coli. J. Bacteriol. 175:921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strohmaier, H., R. Noiges, S. Kotschan, G. Sawers, G. Högenauer, E. L. Zechner, and G. Koraimann. 1998. Signal transduction and bacterial conjugation: characterization of the role of ArcA in regulating conjugative transfer of the resistance plasmid R1. J. Mol. Biol. 277:309-316. [DOI] [PubMed] [Google Scholar]
- 31.Wonderling, L. D., and G. V. Stauffer. 1999. The cyclic AMP receptor protein is dependent on GcvA for regulation of the gcv operon. J. Bacteriol. 181:1912-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wösten, M. M. S. M., and E. A. Groisman. 1999. Molecular characterization of the PmrA regulon. J. Biol. Chem. 274:27185-27190. [DOI] [PubMed] [Google Scholar]
- 33.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]