Abstract
Ralstonia eutropha JMP134(pJP4) degrades 3-chlorobenzoate (3-CB) by using two not completely isofunctional, pJP4-encoded chlorocatechol degradation gene clusters, tfdCIDIEIFI and tfdDIICIIEIIFII. Introduction of several copies of each gene cluster into R. eutropha JMP222, which lacks pJP4 and thus accumulates chlorocatechols from 3-CB, allows the derivatives to grow in this substrate. However, JMP222 derivatives containing one chromosomal copy of each cluster did not grow in 3-CB. The failure to grow in 3-CB was the result of accumulation of chlorocatechols due to the limiting activity of chlorocatechol 1,2-dioxygenase (TfdC), the first enzyme in the chlorocatechol degradation pathway. Micromolar concentrations of 3- and 4-chlorocatechol inhibited the growth of strains JMP134 and JMP222 in benzoate, and cells of strain JMP222 exposed to 3 mM 3-CB exhibited a 2-order-of-magnitude decrease in viability. This toxicity effect was not observed with strain JMP222 harboring multiple copies of the tfdCI gene, and the derivative of strain JMP222 containing tfdCIDIEIFI plus multiple copies of the tfdCI gene could efficiently grow in 3-CB. In addition, tfdCI and tfdCII gene mutants of strain JMP134 exhibited no growth and impaired growth in 3-CB, respectively. The introduction into strain JMP134 of the xylS-xylXYZL genes, encoding a broad-substrate-range benzoate 1,2-dioxygenase system and thus increasing the transformation of 3-CB into chlorocatechols, resulted in derivatives that exhibited a sharp decrease in the ability to grow in 3-CB. These observations indicate that the dosage of chlorocatechol-transforming genes is critical for growth in 3-CB. This effect depends on a delicate balance between chlorocatechol-producing and chlorocatechol-consuming reactions.
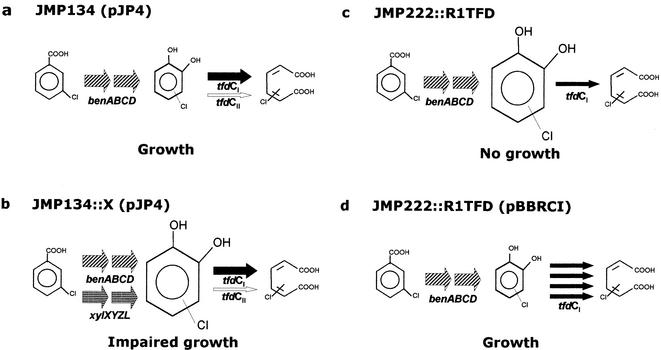
Ralstonia eutropha JMP134(pJP4) grows in pollutant compounds like 3-chlorobenzoate (3-CB) and 2,4-dichlorophenoxyacetic acid. Metabolism of 3-CB is initiated by a chromosomally encoded, low-specificity benzoate dioxygenase and a 1,2-dihydro-1,2-dihydroxybenzoate dehydrogenase and forms 3-chlorocatechol (3-CC) and 4-CC (Fig. 1) (28, 33). The chlorocatechols are metabolized by the enzymes chlorocatechol 1,2-dioxygenase (TfdC), chloromuconate cycloisomerase (TfdD), dienelactone hydrolase (TfdE), and maleylacetate reductase (TfdF) encoded in the tfdCIDIEIFI and tfdDIICIIEIIFII gene clusters from pJP4 (Fig. 1) (9, 20-22, 27, 29, 30). The simultaneous presence of these two apparently isofunctional chlorocatechol-degrading gene clusters (Fig. 1) is very uncommon in bacterial chloroaromatic catabolism (38, 40), which raises a question concerning the specific roles, if any, of each of these operons in chlorocatechol catabolism. Both gene clusters are induced during adaptation to 2,4-dichlorophenoxyacetic acid in cells of R. eutropha JMP134(pJP4) growing in fructose (24) and expressing active enzymes for catabolism of this compound and 2,4-dichlorophenol (21). Introduction of multiple copies of the tfdCIDIEIFI or tfdDIICIIEIIFII genes into strain JMP222, a derivative of strain JMP134 lacking pJP4 and therefore unable to grow in 3-CB, allows the derivatives to grow in this compound (27). However, we observed that derivatives of strain JMP222 harboring single chromosomal copies of each tfd gene module or one copy of the tfdCIDIEIFI module and one copy of the tfdDIICIIEIIFII module did not grow in 3-CB (23). A tfd gene dosage effect has been shown to be important for efficient growth in 3-CB of strain JMP134 derivatives harboring a rearranged form of the pJP4 plasmid (5). In addition, recent evidence from our laboratory indicates that pJP4 occurs naturally at a level of several copies per genome in strain JMP134 (37). These observations led us to hypothesize that expression of single copies of the tfd genes is limiting for growth in 3-CB because toxic intermediates are accumulated. In this study, we obtained evidence that accumulation of chlorocatechols impairs the growth of derivatives of strain JMP134 in 3-CB. This effect is observed when chlorocatechol-producing reactions overtake chlorocatechol-consuming reactions, and it is prevented if several copies of the tfdC genes, encoding chlorocatechol 1,2-dioxygenase, are present.
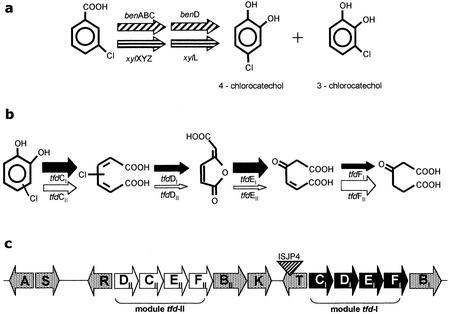
FIG. 1.
