Abstract
Insulin receptor substrate (IRS) proteins are docking proteins that couple growth factor receptors to various effector molecules, including phosphoinositide-3 kinase, Grb-2, Syp, and Nck. Here we show that IRS-1 associates with the loop domain of Bcl-2 and synergistically up-regulates antiapoptotic function of Bcl-2. IRS-2 but not IRS-3 binds to Bcl-2, and IRS-1 associates with Bcl-XL but not with Bax or Bik. Overexpression of IRS-1 suppresses phosphorylation of Bcl-2 induced by stimulation with insulin, and the hypophosphorylation may lead to its enhanced antiapoptotic activity. The binding site for Bcl-2 is located on the carboxyl half-domain of IRS-1. IRS-3, which lacks the corresponding region, dominant-negatively abrogates the survival effects of IRS-1 and Bcl-2. For the antiapoptotic activity of IRS-1, binding to Bcl-2 is more critical than activating phosphoinositide-3 kinase. Our results indicate that IRS proteins transmit signals from the insulin receptor to Bcl-2, thus regulating cell survival probably through regulating phosphorylation of Bcl-2.
INTRODUCTION
Apoptosis is a form of programmed cell death, characterized by chromatin condensation, cytoplasmic blebbing, and DNA fragmentation. During development, programmed cell death plays critical roles in sculpting parts of body, deleting unwanted structures and misplaced, nonfunctional, or harmful cells in animal tissues (Jacobson et al., 1997). Depletion of growth factors is one of the common causes of apoptosis, which probably plays a critical role in controlling cell number and eliminating misplaced cells. Growth factor receptors including receptor tyrosine kinases (RTKs) are considered to generate cell growth signals and cell survival signals to support continuous growth of cells. Although the precise mechanisms of how RTKs generate antiapoptotic signals are not well understood, ligand stimulation of RTKs leads to activation of intracellular signaling cascades including the Ras-MAPK pathway and the phosphoinositide-3 kinase (PI3K) pathway that are considered to possess antiapoptotic activity (Datta et al., 1997; Klesse and Parada, 1998; Kurada and White, 1998; Walker et al., 1998).
In addition to them, several recent studies have shown that RTKs support cell survival by regulating the function of the Bcl-2 family proteins via phosphorylation. There are two types of the Bcl-2 family proteins, namely antiapoptotic members such as Bcl-2 (Cleary et al., 1986) and Bcl-XL (Boise et al., 1993) and proapoptotic members, including Bad (Yang et al., 1995), Bax (Oltvai et al., 1993), and Bik (Boyd et al., 1995). Survival of cells was considered to be regulated by a ratio of expressed amounts of antiapoptotic Bcl-2 family proteins to those of proapoptotic member proteins (Oltvai et al., 1993). Furthermore, the function of Bcl-2 family proteins could be regulated by phosphorylation. For example, activation of RTKs leads to phosphorylation of Bad, a proapoptotic Bcl-2 family member, which results in suppression of apoptosis. The protein kinases that phosphorylate Bad are reported to be c-Akt (Datta et al., 1997; del Peso et al., 1997) and protein kinase A (Harada et al., 1999). Furthermore, several growth factors induce serine/threonine phosphorylation of Bcl-2 (Ito et al., 1997), which modifies the antiapoptotic effects of Bcl-2. Paradoxically, however, because dephosphorylation rather than phosphorylation up-regulates the antiapoptotic activity of Bcl-2 (Haldar et al., 1995; Chang et al., 1997; Maundrell et al., 1997), growth factors that induce phosphorylation of Bcl-2 would be expected to cause apoptotic cell death. Taken together, we hypothesize that there should be a mechanism that regulates phosphorylation of Bcl-2 when RTKs and their signaling pathways are activated. Therefore, we searched for regulatory molecules of Bcl-2 that are present in the signaling cascades of RTKs.
MATERIALS AND METHODS
Antibodies and Cell Culture
For immunoblotting, immunoprecipitation, and immunostaining, we used anti-Bcl-2 antibodies, clones 124 (for immunoblotting and immunostaining; Dako, Glostrup, Denmark), N-19 (for immunoprecipitation and immunoblotting), and C100 (for immunoprecipitation and immunoblotting; Santa Cruz Biotechnology, Santa Cruz, CA), anti-IRS-1 antibodies C-20 (for immunoblotting; Santa Cruz) and J36 (for immunoprecipitation; Yamamoto-Honda et al., 1996), anti-IRS-2 antibody (Upstate Biotechnology, Lake Placid, NY), anti-insulin receptor β chain antibody C-19 (Santa Cruz), anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology), and anti-FLAG M2 (Eastman Kodak, Rochester, NY), anti-myc 9E10 (Babco, Richmond, CA), and anti-hemagglutinin (HA) 12CA5 (Boehringer Mannheim, Mannheim, Germany) antibodies. IM-9 cells were cultured in RPMI 1640 medium containing 10% FCS. Ba/F3 cells were maintained in RPMI 1640 medium containing 10% FCS and 0.25 ng/ml mouse interleukin 3 (IL-3). Chinese hamster ovary (CHO) cells were grown in Ham's F-12 medium containing 10% FCS. Human embryonic kidney 293 cells were maintained in Dulbecco's modified Eagle's medium containing 10% FCS.
Plasmid Construction
To construct the C-1 deletion mutant of murine IRS-1, the XmaI–Eco81I fragment corresponding to amino acids 869-1201 was excised, and the remaining cDNA was blunted and religated. The deletion mutants of IRS-1, C-2 (which encodes aa 1–741), C-3 (aa 1–573), and C-4 (aa 1–421), were constructed by PCR and epitope tagged with the FLAG sequence at the carboxyl terminus. All constructs of the mutants were verified by direct sequencing and subcloned into an expression vector, pUC-CAGGS (Ueno et al., 1995). The ΔBH4 (lacking aa 10–31), the Δloop (lacking aa 35–79), the ΔBH3 (lacking aa 100–118), and the ΔBH1-BH2 (lacking aa 118–239) mutants of human Bcl-2 were constructed by PCR or the site-directed mutagenesis and subcloned into the pcDNA3myc vector (tagged with the myc sequence). All these deletion mutants of Bcl-2 lack the transmembrane domain (aa 219–239). Rat IRS-3 and human Bax and Bik genes were isolated by reverse transcription-PCR of total RNA isolated from rat liver and human peripheral lymphocyte and sequenced by an autosequencer (Applied Biosystems, Foster City, CA). Rat IRS-3 cDNA was tagged with the FLAG sequence (DYLDDDDL) or the HA (YPYDVPDYA) sequence at the 3′ terminus. Human Bcl-2, Bcl-XL, Bax, and Bik genes were subcloned into the pcDNA3myc vector and tagged with the myc sequence (EQKLISEEDLN). Retrovirus vector pSRαSVtkneo was used to transfect cDNAs into Ba/F3 cells, as reported previously (Ueno et al., 1996). To coexpress two cDNAs in Ba/F3 cells, we used an internal ribosomal entry site (IRES) that was subcloned between two cDNAs in pSRαSVtkneo. To construct the loop fragment tagged with the FLAG peptide (LF), the cDNA of human Bcl-2 corresponding to the region aa 31–75 was amplified and tagged with the FLAG sequence by PCR. The construct was subcloned into an expression vector, pMX-puro vector (Onishi et al., 1996). All constructs generated by PCR were verified by sequencing.