Genes involved in 3-CB degradation. (a) Chlorocatechol-producing peripheral reactions for 3-CB encoded in the chromosome of R. eutropha (ben genes), and the 1,2-toluate dioxygenase system (xyl genes) from pWW0. (b) Chlorocatechol 1,2-dioxygenase (TfdC), chloromuconate cycloisomerase (TfdD), dienelactone hydrolase (TfdE), and maleylacetate reductase (TfdF) catalyze the conversion of chlorocatechols to chloromuconate, cis-dienelactone, maleylacetate, and β-ketoadipate, respectively. The arrow thickness indicates the relative specific activity of the enzymes encoded by each module for intermediates of 3-CB metabolism (27, 30). (c) Organization of tfd genes in pJP4, including the tfdCIDIEIFI and tfdDIICIIEIIFII gene clusters. The diagram is not to scale.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. R. eutropha JMP134(pJP4) and 3-CB-mineralizing derivatives of this strain were grown at 30°C in minimal medium (19) with 0.5 to 10 mM 3-CB as the sole carbon source. Growth in 3-CB was determined by measuring the increase in the optical density at 600 nm (OD600). At least three replicates were used for each growth measurement. R. eutropha derivatives not able to proliferate in 3-CB were grown in 3 to 5 mM benzoate plus the appropriate antibiotic (Table 1). Escherichia coli strains were maintained on Luria-Bertani (LB) agar plates containing 50 μg of ampicillin ml−1, 50 μg of kanamycin ml−1, 20 μg of gentamicin ml−1, 20 μg of cloramphenicol ml−1, or 40 μg of tellurite ml−1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant phenotype and/or genotypea | Source or reference |
|---|---|---|
| R. eutropha strains | ||
| JMP134 | 2, 4-D+ 3-CB+, pJP4 | DSMZb |
| JMP134-F3 | 3-CB+, pJP4-F3 | 5 |
| JMP222 | pJP4-free derivative, 2,4-D− 3-CB− Smr | H.-J. Knackmuss |
| Plasmids | ||
| pRK600 | Cfr IncPα tra+ | 15 |
| pBBR1MCS-2 | Kmr, broad host range | 18 |
| pUT | Kmr Apr | 15 |
| pBSL202 | Gmr Apr | 2 |
| pBBRCI | KmrtfdR-Ptfd-ItfdCIDI∇, pBBR1MCS-2 derivative | This study |
| pBBRPI | KmrtfdR-Ptfd-I, pBBR1MCS-2 derivative | This study |
| pSPM100 | Apr TerxyIS-xyIXYZL, pUT derivative | V. de Lorenzo |
| pR1TFD | Gmr AprtfdR-Ptfd-ItfdCIDIEIFI, pBSL202 derivative | 23 |
| pR2TFD | Gmr AprtfdR-Ptfd-IItfdDIICIIEIIFII, pBSL202 derivative | 23 |
| pR12TFD | Gmr AprtfdR-Ptfd-ItfdCIDIEIFI-tfdR-Ptfd-IItfdDIICIIEIIFII, pBSL202 derivative | 23 |
| pBBR1M-I | KmrtfdR-Ptfd-ItfdCIDIEIFI, pBBR1MCS-2 derivative | 27 |
| pBBR1M-II | KmrtfdR-Ptfd-IItfdDIICIIEIIFII, pBBR1MCS-2 derivative | 27 |
| pJP4-ΔtfdCI | tfdCI | This study |
| pJP4-ΔtfdCII | tfdCII | This study |
2,4-D+ and 3-CB+, able to grow in 2,4-dichlorophenoxyacetate and 3-CB respectively; IncPα tra,+ IncP transference functions; tfd, catabolic genes from pJP4; tfdR, regulatory gene of pJP4; Ptfd-I, promoter region for tfdCIDIEIFI cluster; Ptfd-II, promoter region for tfdDIICIIEIIFII cluster; Apr, ampicillin resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Cfr, cloramphenicol resistance; Smr, streptomycin resistance.
DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany.
DNA manipulation.
Restriction, ligation, and dephosphorylation reactions, purification, and electroporation of DNA were performed by standard procedures (3). Derivatives of the broad-host-range plasmid vectors pBBR1MCS-2 (pBBRCI, pBBRPI) and pUT (pSPM100) were mobilized from E. coli to R. eutropha JMP222 by triparental mating with the helper strain E. coli HB101(pRK600), as previously described (27). Transconjugants were selected on minimal medium agar plates supplemented with 3 mM benzoate plus kanamycin or tellurite.
Construction of tfd gene modules.
The two pJP4-encoded chlorocatechol-degrading tfd modules were cloned into the medium-copy-number plasmid vector pBBR1MCS-2 to obtain pBBR1M-I and pBBR1M-II, as described elsewhere (27). To allow insertion into the chromosome, the tfdR-Ptfd-I tfdCIDIEIFI gene module, the tfdR-Ptfd-II tfdDIICIIEIIFII gene module, and both modules were cloned into the mini Tn5-derived plasmid vector pBSL202 to obtain pR1TFD, pR2TFD, and pR12TFD, respectively, as reported elsewhere (23).
Cloning of tfdR-Ptfd-I tfdCI and tfdR-Ptfd-I in multiple copies.
To clone the tfdR-regulated tfdCI gene into pBBR1MCS-2, pBBR1M-I (27) was digested with KpnI to obtain a 2.2-kb fragment containing tfdR-Ptfd-I tfdCIDI▿. This DNA fragment was then introduced into pBBR1MCS-2 to obtain pBBRCI, which harbors a complete tfdCI gene and a truncated tfdDI gene (tfdDI▿). For control purposes, plasmid pBBR1M-I was digested with PmlI and XbaI to delete a 3.9-kb fragment containing the tfdCIDIEIFI genes. The XbaI end of the plasmid backbone was blunt ended by using Mung bean nuclease. The plasmid was religated to obtain pBBRPI, which encodes only the tfdR-Ptdf-I region. The pBBR1MCS-2 derivatives containing tfd genes were shown to be present at levels of about 10 copies per cell (37).
Inactivation of the tfdCI and tfdCII genes in pJP4.
The tfdCI and tfdCII genes were independently inactivated in E. coli cells harboring the pJP4 plasmid by using the method of Datsenko and Wanner (7). PCR primers MutC1FW (5′-TCATGACGGAGGCAAAGTGAACAAAAGAGTCAAGGATGTTGTGTAGGCTGGAGCTGCTTC-3′) and MutC1RE (5′-GGGTTTGCCCCCGCCTGCGCACGCGCGGGCTCGATAACGAATTCCGGGGATCCGTCGACC-3′) and primers MutC2FW (5′-TTCATCTTTTTTGAAGAGAAAGCACCATGACAAATCCCCGGTGTAGGCTGGAGCTGCTTC-3′) and MutC2RE (5′-GCCTTCGCCGTCTCAGGCGCGGGACTTCTCGATGACGAAGATTCCGGGGATCCGTCGACC-3′), which contain 40-bp homology extensions on tfdCI or tfdCII sequences and 20-bp priming sequences for pKD13 (7), were synthesized. These primer pairs were used with pKD13 as the template to amplify the kanamycin-resistant gene flanked by 40 bp of the tfdCI or tfdCII gene sequences. The following PCR program was used: 95°C for 5 min, 28 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 90 s, and then 72°C for 10 min. The PCR products were used to inactivate tfdCI or tfdCII genes in an E. coli(pJP4) strain harboring RecBCD recombinase by a previously described procedure (7). pJP4 derivatives containing inactivated tfdCI or tfdCII genes were transferred to strain JMP222 by biparental conjugation (6).
Toxicity tests for chlorocatechols.
The MICs of 3-CC and 4-CC were determined for strains JMP134 and JMP222 growing in 5 mM benzoate or in LB broth. Growth was determined by determining the increase in OD600 after 24 h. To avoid interference by colored metabolic products, net OD600 values were calculated after subtraction of the OD600 determined for the cell-free supernatants from cultures after centrifugation of cells. The effect of chlorocatechols produced from 3-CB in cells growing in benzoate was studied by determining the viability in a 5 mM benzoate culture of strain JMP222 or JMP222(pBBRCI) to which 1 mM 3-CB was added at the late exponential phase and comparing the results with the results for a control which received no 3-CB. Samples were taken every 2 to 6 h for up to 24 h, and appropriate dilutions were plated on LB agar plates to determine the CFU.
Enzyme activity assays.
For enzyme activity assays, cells were grown in minimal medium containing 3 mM benzoate until the late exponential growth phase and were induced with 1 mM 3-CB for 3 h. Crude extracts were prepared and the activities of chlorocatechol 1,2-dioxygenase (TfdC) and chloromuconate cycloisomerase (TfdD) were measured as reported previously (27).
HPLC analysis.
Accumulation of chlorinated compounds was determined by high-performance liquid chromatography (HPLC) analysis by using cell-free supernatants from cell suspensions grown in 5 mM benzoate and induced for at least 2 h with 0.5 mM 3-CB. Cells were pelleted, washed twice with minimal medium, and resuspended in minimal medium to an OD600 of 0.5. 3-CB (0.2 mM) was added, and the cells were incubated at 30°C in an orbital shaker. Samples (10 μl) from cell-free supernatants were taken at different times and injected into a Shimadzu LC-10AD liquid chromatograph system equipped with an SC125 Lichrospher 5 μm column (Bischoff, Leonberg, Germany). A methanol-H2O (60:40) mixture containing 0.1% (vol/vol) phosphoric acid was used as the solvent at a flow rate of 1 ml min−1. The column effluent was monitored simultaneously at 210, 260, and 270 nm with an SPD-M10A diode array detector. The retention volumes were as follows: 2-chloro-cis,cis-muconate, 0.7 ml; 3-chloro-cis,cis-muconate, 0.5 ml; 3-CB, 4.6 ml; 3-CC, 1.4 ml; and 4-CC, 1.8 ml.
RESULTS
Growth in 3-CB of R. eutropha JMP222 derivatives containing the tfdCIDIEIFI or tfdDIICIIEIIFII gene cluster.
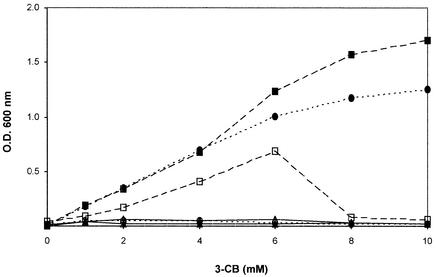
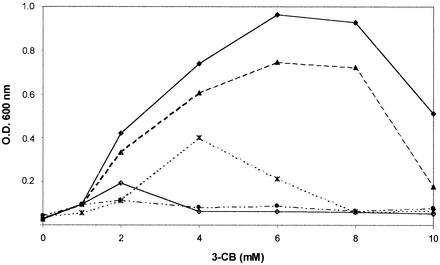
Derivatives containing about 10 copies (37) of tfdR-Ptdf-I tfdCIDIEIFI [R. eutropha JMP222(pBBR1M-I)] or tfdR-Ptdf-II tfdDIICIIEIIFII [R. eutropha JMP222(pBBR1M-II)] were previously reported to grow in liquid cultures with different concentrations of 3-CB as the sole carbon and energy source (27) (Fig. 2). The effect of the presence of single copies of these tfd gene modules was also tested. The mini Tn5-derived suicide plasmid vector pBSL202 was used to insert tfdR-Ptdf-I tfdCIDIEIFI (pR1TFD), tfdR-Ptdf-II tfdDIICIIEIIFII (pR2TFD), or both gene clusters (pR12TFD) into the chromosome of strain JMP222, as recently reported (23). The presence of each gene module in the chromosome of JMP222 transconjugants was confirmed by PCR and Southern blot analysis (23). None of the transconjugants, including strains JMP222::tfdR-Ptdf-I tfdCIDIEIFI (JMP222::R1TFD), JMP222::tfdR-Ptdf-II tfdDIICIIEIIFII (JMP222::R2TFD), and JMP222::tfdR-Ptdf-I tfdCIDIEIFI-tfdR-Ptdf-II tfdDIICIIEIIFII (JMP222::R12TFD), was able to proliferate in liquid cultures containing 0.5 to 10 mM 3-CB (Fig. 2 and results not shown). The possibility of a position effect of the inserted tfd gene clusters was eliminated because all transconjugants showed the same growth behavior in 3-CB. Determination of the copy numbers of tfd genes in selected transconjugants, based on a previously described procedure (37), indicated that there was single-copy gene dosage. The inability of derivatives harboring single copies of the tfd gene clusters to grow in 3-CB may have been due to differences in enzyme activity compared with the activities of derivatives containing multiple copies. Therefore, the specific activities of TfdC and TfdD were determined in crude extracts prepared from cells grown in benzoate and induced with 3-CB. The TfdC specific activities of the derivatives harboring chromosomal insertions were 4- to 10-fold lower than those observed for strain JMP222 containing multiple plasmid copies of each tfd gene module or the wild-type strain (Table 2). The same pattern was observed with chloromuconate cycloisomerase specific activities (data not shown). These results also indicate that the tfd genes were active in each derivative.
FIG. 2.