Transfection, Immunoprecipitation, Immunoblotting, and Kinase Assay
Transfection into 293 cells was carried out by the calcium phosphate method as described previously (Ueno et al., 1995). To establish CHO-IR/IRS-1/Bcl-2 cells, Bcl-2/pSSRα-bsr (carrying the blasticidin-resistant gene) was introduced into the CHO-IR/IRS-1 cells (Yonezawa et al., 1994), and stable clones were selected in the presence of 5 μg/ml blasticidin (Funakoshi, Tokyo, Japan) as reported (Ueno et al., 1995). Immunoprecipitation and immunoblotting were performed as described (Ueno et al., 1995). To detect serine/threonine phosphorylation of Bcl-2 by insulin stimulation, cells were labeled with [32P]orthophosphoric acid as described previously (Tobe et al., 1993). Then cells were stimulated with insulin (100 nM) at 37°C for 5 min, lysed, and immunoprecipitated with anti-Bcl-2 antibody. The kinase activity of PI3K was measured as described previously (Yamamoto-Honda et al., 1996).
Immunostaining
Cells were fixed in 3.7% formaldehyde in PBS for 10 min, permeabilized with 0.1% Nonidet P-40 in PBS for 10 min, and blocked in PBS containing 5% normal goat serum (Life Technologies, Gaithersburg, MD) and 1 mg of BSA fraction V (Sigma, St. Louis, MO)/ml for 1 h. Then the cells were incubated with indicated antibodies for 1 h, followed by incubation with FITC- or Texas Red-conjugated goat secondary antibodies for 1 h. The cells were visualized under a Bio-Rad (Hercules, CA) MRC 1024 confocal microscope.
Survival Assay
For flow cytometry, cells were fixed with 70% ethanol for 5 h at −20°C, treated with 100 μg/ml RNase A at 37°C for 30 min, and stained with 50 μg/ml propidium iodide for 30 min. Then cells were subjected to fluorescence-activated cell sorting (FACS) analysis by a FACSort (Becton Dickinson, Mountain View, CA). The survival ratio was calculated as the rate of a number of viable cells to the total number of viable and apoptotic cells, which were counted after trypan blue staining.
RESULTS
Association between IRS Proteins and the Bcl-2 Family Proteins
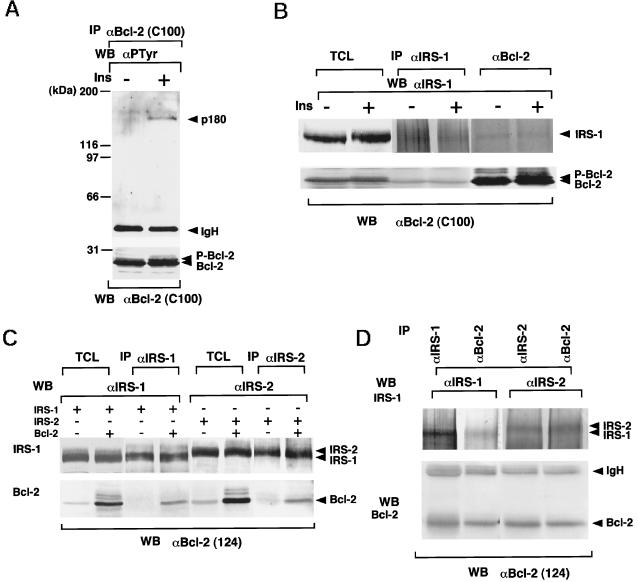
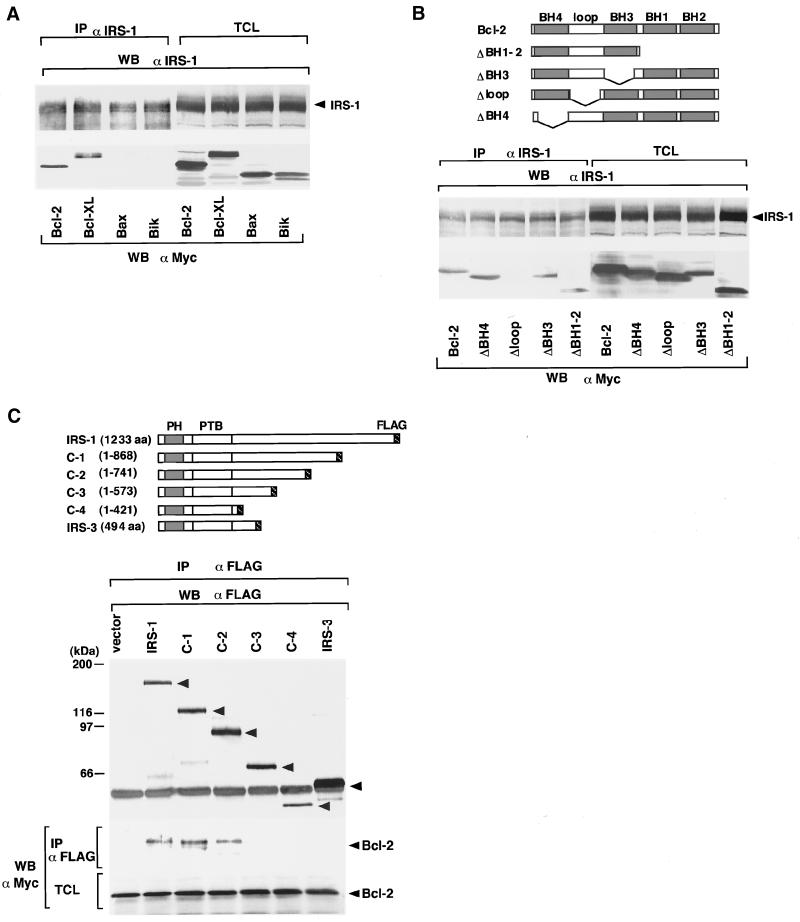
We first examined the tyrosine-phosphorylated proteins associated with Bcl-2 when RTKs are activated by ligand binding. We used IM-9 cells for these experiments, because IM-9 cells, derived from B lymphocytes, express relatively large amounts of insulin receptors (Van et al., 1976) and Bcl-2 (our unpublished results). When cells were stimulated with insulin, an ∼180-kDa tyrosine-phosphorylated protein was coprecipitated with Bcl-2 (Figure 1A). Because the ∼180-kDa proteins tyrosine phosphorylated with insulin stimulation are known to be IRS-1 (Sun et al., 1991) and IRS-2 (Sun et al., 1995), and IM-9 cells express more IRS-1 than IRS-2 (our unpublished results), we assumed that this phosphoprotein could be IRS-1. IRS proteins are substrates for many growth factors such as insulin (Sun et al., 1991), insulin-like growth factor-1 (IGF-1) (Chuang et al., 1993; Myers et al., 1993), IL-4 (Keegan et al., 1994), IL-9 (Vin et al., 1995), IL-13 (Welham et al., 1995), and growth hormone (GH) (Souza et al., 1994; Ridderstrale et al., 1995). So far, four IRS proteins, namely IRS-1, IRS-2, IRS-3 (Lavan et al., 1997b), and IRS-4 (Lavan et al., 1997a), have been identified. Moreover, GAB1 (Holgado-Madruga et al., 1996) and GAB2 (Gu et al., 1998) genes have been cloned that are structurally related to IRS proteins. IRS-1, IRS-2, and IRS-4 are structurally closely related to each other (Lavan et al., 1997a). However, IRS-3 lacks the region corresponding to the carboxyl half-domain of IRS-1 (Lavan et al., 1997b). As shown in Figure 1B, IRS-1 and Bcl-2 are constitutively associated in IM-9 cells, and such association