Growth with different 3-CB concentrations of R. eutropha derivatives harboring different copy numbers of ortho ring cleavage pathway tfd gene modules. Symbols: ▪, R. eutropha JMP222(pBBR1M-I); □, R. eutropha JMP222(pBBR1M-II); ▴, R. eutropha JMP222::R1TFD; ▵, R. eutropha JMP222::R2TFD; •, R. eutropha JMP222::R1TFD(pBBRCI); ○, R. eutropha JMP222::R1TFD(pBBRPI). OD600 was measured at the stationary phase. The values are means based on triplicate experiments. The deviations (not shown for clarity) were less than 5 to 10%.
TABLE 2.
Chlorocatechol 1,2-dioxygenase specific activities in crude extracts of R. eutropha strains grown in benzoate and induced with 3-CB
| R. eutropha strain | Chlorocatechol 1,2-dioxygenase sp act (U/mg)a |
|---|---|
| JMP134(pJP4) | 0.24 ± 0.01 |
| JMP222(pBBR1M-I) | 0.71 ± 0.03 |
| JMP222(pBBR1M-II) | 0.22 ± 0.02 |
| JMP222::R1TFD | 0.06 ± 0.01 |
| JMP222::R2TFD | 0.02 ± 0.01 |
| JMP222::R1TFD(pBBRCI) | 0.80 ± 0.07 |
| JMP222::R1TFD(pBBRPI) | 0.08 ± 0.07 |
| JMP134(pJP4ΔtfdCI) | 0.11 ± 0.02 |
| JMP134(pJP4ΔtfdCII) | 0.17 ± 0.02 |
Chlorocatechol 1,2-dioxygenase activity was assayed with 3,5-dichlorocatechol. The values are averages of two determinations.
Multiple copies of the tfdCI gene prevent toxicity of chlorocatechols and stimulate derivatives of R. eutropha JMP222 to grow in 3-CB.
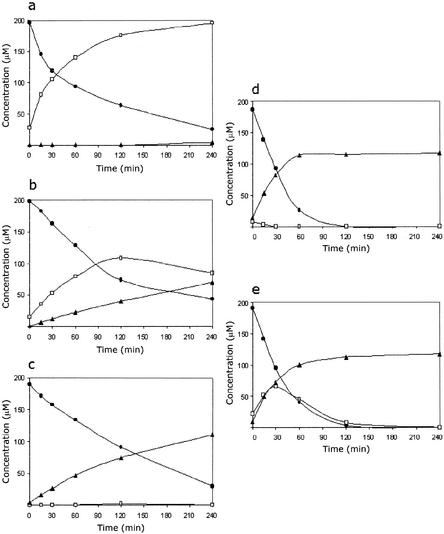
Low specific activities of enzymes that catalyze rate-limiting steps should produce slow growth but do not necessarily mean that there is a lack of growth phenotypes. Therefore, we assumed that lower enzyme activities of derivatives of strain JMP222 harboring chromosomal copies of the tfd gene clusters could produce an accumulation of toxic intermediates. Chlorocatechols are compounds that affect bacterial growth (35, 36). We hypothesized that chlorocatechols accumulate and produce toxic effects in R. eutropha, and this possibility was studied. The accumulation of chlorocatechols after exposure of different JMP222 derivatives to 3-CB was determined by HPLC analysis. Strain JMP222 quantitatively transformed 0.2 mM 3-CB into 3-CC and 4-CC (Fig. 3a). These chlorocatechols are not transformed further because this strain lacks any chlorocatechol-degrading genes. A characteristic deep brown color was clearly observed after extended incubation. Strain JMP222::R1TFD accumulated about 50% of the added 3-CB as chlorocatechols (Fig. 3b). Because of the presence of the tfd gene cluster in the chromosome, this strain was capable of further transformation of chlorocatechols. However, due to the poor activity of TfdDI with 2-chloromuconate (20, 41), about one-third of the 3-CB added was excreted as 2-chloromuconate (Fig. 3b). To study if accumulation of chlorocatechols occurred because of a limiting specific activity of chlorocatechol 1,2-dioxygenase, the gene dosage of tfdCI was increased by introducing pBBRCI into strain JMP222::R1TFD. In this derivative, harboring about 10 copies of the tfdCI gene per cell, no accumulation of chlorocatechols was detected (Fig. 3c), and the 2-chloromuconate accumulation increased to one-half of the 3-CB carbon input. Strain JMP222::R1TFD containing the control plasmid pBBRPI, encoding tfdR plus the upstream noncoding region of tfdCI, did accumulate chlorocatechols and 2-chloromuconate (data not shown) at rates and quantities similar to those of strain JMP222::R1TFD, showing that the effect of pBBRCI is not due to activation of the chromosomal copy of the tfdCI gene driven by the higher tfdR gene dosage.
FIG. 3.
Accumulation of chlorocatechols and 2-chloromuconate from 3-CB. Chlorinated intermediates were detected by HPLC by using samples of supernatants after incubation of 0.2 mM 3-CB with preinduced cell suspensions (OD600, 0.5) of strains JMP222 (a), JMP222::R1TFD (b), JMP222::R1TFD(pBBRCI) (c), JMP134(pJP4) (d), and JMP134::X(pJP4) (e). Symbols: •, 3-CB; □, 3-CC plus 4-CC; ▴, 2-chloromuconate.
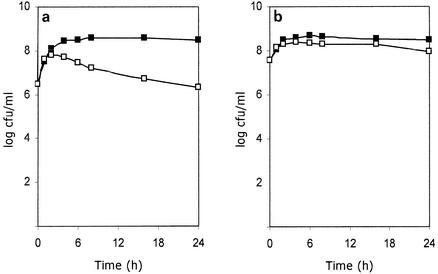
To study the possibility that accumulation of chlorocatechols intoxicates R. eutropha cells, the MIC of the two chlorocatechols produced during growth in 3-CB (Fig. 1a) was determined. The MIC of chlorocatechols for growth of R. eutropha JMP222 in 5 mM benzoate was 40 μM. The MIC for strain JMP134 was higher, 200 μM. When LB broth was used, the MICs were 200 and 500 μM for strains JMP222 and JMP134, respectively. No significant differences between the effect of 3-CC and the effect of 4-CC were found. The effect of in situ formation of chlorocatechols on cell viability, measured by determining the CFU, was also tested. With cells of strain JMP222 growing on 5 mM benzoate and exposed to 1 mM 3-CB, a 2-order-of-magnitude decrease in cell viability was observed (Fig. 4a). When the same experiment was performed with strain JMP222(pBBRCI), harboring multiple copies of tfdCI, no effect on cell viability was observed (Fig. 4b). These observations strongly indicate that 3-CC and 4-CC actually provoke a toxic effect in R. eutropha derivatives.
FIG. 4.
Effect of 3-CB on cell viability of R. eutropha JMP134 derivatives. Cell suspensions of strain JMP222 (a) or JMP222(pBBRC1) (b) previously grown in 5 mM benzoate were exposed (□) or not exposed (▪) to 1 mM 3-CB, and samples were analyzed to determine the number of CFU at different times. The values are averages based on two replicates.
If multiple copies of the tfdCI gene prevent accumulation of toxic chlorocatechols in R. eutropha derivatives that do not grow in 3-CB because they possess only a chromosomal copy of the chlorocatechol-degrading tfd genes, the presence of several copies of tfdCI in such derivatives should also allow growth in 3-CB. Accordingly, strain JMP222::R1TFD(pBBRC1) could grow in 1 to 10 mM 3-CB as well as strain JMP222(pBBR1M-I) (Fig. 2), but it grew slowly. The presence of pBBRC1 provided levels of chlorocatechol 1,2-dioxygenase similar to that observed when the complete tfdCIDIEIFI gene module in plasmid pBBR1M-I was present (Table 2). This growth-proficient effect was due to the tfdR-driven expression of tfdCI and not to expression of the chloromuconate cycloisomerase activity encoded by the truncated tfdDI▿ gene (data not shown). This effect was also not due to expression (driven by a higher tfdR gene dosage) of any chromosomally encoded enzyme activity using chlorocatechols, because strain JMP222::R1TFD(pBBRPI) was not able to grow in 3-CB (Fig. 2) and did not express higher levels of chlorocatechol 1,2-dioxygenase (Table 2).
Imbalance between chlorocatechol-producing and chlorocatechol-consuming reactions in R. eutropha JMP134(pJP4) affects growth in 3-CB.
As the level of chlorocatechol 1,2-dioxygenase activity is important for growth of R. eutropha in 3-CB, inactivation of either of the two tfdC genes present in the wild-type strain should affect the ability to grow in 3-CB. To explore this possibility, tfdCI or tfdCII gene mutants were constructed in strain JMP134(pJP4) by using a procedure that inactivates genes by allelic exchange, followed by nearly precise excision of an antibiotic resistance gene (7). Inactivation of the tfdC genes was demonstrated by enzyme assays (Table 2). The derivative with the inactivated tfdCI gene completely lost the ability to grow in 3-CB (Fig. 5), whereas the mutant with the inactivated tfdCII gene exhibited a less severe effect during growth with this compound (Fig. 5). Both derivatives fully recovered the ability to grow in 3-CB when plasmid pBBRCI was present (data not shown).
FIG. 5.
Growth with different 3-CB concentrations of R. eutropha derivatives harboring xyl genes or inactivated tfdC genes. Symbols: ⧫, R. eutropha JMP134(pJP4); ◊, R. eutropha JMP134::X(pJP4); •, R. eutropha JMP134(pJP4-ΔtfdCI); ∗, R. eutropha JMP134(pJP4-ΔtfdCII); ▴, R. eutropha JMP134::X(pJP4-F3). OD600 was measured at the stationary phase. The values are means based on triplicate experiments. The deviations (not shown for clarity) were less than 5 to 10%.
Results described above indicate that a decrease in chlorocatechol-transforming activity impairs growth in 3-CB. Therefore, an increase in a chlorocatechol-producing enzyme activity should also have a negative effect on growth with this substrate. To test this possibility, the pWW0-encoded toluate dioxygenase system was introduced into derivatives of strains JMP134(pJP4) and JMP222. The toluate dioxygenase system (xylS-xylXYZL gene cluster) encodes the broad-substrate-range benzoate 1,2-dioxygenase (xylXYZ) and the 1,2-dihydro-1,2-dihydroxytoluate dehydrogenase (xylL), regulated by the transcriptional activator xylS (Fig. 1a). This enzyme system efficiently catalyzes the conversion of 3-CB to 3-CC and 4-CC (33). By using pSPM100, a pUT derivative containing the xylS-xylXYZL genes, several R. eutropha transconjugants containing chromosomal insertions of the xyl gene cluster were obtained (23). One of the derivatives, strain JMP134::X(pJP4), exhibited significantly less growth in 3-CB than the wild-type strain (Fig. 5). The possibility of a position effect of the xyl gene cluster insertion was eliminated because all of the approximately 100 transconjugants tested behaved similarly; this included some transconjugants that contained the insertion of xyl genes in the pJP4 backbone. As expected, transient accumulation of chlorocatechols was detected only in strain JMP134::X(pJP4), whereas 2-chloromuconate accumulated almost equally in the wild-type strain and the derivative containing xyl genes (Fig. 3d and e). Although adequate expression of XylXYZL activities was observed in strain JMP134::X(pJP4) (23; unpublished data), expected differences in the rates of 3-CB degradation between this strain and strain JMP134 were not detected by using cell suspensions and HPLC analysis and, therefore, are not visible in Fig. 3. Impairment of growth in 3-CB was also observed for strain JMP222::X(pBBR1M-I) and, especially, for strain JMP222::X(pBBR1M-II) (data not shown). The negative effect of introduction of an additional chlorocatechol-producing enzyme activity in strain JMP134(pJP4) should be balanced by a simultaneous increase in a chlorocatechol-consuming activity. This idea was explored by introducing the xyl gene cluster into strain JMP134 harboring pJP4-F3. This pJP4 derivative has a long duplication that includes both the tfdCIDIEIFI and tfdDIICIIEIIFII gene clusters and, therefore, has increased TfdC enzyme activity (5). Accordingly, strain JMP134::X(pJP4-F3) grew in 3-CB much better than strain JMP134::X(pJP4) grew, at levels fairly similar to those of the wild-type strain (Fig. 5).
DISCUSSION
In this study we showed that the ability of R. eutropha JMP134(pJP4) to grow in 3-CB is affected by the gene dosage of the chlorocatechol-degrading tfd gene clusters, tfdCIDIEIFI and tfdDIICIIEIIFII. Here we provide evidence that impairment of growth is primarily due to accumulation of toxic chlorocatechols when a low tfdCDEF gene dosage is present. In addition to the effect of the tfdCIDIEIFI and tfdDIICIIEIIFII modules (5, 23, 27, 37; this study), a growth-proficient effect due to an increase in the copy number of chlorocatechol degradation gene clusters has been shown for the clc element of Pseudomonas sp. strain B13 (31) and the tcbCDEF gene cluster of Pseudomonas sp. strain P51 (17). In the latter cases, no explanation was provided.