was not affected by insulin stimulation. As reported previously, phosphorylated Bcl-2 can be detected as a shifted band by using anti-Bcl-2 monoclonal antibody C100 (Figure 1, A and B) (Scatena et al., 1998). In Figure 1B, we found that IRS-1 coprecipitated nonphosphorylated but not phosphorylated Bcl-2, indicating that IRS-1 does not bind to phosphorylated Bcl-2. The reason will be discussed later. By cotransfection, IRS-2 also associated with Bcl-2 (Figure 1C). The association was also observed in the lysates from murine hepatocytes (Figure 1D), indicating that not only transfected but also endogenous IRS-1/IRS-2 and Bcl-2 associate with each other. IRS-1 also bound to Bcl-XL but not to Bax or Bik (Figure 2A). Interestingly, Bcl-2 and Bcl-XL are antiapoptotic members, and Bax and Bik are proapoptotic members of the Bcl-2 family proteins. To determine the domains of Bcl-2 and IRS-1 that bind with each other, we used deletion mutants of Bcl-2 and IRS-1 (Figure 2, B and C). As shown in Figure 2B, only the Δloop mutant of Bcl-2 failed to bind to IRS-1, indicating that IRS-1 binds to the loop domain of Bcl-2. This result is reasonable because Bcl-2 and Bcl-XL possess the loop domain, whereas Bax and Bik do not. On the other hand, using deletion mutants of IRS-1, we found that Bcl-2 binds to the carboxyl half-region of IRS-1 (amino acids 574-1233; Figure 2C). IRS-3 that lacks the corresponding domain of IRS-1 could not associate with Bcl-2 (Figure 2C).
Figure 1.
Association among IRS-1, IRS-2, and Bcl-2. (A) A 180-kDa tyrosine phosphoprotein associated with Bcl-2 in insulin-stimulated IM-9 cells. IM-9 cells stimulated with insulin (100 nM) at 37°C for 5 min were lysed, immunoprecipitated (IP) with C100 anti-Bcl-2 antibody, and immunoblotted (WB) with anti-phosphotyrosine antibody 4G10 (upper panel) or C100 (lower panel). P-Bcl-2, phosphorylated Bcl-2. (B) A 180-kDa tyrosine phosphoprotein associated with Bcl-2 in insulin-stimulated IM-9 cells is IRS-1. IM-9 cells were lysed, immunoprecipitated (IP) with J36 anti-IRS-1 antibody or C100 anti-Bcl-2 antibody, and immunoblotted (WB) with C-20 anti-IRS-1 antibody or C100 anti-Bcl-2 antibody. TCL, total cell lysates. (C) IRS-1 and IRS-2 associate with Bcl-2. Murine IRS-1 or IRS-2 in pRcCMV was cotransfected with human Bcl-2/pUC-CAGGS in 293 cells. Then lysates were immunoprecipitated and immunoblotted with indicated antibodies. +, transfected; −, not transfected. (D) IRS-1 and IRS-2 associate with Bcl-2 in murine hepatocytes. Two milligrams of lysates from murine hepatocytes (C57BL/6J Jcl mice mice) were immunoprecipitated and immunoblotted with the indicated antibodies.
Figure 2.
(A) IRS-1 associates with Bcl-2 and Bcl-XL but not with Bax or Bik. Human Bcl-2, Bcl-XL, Bax, and Bik in the pcDNA3myc vector were cotransfected with IRS-1/pRcCMV in 293 cells. Then lysates were immunoprecipitated (IP) and immunoblotted (WB) with the indicated antibodies. (B) IRS-1 associates with the loop domain of Bcl-2. Upper panel, schematic representation of deletion mutants of Bcl-2. Lower panel, the deletion mutants of Bcl-2, ΔBH4, Δloop, ΔBH3, and ΔBH1-BH2 (ΔBH1–2) in pcDNA3myc vector were cotransfected with IRS-1/pRcCMV in 293 cells. Then lysates were immunoprecipitated and immunoblotted with the indicated antibodies. (C) Bcl-2 associates with the carboxyl half-region of IRS-1. Upper panel, schematic representation of deletion mutants of IRS-1. PH, pleckstrin homology domain; PTB, phosphotyrosine binding domain. Lower panel, the deletion mutants of IRS-1 (C-1, -2, -3, and 4) and IRS-3 tagged with the FLAG sequence in pUC-CAGGS were cotransfected with Bcl-2/pcDNA3myc in 293 cells. Then lysates were immunoprecipitated and immunoblotted with the indicated antibodies.