Lower gene dosages of tfd gene clusters result in lower Tfd enzyme activities. It is evident that activities encoded by the chlorocatechol degradation genes can become rate limiting. The transformation of 2-chloromuconate has been described previously as a rate-limiting step during degradation of 3-CB by the wild-type strain (28). Both TfdDI (20, 41) and, more drastically, TfdDII (22, 27, 30) are only poorly suited to transform this substrate. Moreover, cis-dienelactone transformation is rate limiting in strains containing only tfdDIICIIEIIFII (22, 27, 30). Theoretically, accumulation of a metabolite should produce lower growth rates and/or growth yields (as in the cases mentioned above) but not necessarily a lack of growth, as reported here for R. eutropha derivatives with single copies of the tfd modules. This is the case for the growth yield of strain JMP222 harboring each tfd gene module. Strain JMP222 harboring pBBR1M-II and, therefore, encoding a TfdD enzyme activity which is unable to metabolize 2-chloromuconate (22, 30) produced one-half the growth yield produced by strain JMP222 harboring pBBR1M-I, in which the level of the TfdD enzyme is low but the enzyme is active (Fig. 2). However, if the accumulated metabolite is toxic, a significant loss of the ability to grow, especially at high concentrations, can be expected. As the wild-type strain is able to grow in 3-CB while it accumulates significant amounts of 2-chloromuconate, the possible toxic effect of this metabolite, if there is any, should not be strong enough to prevent growth in 3-CB. In contrast, toxicity of chlorocatechols for bacterial cells has been reported for several organisms (1, 10, 13, 35, 36), including R. eutropha (this study). Therefore, the presence of adequate levels of chlorocatechol-consuming enzyme activities should be essential for growth in 3-CB (Fig. 6) and other chloroaromatic compounds as well. These levels should be high enough to prevent chlorocatechol accumulation and avoid a death rate higher than the growth rate. Although some activity with chlorocatechols has been reported for the chromosomally encoded catechol 1,2-dioxygenase (29), transformation of chlorocatechols into the corresponding chloromuconates is performed mainly by the chlorocatechol 1,2-dioxygenase encoded by the tfdCI and tfdCII genes. The activity of TfdCI with 3-CC or 4-CC is about two to three times higher than that of TfdCII (27, 30). In this context, it is interesting to propose that differences in TfdC activity are the main reason for impairment of growth at high 3-CB concentrations for R. eutropha JMP222 harboring tfdCIDIEIFI or tfdDIICIIEIIFII (27) (Fig. 2). Although differences in growth rates between the two types of derivatives may be explained in part by differences in the corresponding TfdD and TfdE activities (21, 22, 27, 30), derivatives possessing the more active TfdCI enzyme exhibit higher growth yields and use higher concentrations of 3-CB than derivatives with the less active TfdCII enzyme. The catabolic phenotypes of the tfdCI and tfdCII mutants agree perfectly with their differential contributions to chlorocatechol turnover (Fig. 5). The growth-proficient effects of the presence of multiple copies of the tfdCI gene (pBBRCI) in strains harboring pJP4ΔtfdCI, pJP4ΔtfdCII, or chromosomal insertions of a tfd gene cluster strongly support the idea that chlorocatechol-consuming reactions play the main role in the catabolic performance of R. eutropha derivatives with 3-CB. Recent evidence indicates that relatively small differences in TfdC activity result in significant differences in the ability to grow in 3-CB, as observed when growth of the wild-type strain JMP134(pJP4) in 3-CB was compared with growth of strain JMP134-F3 having a duplication of most tfd genes (5). Integration of a single copy of pJP4 into the chromosome of strain JMP134, which normally harbors about five copies of pJP4 per genome, did not allow growth of this derivative in 3-CB (37). This finding further supports the observation that a single copy of both tfd gene modules is not enough for growth in chlorobenzoate, as also observed for strain JMP222::R12TFD (this study). These observations clearly indicate that two- to fivefold differences in TfdC activity have a dramatic effect on growth with 3-CB. A threshold copy number higher than two was determined for the clc element to allow growth of Pseudomonas putida F1 in chlorobenzene (31), and at least two copies of the tcb gene cluster are required for growth of P. putida KT2442 in 3-CB (17). In this context, it is worth mentioning that it has been speculated that the main reason for the presence of the tfdR-tfdDIICIIEIIFII gene cluster in pJP4 is a requirement for an active regulatory gene (tfdR) for tfd genes (21, 22). However, results reported here indicate that the presence of two tfd gene modules for chlorocatechol degradation that code for the same functions in the pJP4 plasmid, a very unusual case in chloroaromatic compound degradation (38, 40), might also be due to a requirement for higher TfdC activity levels.
FIG. 6.
Imbalance between chlorocatechol-producing and chlorocatechol-consuming reactions results in accumulation of toxic metabolites and in an inability to grow in 3-CB. The relative amounts of chlorocatechols, indicated by the different sizes of molecule diagrams, and the growth phenotypes in 3-CB are shown for strains JMP134(pJP4) (a), JMP134::X(pJP4) (b), JMP222::R1TFD (c), and JMP222::R1TFD(pBBRCI) (d). The arrow thickness indicates the relative specific activity of TfdC proteins.
Limiting activities of TfdC enzymes may produce not only toxic effects due to accumulation of chlorocatechols but also less expression of tfd genes due to lower concentrations of chloromuconates, the inducer molecules for tfd genes (24). Therefore, it can be argued that differences in growth in 3-CB may be due to differential expression of tfd genes and not to toxic effects of chlorocatechols. However, the available evidence indicates that limited tfd expression is a less important factor in the accumulation of chlorocatechols than copy number changes are. Accumulation of chlorocatechols does not necessarily mean no formation of chloromuconates in strains having tfdC genes. The low or null activities of TfdD proteins with 2-chloromuconate result in accumulation of inducer levels that are up to 50% of the 3-CB input (Fig. 3), which are enough for induction, as has been shown for chlorocatechol-degrading gene systems (26; L. Guzmán and B. González, unpublished results). On the other hand, strains JMP134 and JMP134::X(pJP4) accumulate essentially the same levels of chloromuconates (Fig. 3d and e) and, therefore, presumably have similar levels of activation of tfd gene expression. However, only strain JMP134::X(pJP4) accumulates chlorocatechols because it has a higher copy number of chlorocatechol-producing genes. In addition, when a single copy of the tfdCIDIEIFI gene module, cloned under control of the Ptac/LacI heterologous regulatory system and therefore independent of chloromuconates, was introduced into strain JMP222, the cells expressed higher TfdC levels than strain JMP222::R1TFD expressed (4). These cells also produced chloromuconate at fairly normal levels but still did not grow in 3-CB (4). Similar results were obtained for JMP222 derivatives harboring the tfdCIDIEIFI gene module under the control of another heterologous regulatory system, Psal/NahR (C. Varela, R. Céspedes, and B. González, unpublished results).