Colocalization of IRS-1 and Bcl-2
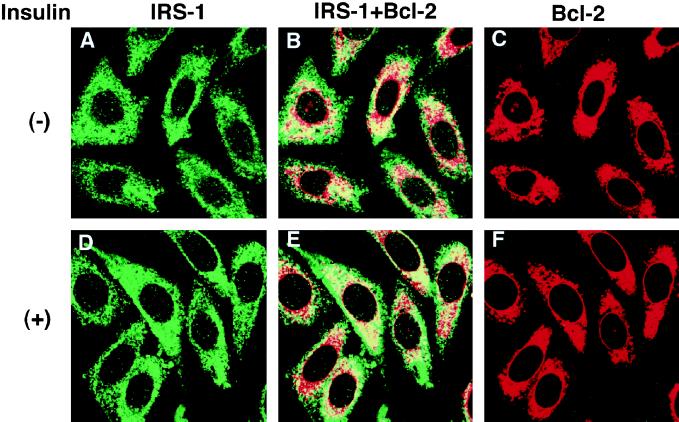
It has been reported that IRS-1 localizes to the cytoplasm and that stimulation with insulin induces its association with the insulin receptor through its phosphotyrosine binding domain (Craparo et al., 1995; Eck et al., 1996), whereas Bcl-2 localizes to the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane (Lithgow et al., 1994). Because IRS-1 and Bcl-2 associated with each other (Figure 1B), we examined the subcellular localization of both molecules and the effects of growth factor stimulation. To this end, we established a stable transfectant of CHO cells expressing the insulin receptor, IRS-1, and Bcl-2 (CHO-IR/IRS-1/Bcl-2 cells). By immunostaining with anti-IRS-1 and anti-Bcl-2 antibodies, IRS-1 and Bcl-2 were revealed to colocalize at the perinuclear region (Figure 3, A–F). Similar results were obtained when IRS-1 and Bcl-2 were cotransfected into NIH3T3 and COS7 cells (our unpublished results). When these cell lines were stimulated with insulin, however, there was no alteration in the subcellular localization of either molecule. The similar results were obtained when these cells were stimulated with insulin for a shorter or longer period (from 1 min up to 12 h) at a lower temperature (up to 4°C). Consistent with our data, a previous report regarding the subcellular localization of IRS-1 indicated that, after insulin stimulation, IRS-1 did not translocate to the plasma membrane and was tyrosine phosphorylated at the cytoplasm by internalization of activated insulin receptors (Kublaoui et al., 1995).
Figure 3.
Colocalization of IRS-1 and Bcl-2. A part of IRS-1 colocalizes with Bcl-2, and insulin stimulation of cells does not affect localization of IRS-1 and Bcl-2. CHO-IR/IRS-1/Bcl-2 cells were treated with (D–F) or without (A–C) insulin (100 nM) at 37°C for 10 min. Then cells were fixed and incubated with anti-IRS-1 antibody (C-20; A, B, D, and E) and anti-Bcl-2 antibody (clone 124; B, C, E, and F), followed by FITC-conjugated antirabbit (for C-20) or Texas Red-conjugated anti-mouse (for anti-Bcl-2 antibody) secondary antibodies.
IRS-1 Enhances Antiapoptotic Activity of Bcl-2
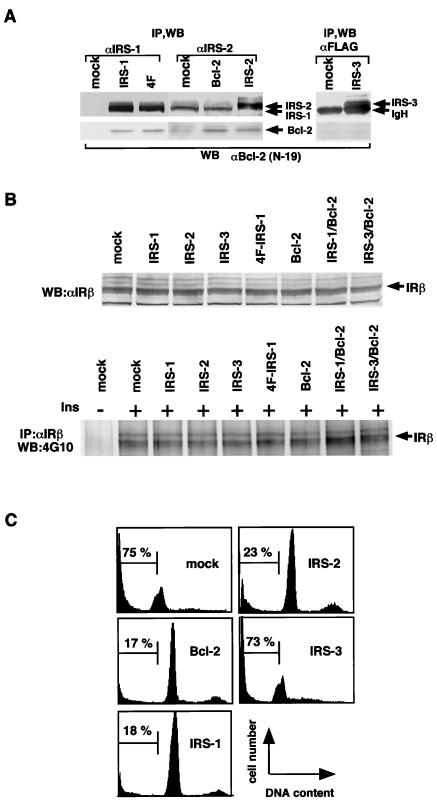
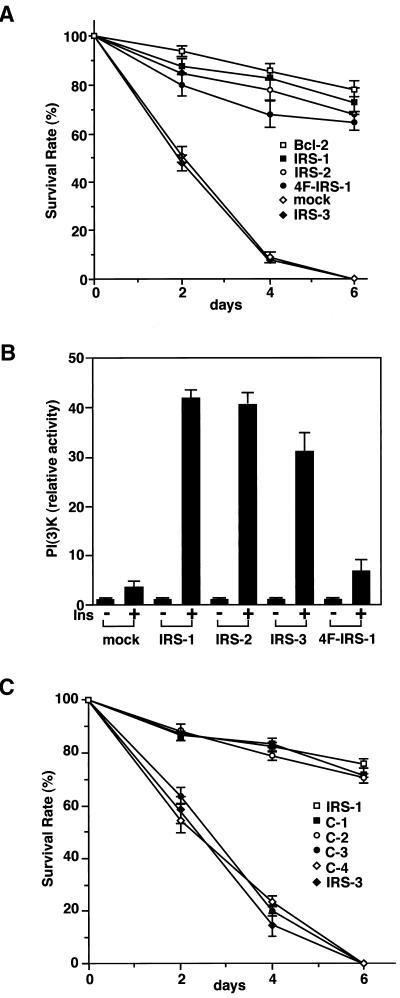
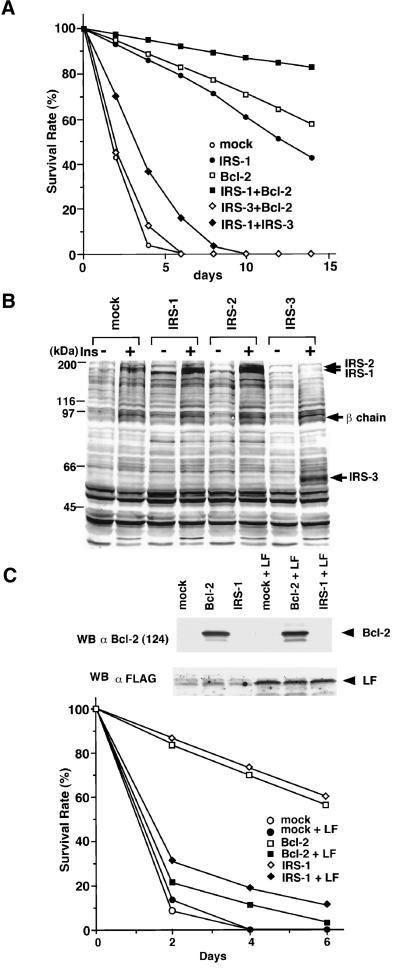
From the observations described above, we presumed that IRS proteins regulate the apoptosis of cells by modulating the Bcl-2 function. It has been shown that overexpression of Bcl-2 suppresses apoptosis of Ba/F3 cells when deprived of IL-3. To analyze the survival effects of IRS proteins and their regulatory mechanisms for Bcl-2, we examined whether they could suppress apoptosis of cells induced by IL-3 withdrawal. Ba/F3 cells express endogenous IRS-2 and Bcl-2; however, the expression levels of them are relatively low. Therefore, we established stable transfectants of IRS-1, IRS-2, IRS-3, and Bcl-2, in which IRS-1 and IRS-2 but not IRS-3 associate with Bcl-2 (Figure 4A). We confirmed that the Ba/F3-derived cell lines used in these experiments express comparable levels of insulin receptors, and when treated with insulin, the levels of autophosphorylation of the insulin receptor were also approximately equal (Figure 4B). Interestingly, IRS-1 and IRS-2 could suppress apoptosis, but IRS-3 could not (Figures 4C and 5A). Because the results of the survival rate, calculated as described in MATERIALS AND METHODS, correlated well with those of the PI staining and the FACS analysis of cells (Figures 4C and 5A), we used the survival rate as an antiapoptotic level in the subsequent experiments. In the presence of a tyrosine kinase inhibitor, genistein (50 μg/ml), or in the absence of FCS, IRS-1 and IRS-2 could not suppress the apoptosis of cells (our unpublished results). We thus deduced that the IRS-1-mediated signals, which were activated by growth factors such as insulin and IGF-1 included in FCS, could be required to exert survival effects. In fact, the FCS we used in this study contained 15 nM insulin quantified by the immunoradiometric assay (IRMA). Moreover, even if the FCS concentration was reduced to 0.5%, we obtained similar results when insulin was added to the medium (10 nM; our unpublished results). Because IRS-1 and IRS-2 can interact with the PI3K-Akt pathway, it is reasonable to assume that they suppress apoptosis by activating the PI3K-Akt pathway. However, IRS-3 also has binding sites for the p85 subunit of PI3K (Lavan et al., 1997b) and can activate PI3K (Figure 5B) but cannot suppress apoptosis (Figures 4C and 5A). On the other hand, a quadruple tyrosine-phenylalanine mutant of IRS-1, 4F-IRS-1, which lacks all the major binding sites for p85 (Tyr-460, Tyr-608, Tyr-939, and Tyr-987) and significantly loses the ability to activate PI3K (Yamamoto-Honda et al., 1996; Figure 5B), can bind to Bcl-2 (Figure 4A) and suppressed apoptosis at a level similar to that of IRS-1 (Figure 5A). We next examined whether the series of truncated mutants of IRS-1 could suppress apoptosis and found that C-1 and C-2 could suppress apoptosis but C-3 and C-4 mutants could not (Figure 5C). Because C-1 and C-2 can bind to Bcl-2, whereas C-3 and C-4 cannot (Figure 2C), these findings suggest that, to exert the survival effect of IRS-1, association with Bcl-2 is more important than association with the PI3K pathway. The next issue to be addressed is whether IRS-3 is merely unable to suppress apoptosis or whether it interferes with the antiapoptotic effect of the IRS-1/2-Bcl-2 pathway. To test this, we established three Ba/F3 lines coexpressing IRS-1/Bcl-2, IRS-3/Bcl-2, and IRS-1/IRS-3. Although the cells coexpressing IRS-1/Bcl-2 showed an enhanced survival rate compared with the cells expressing either one of them alone, the cells coexpressing IRS-3 and Bcl-2 underwent apoptosis as rapidly as the mock cells, and the cells coexpressing IRS-1 and IRS-3 showed a significantly reduced survival rate compared with the cells expressing IRS-1 alone (Figure 6A), indicating that IRS-3 abrogates the survival effects of IRS-1 and Bcl-2. Consistent with this, tyrosine phosphorylation of endogenous IRS-2 was severely impaired in the cells expressing IRS-3 when stimulated with insulin (Figure 6B). This result was confirmed in the cells coexpressing IRS-1 and IRS-3 by the finding that phosphorylation of IRS-1 was significantly suppressed compared with that in the cells expressing IRS-1 alone (our unpublished results).
Figure 4.
IRS-1 and IRS-2 but not IRS-3 suppress apoptotic cell death. (A) IRS-1, 4F-IRS-1 (4F), and IRS-2 but not IRS-3 associate with Bcl-2 in Ba/F3 cells. Lysates from the indicated cell lines were immunoprecipitated and immunoblotted with the indicated antibodies. P-Bcl-2, phosphorylated Bcl-2. (B) Upper panel, lysates from the indicated cell lines were immunoblotted with anti-insulin receptor β chain (αIRβ). Lower panel, lysates from the indicated cell lines were immunoprecipitated and immunoblotted with indicated antibodies. (C) IRS-1 and IRS-2 but not IRS-3 suppress apoptosis of Ba/F3 cells deprived of IL-3. Stable transfectants of IRS-1, IRS-2, IRS-3, 4F-IRS-1, and Bcl-2 were deprived of IL-3 and cultured in RPMI 1640 medium containing 5% FCS. FCS used in this study contained 15 nM insulin quantified by IRMA. The FACS analysis was performed 48 h after IL-3 depletion.
Figure 5.
(A) IRS-1 and IRS-2 but not IRS-3 suppress apoptosis of Ba/F3 cells deprived of IL-3. Stable transfectants of IRS-1, IRS-2, IRS-3, 4F-IRS-1, Bcl-2, and mock cells were deprived of IL-3 and cultured in RPMI 1640 medium containing 5% FCS. FCS used in this study contained 15 nM insulin quantified by IRMA. (B) PI3K activity of insulin-stimulated cell lines. Stable transfectants of IRS-1, IRS-2, IRS-3, 4F-IRS-1, and mock cells treated with or without insulin were lysed and immunoprecipitated with 4G10. The immunoprecipitates were subjected to the PI3K assay. (C) Survival rate of stable transfectants expressing deletion mutants of IRS-1, C-1, C-2, C-3, and C-4.
Figure 6.
(A) Survival rate of double stable transfectants of Ba/F3 cells established by using retrovirus vectors carrying IRS-1-IRES-Bcl-2, IRS-3-HA-IRES-Bcl-2,or IRS-1-IRES-IRS-3, compared with those of Bcl-2, IRS-1, and Ba/F3 cells. (B) Stable transfectants expressing IRS-1, IRS-2, IRS-3, and mock cells were serum starved and stimulated with insulin (100 nM). Lysates from these cells were subjected to immunoblotting with 4G10. β chain, β subunit of the insulin receptor. (C) Coexpression of the loop fragment of Bcl-2 in Bcl-2 and IRS-1 cells severely impairs the survival of these cells. Upper panel, the lysates from indicated cell lines were subjected to the immunoblotting with anti-Bcl-2 (clone 124) or anti-FLAG M2 antibody. Lower panel, survival rate of indicated cell lines. LF, loop fragment tagged with the FLAG peptide.
To ascertain the importance of association between IRS-1 and Bcl-2 in survival of cells, we constructed a deletion mutant of Bcl-2, which consists of the loop domain of human Bcl-2 (aa 31–75) epitope tagged with the FLAG peptide (LF). We expect that the LF peptide dominant-negatively blocks signals from IRS-1 to Bcl-2 and thus abolishes the antiapoptotic effects of IRS-1 and Bcl-2. We cotransfected the construct into mock, Bcl-2, and IRS-1 cells and examined the effect on the survival of cells. As expected, we found in the experiment that the cells transfected with the LF peptide become sensitive to apoptosis induced by IL-3 depletion under the same conditions of Figure 6A (Figure 6C).