If chlorocatechol-consuming conversions are important for accumulation of the intermediates, chlorocatechol-producing reactions should also play a role. R. eutropha strains possess a chromosomally encoded benzoate dioxygenase system that is fairly active with 3-CB (28, 33). In fact, when strain JMP222 (but not strain JMP134) was exposed to 3-CB, chlorocatechols rapidly accumulated (Fig. 3). This behavior has also been reported for another pJP4-free strain JMP134 derivative, strain JMP289 (25). Chlorocatechol production should be essentially the same in derivatives harboring single and multiple copies of the chlorocatechol-degrading genes, thus leading to greater accumulation of chlorocatechols if single copies of tfd genes are present (Fig. 6a and c). Such an imbalance is corrected when additional copies of a tfdC gene are provided (Fig. 6d). In this context, it was interesting to study the effect of the introduction into R. eutropha of an additional enzyme system producing chlorocatechols from 3-CB, i.e., the xylS-xylXYZL system from plasmid pWW0. The plasmid-encoded activity reported for P. putida mt-2 has an activity with 3-CB that is higher than the chromosomally encoded R. eutropha enzyme activity (33). Our results clearly show that the xyl gene-encoded toluate dioxygenase system produces an imbalance in chlorocatechol turnover, leading to accumulation of chlorocatechols and impairment of growth in the presence of 3-CB (Fig. 6b).
The results reported here have significant implications concerning the evolution of chloroaromatic compound metabolism pathways and also construction of chloroaromatic compound-degrading bacterial strains. For a long time, it has been known that many aerobic chloroaromatic compound catabolic pathways consist of diverse peripheral reactions that produce the corresponding chlorocatechols and central pathways which channel the chlorocatechols into the energy-producing intermediate metabolism (32, 34). In such a way, a microorganism can use a chloroaromatic compound as a sole carbon and energy source. Therefore, it has been assumed in several cases that acquisition of a chlorocatechol-degrading gene cluster by bacterial strains having peripheral, chlorocatechol-producing reactions may allow use of a specific chloroaromatic compound as a carbon source. There are several cases which show that this assumption is correct (32). However, there are also reports indicating that the expected growth property was not observed (8, 11, 12, 14, 16, 17, 32). In some of these cases, it has been proposed that impaired gene expression of catabolic genes in heterologous hosts is the reason for such failures (8, 11, 12, 16). Our results clearly suggest that intoxication due to unbalanced turnover of chlorocatechols should also be considered an important factor. In this context, it is worth noting that the catabolic plasmid pP51 codes for both the chlorocatechol-producing and chlorocatechol-consuming enzyme activities (39). Thus, if changes in copy number occur, the normal balance is not altered.
Acknowledgments
This work was supported by grant 8990004 from FONDECYT-Chile and by a collaborative grant from CONICYT, Chile/BMBF-IB, Germany. D.P-P. is a CONICYT Ph.D. fellow. T.L. is a MECESUP Ph.D. fellow.
REFERENCES
- 1.Acevedo, C., R. Brezny, T. W. Joyce, and B. González. 1995. Metabolism of mono and dichlorinated guaiacols by Rhodococcus ruber CA16. Curr. Microbiol. 30:63-67. [Google Scholar]
- 2.Alexeyev, M. F., I. N. Shokolenko, and T. P. Crighan. 1995. New mini-Tn5 derivatives for insertion mutagenesis and genetic engineering in Gram-negative bacteria. Can. J. Microbiol. 41:1053-1055. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl (ed.). 1992. Short protocols in molecular biology, 2nd ed. Greene Publishing Associates, New York, N.Y.
- 4.Bobadilla, R., C. Varela, R. Céspedes, and B. González. 2002. Engineering bacterial strains through the chromosomal insertion of the chlorocatechol catabolism tfdICDEF gene cluster, to improve degradation of typical bleached Kraft pulp mill effluent pollutants. Electronic J. Biotechnol. 5:162-172. [Google Scholar]
- 5.Clément, P., D. H. Pieper, and B. González. 2001. Molecular characterization of a deletion/duplication rearrangement in tfd genes from Ralstonia eutropha JMP134(pJP4) that improves growth on 3-chlorobenzoic acid but abolishes growth on 2,4-dichlorophenoxyacetic acid. Microbiology 147:2141-2148. [DOI] [PubMed] [Google Scholar]
- 6.Clément, P., D. Springael, and B. González. 2000. Deletions of mob and tra pJP4 transfer functions after mating of Ralstonia eutropha JMP134(pJP4) with Escherichia coli harboring F′::Tn10. Can. J. Microbiol. 46:485-489. [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Don, R. H., and J. M. Pemberton. 1981. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J. Bacteriol. 145:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Don, R. H., A. J. Weightman, H.-J. Knackmuss, and K. N. Timmis. 1985. Transposon mutagenesis and cloning analyses of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J. Bacteriol. 161:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz, H., W. Reineke, and E. Schmidt. 1991. Toxicity of chlorobenzene on Pseudomonas sp. strain RHO1, a chlorobenzene-degrading strain. Biodegradation 2:165-170. [DOI] [PubMed] [Google Scholar]
- 11.Ghosal, D., and I.-S. You. 1988. Gene duplication in haloaromatic degradative plasmids pJP4 and pJP2. Can. J. Microbiol. 34:709-715. [DOI] [PubMed] [Google Scholar]