IRS-1 Suppresses Phosphorylation of Bcl-2 Induced by Insulin
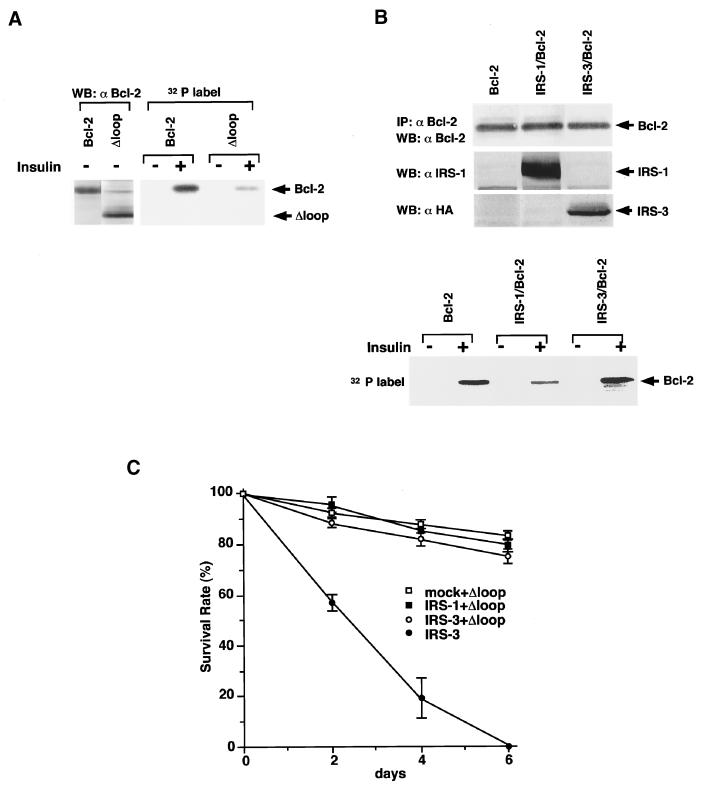
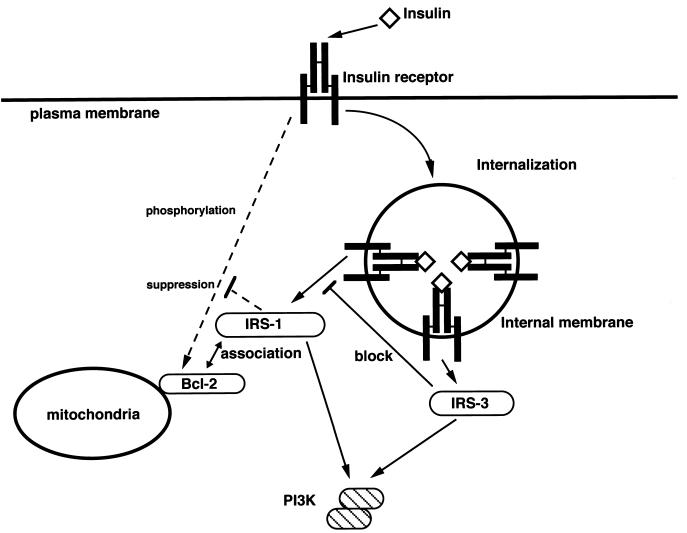
To investigate the mechanisms involved in the regulation of the antiapoptotic function of Bcl-2 by IRS proteins, we examined their effects on phosphorylation of Bcl-2, because serine/threonine phosphorylation sites are present within the loop domain of Bcl-2 (Chang et al., 1997; Maundrell et al., 1997). To confirm that the insulin treatment induces phosphorylation of Bcl-2, and the phosphorylation site is located within the loop domain, we labeled Ba/F3 cells expressing Bcl-2 and Δloop-Bcl-2 with [32P]orthophosphoric acid and stimulated with insulin. The deletion mutant of Bcl-2 that lacks the loop domain (Δloop-Bcl-2) was not phosphorylated by insulin treatment (Figure 7A), indicating that the phosphorylation sites of Bcl-2 by insulin were located within the loop domain. Because the expression level of endogenous Bcl-2 in Ba/F3 cells is relatively low, we could not clearly compare the phosphorylation levels induced by insulin treatment (our unpublished results). Therefore, we used double transfectants of Ba/F3, IRS-1/Bcl-2, IRS-3/Bcl-2, and Bcl-2 cells that express approximately the same amount of Bcl-2 (Figure 7B). Insulin treatment of these cells induced phosphorylation of Bcl-2, but coexpression of IRS-1 significantly suppressed insulin-induced phosphorylation, whereas phosphorylation of Bcl-2 in IRS-3/Bcl-2 cells was rather enhanced compared with that in Bcl-2 cells (Figure 7B). Because the phosphorylation levels of Bcl-2 correlate well with the results of Figure 6A, they support our hypothesis that IRS-3 blocks activation of the IRS-1/2-Bcl-2 pathway in a dominant-negative manner and thus abrogates the survival effects of Bcl-2. To confirm the importance of the phosphorylation level of Bcl-2 in the regulation of apoptosis by IRS proteins, we next generated stable transfectants that coexpress the Δloop mutant with mock, IRS-1, and IRS-3 cells. The Δloop mutant lacks the phosphorylation site(s) by insulin stimulation (Figure 7A), and possesses an enhanced ability to inhibit apoptosis compared with the full-length protein (Chang et al., 1997; Maundrell et al., 1997). As expected, IRS-3 cells coexpressing the Δloop mutant became resistant to apoptosis. This result supports our hypothesis that the dominant-negative effect of IRS-3 is exerted through up-regulating phosphorylation of Bcl-2. In the Figure 8, we present our hypothesis on the mechanism of how IRS proteins regulate survival of cells through controlling phosphorylation of Bcl-2. We could not obtain data as to whether the association between IRS-1 and Bcl-2 is direct; therefore, it is possible that internal molecule(s) exist between them.
Figure 7.
Effects of IRS proteins on phosphorylation of Bcl-2. (A) Insulin induces phosphorylation of Bcl-2 but not the Δloop mutant of Bcl-2. Left panel, total cell lysates from Bcl-2 and Δloop cells were subjected to immunoblotting with anti-Bcl-2 antibody (N-19). Right panel, Bcl-2 and Δloop cells were serum starved and labeled with [32P]orthophosphoric acid. Then cells were stimulated with or without insulin (100 nM), lysed, immunoprecipitated with anti-Bcl-2 antibody, and subjected to SDS-PAGE. The phosphorylated proteins were visualized by autoradiography. Δloop, deletion mutant of Bcl-2 lacking the loop domain. (B) IRS-1 but not IRS-3 suppresses insulin-induced phosphorylation of Bcl-2. Bcl-2, IRS-1/Bcl-2, and IRS-3-HA/Bcl-2 cells were serum starved and labeled with [32P]orthophosphoric acid. Phosphorylation of Bcl-2 was detected as in A. The expression levels of IRS-1, IRS-3, and Bcl-2 were indicated by immunoblotting with the indicated antibodies. (C) Survival rate of double stable transfectants of Ba/F3 cells established by using retrovirus vectors carrying IRS-1-HA-IRES-Δloop and IRS-3-HA-IRES-Δloop, compared with those of Δloop and IRS-3 cells. Δloop, deletion mutant of Bcl-2 lacking the loop domain.
Figure 8.
Schematic representation of our proposed model for the role of association between IRS-1 and Bcl-2 (see DISCUSSION). The pathways activated by unknown mechanisms are indicated by dotted lines.
DISCUSSION
In this study, we searched for regulatory molecules of Bcl-2 in the signaling pathway of the insulin receptor and found that IRS-1 binds to the loop domain of Bcl-2. The association between IRS-1 and Bcl-2 was observed regardless of insulin stimulation (Figure 1B). However, IRS-1 could not coprecipitate phosphorylated Bcl-2, as shown in Figure 1B. We can raise two possibilities to explain these data. First, when phosphorylated, Bcl-2 dissociates from IRS-1. Second, a part of Bcl-2 not bound to IRS-1 is mainly phosphorylated with insulin treatment, whereas the rest of Bcl-2 constitutively associated with IRS-1 is not phosphorylated. Because nonphosphorylated Bcl-2 coprecipitated with IRS-1 and IRS-1 coprecipitated with Bcl-2 did not decrease with insulin stimulation (Figure 1B), we concluded that the latter possibility is more probable. Constitutive association between IRS-1 and Bcl-2 does not conflict with previous data as described in RESULTS. Kublaoui et al. (1995) reported that the distribution of IRS-1 is 80% cytosolic, 20% internal membrane associated, and essentially undetectable in the plasma membrane. After insulin stimulation, the phosphorylation state of IRS-1 in the internal membrane parallels that of the insulin receptor, but cytosolic IRS-1 phosphorylation remains constant. They hypothesized that insulin action is mediated by receptor internalization (Kublaoui et al., 1995).
As shown in Figures 4 and 5, IRS-1 suppresses apoptotic cell death induced by growth factor withdrawal. The antiapoptotic effects of IRS-1 and Bcl-2 were synergistic (Figure 6A). Our results that IRS-3 and the deletion mutants of IRS-1 that fail to bind to Bcl-2 (Figure 2C, C-3 and C-4) cannot suppress apoptosis (Figure 5C) indicate that the association between IRS-1 and Bcl-2 is important to suppress apoptosis. The data of Figure 6C support our assumption. Because IRS-1 binds to the loop domain of Bcl-2 that is the region of serine/threonine phosphorylation, it was most likely that IRS proteins regulate the phosphorylation of Bcl-2. As expected, we found that overexpression of IRS-1 suppresses IRS proteins regulate the phosphorylation of Bcl-2. As expected, we found that overexpression of IRS-1 suppresses phosphorylation of Bcl-2 and that IRS-3 has an opposite effect (Figure 7B).
The roles of phosphorylation of Bcl-2 are controversial. Many papers argue that dephosphorylation of Bcl-2 enhances the antiapoptotic function of Bcl-2 and phosphorylation inactivates its effects (Haldar et al., 1995; Chang et al., 1997; Maundrell et al., 1997), although some reports contradict them (Ito et al., 1997; Ruvolo et al., 1998). Moreover, several recent studies insist that phosphorylation of Bcl-2 is a marker of M phase events (Ling et al., 1998; Scatena et al., 1998). However, the well-described phenomena that the loop deletion mutants of Bcl-2 possesses enhanced antiapoptotic activity than wild-type Bcl-2 (Chang et al., 1997; Maundrell et al., 1997; Srivastava et al., 1999; Wang et al., 1999) cannot be explained only by their hypothesis. From the standpoint of the first group, it can be interpreted as follows; the loop-deleted mutant is not phosphorylated; therefore, its antiapoptotic activity is up-regulated. Because phosphorylation of Bcl-2 and cell survival inversely correlate well (Figures 4C, 5A, and 7B) and, by coexpressing the Δloop mutant, IRS-3 cells became resistant to apoptosis (Figure 7C), our results support the first hypothesis. However, it is possible that phosphorylation of Bcl-2 can be independently regulated by the signal transduction pathway downstream of growth factor receptors and by cell cycle.
We could not present data indicating the mechanism for how IRS-1 suppresses phosphorylation of Bcl-2. From Figure 7, A and B, we can conclude that 1) insulin stimulation result in serine/threonine phosphorylation of Bcl-2 at the loop domain; 2) the expression level of IRS-1 is in inverse proportion to phosphorylation of Bcl-2; and 3) IRS-3 opposes the effect of IRS-1. Because, without insulin and FCS, the survival effects of IRS-1 were not exerted, and the tyrosine kinase inhibitor genistein abolished its effects, we expect that the signals mediated through IRS-1 enhance the activity of serine/threonine phosphatases that dephosphorylate Bcl-2. Indeed, we have preliminary data that serine/threonine phosphatase inhibitors such as okadaic acid and cypermethline induce apoptosis of IRS-1/Bcl-2 cells at the same condition as Figure 6A (our unpublished results).
Several reports have already indicated that IRS-1 possesses an antiapoptotic activity. Growth factors such as insulin (Sun et al., 1991), IGF (Chuang et al., 1993; Myers et al., 1993), and IL-4 (Keegan et al., 1994) that use IRS-1 as a substrate for intracellular signaling are known to suppress apoptotic cell death (Barres et al., 1992, 1993; Parry et al., 1994). Hepatocellular carcinoma cell lines overexpressing IRS-1 are resistant to apoptosis (Tanaka and Wands, 1996b). However, when an IRS-1 mutant lacking the carboxyl-terminal domain is introduced into such cell lines, the cell becomes sensitive to apoptosis (Tanaka and Wands, 1996a). These reports suggest that IRS-1 has a critical role in tumorigenesis, and the carboxyl-terminal domain is essential for these activities. From our data (Figures 5C and 6A), we can deduce that the carboxyl-terminal–truncated mutant of IRS-1 dominant-negatively blocks the IRS-1/Bcl-2 cascade to induce apoptosis of hepatocellular carcinoma cells.
Recently, CHICO, a Drosophila homologue of vertebrate IRS-1–4, was reported to play an essential role in the control of cell size and growth of flies (Bohni et al., 1999). Moreover, a recent study demonstrated that knockout mice lacking both IRS-1 and IRS-2 (Irs-1−/−Irs-2−/−) are lethal. The heterozygotes (Irs-1−/−Irs-2+/−) mice showed severe growth retardation. They also showed that apoptosis of the islet β cells of IRS-2-deficient mice were increased. These data indicate that, in mammals, IRS proteins are critical for development and regulation of apoptosis (Withers et al., 1999). Taken together, our findings that IRS proteins associate with Bcl-2 and modulate its antiapoptotic function might provide a clue to understand the mechanism of how growth factors regulate cell survival, apoptosis, and organ development.
ACKNOWLEDGMENTS
We thank O.N. Witte for the expression vector pSRαSVtkneo. This work was supported in part by a grant-in-aid from the Ministry of Education, Science, and Culture of Japan and from the Ministry of Health and Welfare of Japan.
Abbreviations used:
- CHO
Chinese hamster ovary
- FACS
fluorescence-activated cell sorting
- HA
hemagglutinin
- IGF
insulin-like growth factor
- IL
interleukin
- IRES
internal ribosomal entry site
- IRMA
immunoradiometric assay
- IRS
insulin receptor substrate
- LF
loop fragment
- PI3K
phosphoinositide-3 kinase
- RTK
receptor tyrosine kinase
REFERENCES
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Boyd JM, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LM, Myers MJ, Seidner GA, Birnbaum MJ, White MF, Kahn CR. Insulin receptor substrate 1 mediates insulin and insulin-like growth factor I-stimulated maturation of Xenopus oocytes. Proc Natl Acad Sci USA. 1993;90:5172–5175. doi: 10.1073/pnas.90.11.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Craparo A, O'Neill TJ, Gustafson TA. NonSH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez GM, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Eck MJ, Dhe PS, Trub T, Nolte RT, Shoelson SE. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell. 1996;85:695–705. doi: 10.1016/s0092-8674(00)81236-2. [DOI] [PubMed] [Google Scholar]
- Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for antiapoptosis function, J. Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Klesse LJ, Parada LF. p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neurosci. 1998;18:10420–10428. doi: 10.1523/JNEUROSCI.18-24-10420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui B, Lee J, Pilch PF. Dynamics of signaling during insulin-stimulated endocytosis of its receptor in adipocytes, J. Biol Chem. 1995;270:59–65. doi: 10.1074/jbc.270.1.59. [DOI] [PubMed] [Google Scholar]
- Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- Lavan BE, Fantin VR, Chang ET, Lane WS, Keller SR, Lienhard GE. A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family, J. Biol Chem. 1997a;272:21403–21407. doi: 10.1074/jbc.272.34.21403. [DOI] [PubMed] [Google Scholar]
- Lavan BE, Lane WS, Lienhard GE. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem. 1997b;272:11439–11443. doi: 10.1074/jbc.272.17.11439. [DOI] [PubMed] [Google Scholar]
- Ling YH, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- Lithgow T, Van DR, Bertram JF, Strasser A. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth & Differ. 1994;5:411–417. [PubMed] [Google Scholar]
- Maundrell K, et al. Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J Biol Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- Myers MJ, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3′-kinase. Endocrinology. 1993;132:1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Onishi M, et al. Applications of retrovirus-mediated expression cloning, Exp. Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- Parry SL, Hasbold J, Holman M, Klaus GG. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with IL-4 and anti-CD40. J Immunol. 1994;152:2821–2829. [PubMed] [Google Scholar]
- Ridderstrale M, Degerman E, Tornqvist H. Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes, J. Biol Chem. 1995;270:3471–3474. doi: 10.1074/jbc.270.8.3471. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;3:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, Pietenpol JA. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest, J. Biol Chem. 1998;273:30777–30784. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- Souza SC, Frick GP, Yip R, Lobo RB, Tai LR, Goodman HM. Growth hormone stimulates tyrosine phosphorylation of insulin receptor substrate-1. J Biol Chem. 1994;269:30085–30088. [PubMed] [Google Scholar]
- Srivastava RK, Mi QS, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MJ, Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signaling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype, J. Clin Invest. 1996a;98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996b;56:3391–3394. [PubMed] [Google Scholar]
- Tobe K, et al. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993;268:11167–11171. [PubMed] [Google Scholar]
- Ueno H, Hirano N, Kozutsumi H, Sasaki K, Tanaka T, Yazaki Y, Hirai H. An epidermal growth factor receptor-leukocyte tyrosine kinase chimeric receptor generates ligand-dependent growth signals through the Ras signaling pathway. J Biol Chem. 1995;270:20135–20142. doi: 10.1074/jbc.270.34.20135. [DOI] [PubMed] [Google Scholar]
- Ueno H, Sasaki K, Kozutsumi H, Miyagawa K, Mitani K, Yazaki Y, Hirai H. Growth and survival signals transmitted via two distinct NPXY motifs within leukocyte tyrosine kinase, an insulin receptor-related tyrosine kinase. J Biol Chem. 1996;271:27707–27714. doi: 10.1074/jbc.271.44.27707. [DOI] [PubMed] [Google Scholar]
- Van OE, De MP, Roth J. Cell surface receptors for insulin and human growth hormone. Effect of microtubule and microfilament modifiers. J Biol Chem. 1976;251:6844–6851. [PubMed] [Google Scholar]
- Vin T, Keller SR, Quelle FW, Witthuhn BA, Tsang ML, Lienhard GE, Ihle JN, Yang YC. Interleukin-9 induces tyrosine phosphorylation of insulin receptor substrate-1 via JAK tyrosine. J Biol Chem. 1995;270:20497–20502. doi: 10.1074/jbc.270.35.20497. [DOI] [PubMed] [Google Scholar]
- Walker F, Kato A, Gonez LJ, Hibbs ML, Pouliot N, Levitzki A, Burgess AW. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol Cell Biol. 1998;18:7192–7204. doi: 10.1128/mcb.18.12.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang Z, Boise L, Dent P, Grant S. Loss of the bcl-2 phosphorylation loop domain increases resistance of human leukemia cells (U937) to paclitaxel-mediated mitochondrial dysfunction and apoptosis. Biochem Biophys Res Commun. 1999;259:67–72. doi: 10.1006/bbrc.1999.0669. [DOI] [PubMed] [Google Scholar]
- Welham MJ, Learmonth L, Bone H, Schrader JW. Interleukin-13 signal transduction in lymphohemopoietic cells. Similarities and differences in signal transduction with interleukin-4 and insulin, J. Biol Chem. 1995;270:12286–12296. doi: 10.1074/jbc.270.20.12286. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signaling. Nat Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Honda R, et al. Mutant of insulin receptor substrate-1 incapable of activating phosphatidylinositol 3-kinase did not mediate insulin-stimulated maturation of Xenopus laevis oocytes, J. Biol Chem. 1996;271:28677–28681. doi: 10.1074/jbc.271.45.28677. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yonezawa K, et al. Signal transduction pathways from insulin receptors to Ras. Analysis by mutant insulin receptors. J Biol Chem. 1994;269:4634–4640. [PubMed] [Google Scholar]