- 12.Ghosal, D., I.-S. You, D. K. Chatterjee, and A. M. Chakrabarty. 1985. Genes specifying degradation of 3-chlorobenzoic acid in plasmids pAC27 and pJP4. Proc. Natl. Acad. Sci. USA 82:1638-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González, B., C. Acevedo, R. Brezny, and T. Joyce. 1993. Metabolism of chlorinated guaiacols by a guaiacol-degrading Acinetobacter junii strain. Appl. Environ. Microbiol. 59:3424-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haro, M. A., and V. de Lorenzo. 2001. Metabolic engineering of bacteria for environmental applications: construction of Pseudomonas strains for biodegradation of 2-chlorotoluene. J. Biotechnol. 85:103-113. [DOI] [PubMed] [Google Scholar]
- 15.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic selection markers for cloning and stable chromosomal insertion of foreign DNA in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinsteuber, S., R. H. Müller, and W. Babel. 2001. Expression of the 2,4-D degradative pathway of pJP4 in an alkaliphilic, moderately halophilic soda lake isolate, Halomonas sp. EF43. Extremophiles 5:375-384. [DOI] [PubMed] [Google Scholar]
- 17.Klemba, M., B. Jakobs, R. M. Wittich, and D. H. Pieper. 2000. Chromosomal integration of tcb chlorocatechol degradation pathway genes as a means of expanding the growth substrate range of bacteria to include haloaromatics. Appl. Environ. Microbiol. 66:3255-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 19.Kröckel, L., and D. Focht. 1987. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl. Environ. Microbiol. 53:2470-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhm, A. E., M. Schlömann, H.-J. Knackmuss, and D. H. Pieper. 1990. Purification and characterization of dichloromuconate cycloisomerase from Alcaligenes eutrophus JMP134. Biochem. J. 266:877-883. [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, C. M., J. H. Leveau, A. J. B. Zehnder, and J. R. van der Meer. 2000. Characterization of a second tfd gene cluster for chlorophenol and chlorocatechol metabolism on plasmid pJP4 in Ralstonia eutropha JMP134(pJP4). J. Bacteriol. 182:4165-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli, C. M., R. Schönenberger, M., Suter, A. J. B. Zehnder, and J. R. van der Meer. 2002. TfdDII, one of the two chloromuconate cycloisomerases of Ralstonia eutropha JMP134(pJP4), cannot efficiently convert 2-chloro-cis,cis-muconate to trans-dienelactone to allow growth on 3-chlorobenzoate. Arch. Microbiol. 178:13-25. [DOI] [PubMed] [Google Scholar]
- 23.Ledger, T., D. H. Pieper, D. Pérez-Pantoja, and B. González. 2002. Novel insights into interplay between xyl genes-encoded peripheral reactions and tfd genes-encoded chlorocatechol pathway for degradation of chlorobenzoates by Ralstonia eutropha JMP134. Microbiology 148:3431-3440. [DOI] [PubMed] [Google Scholar]
- 24.Leveau, J. H., F. Konig, H. Fuchslin, C. Werlen, and J. R. van der Meer. 1999. Dynamics of multigene expression during catabolic adaptation of Ralstonia eutropha JMP134(pJP4) to the herbicide 2,4-dichlorophenoxyacetate. Mol. Microbiol. 33:396-406. [DOI] [PubMed] [Google Scholar]
- 25.Leveau, J. H., and J. R. van der Meer. 1996. The tfdR gene product can successfully take over the role of the insertion element-inactivated tfdT protein as a transcriptional activator of the tfdCDEF gene cluster, which encodes chlorocatechol degradation in Ralstonia eutropha JMP134(pJP4). J. Bacteriol. 178:6824-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFall, S. M., M. R. Parsek, and A. M. Chakrabarty. 1997. 2-Chloromuconate and ClcR-mediated activation of the clcABD operon: in vitro transcriptional and DNAse I footprint analyses. J. Bacteriol. 179:3655-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Pantoja, D., L. Guzmán, M. Manzano, D. H. Pieper, and B. González. 2000. Role of tfdCIDIEIFI and tfdDIICIIEIIFII gene modules in catabolism of 3-chlorobenzoate by Ralstonia eutropha JMP134(pJP4). Appl. Environ. Microbiol. 66:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieper, D. H., H.-J. Knackmuss, and K. N. Timmis. 1993. Accumulation of 2-chloromuconate during metabolism of 3-chlorobenzoate by Alcaligenes eutrophus JMP134. Appl. Microbiol. Biotechnol. 39:563-567. [Google Scholar]
- 29.Pieper, D. H., W. Reineke, K. H. Engesser, and H.-J. Knackmuss. 1988. Metabolism of 2,4-dichlorophenoxyacetic acid, 4-chloro-2-methylphenoxyacetic acid and 2-methylphenoxyacetic acid by Alcaligenes eutrophus JMP134. Arch. Microbiol. 150:95-102. [Google Scholar]
- 30.Plumeier, I., D. Pérez-Pantoja, S. Heim, B. González, and D. H. Pieper. 2002. The importance of different tfd genes for the degradation of chloroaromatics by Ralstonia eutropha JMP134. J. Bacteriol. 184:4054-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravatn, R., S. Studer, D. Springael, A. J. B. Zehnder, and J. R. van der Meer. 1998. Chromosomal integration, tandem amplification, and deamplification in Pseudomonas putida F1 of a 105-kilobase genetic element containing the chlorocatechol degradative genes from Pseudomonas sp. strain B13. J. Bacteriol. 180:4360-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reineke, W. 1998. Development of hybrid strains for the mineralization of chloroaromatics by patchwork assembly. Annu. Rev. Microbiol. 52:287-331. [DOI] [PubMed] [Google Scholar]
- 33.Reineke, W., and H.-J. Knackmuss. 1978. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim. Biophys. Acta 542:412-433. [DOI] [PubMed] [Google Scholar]
- 34.Reineke, W., and H.-J. Knackmuss. 1988. Microbial degradation on haloaromatics. Annu. Rev. Microbiol. 42:263-287. [DOI] [PubMed] [Google Scholar]
- 35.Schweigert, N., R. W. Hunziker, B. I. Escher, and R. I. L. Eggen. 2001. Acute toxicity of (chloro-)catechols and (chloro-)catechols-copper combinations in Escherichia coli corresponds to their membrane toxicity in vitro. Environ. Toxicol. Chem. 20:239-247. [PubMed] [Google Scholar]
- 36.Schweigert, N., A. J. B. Zehnder, and R. I. L. Eggen. 2001. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 3:81-91. [DOI] [PubMed] [Google Scholar]
- 37.Trefault, N., P. Clément, M. Manzano, D. H. Pieper, and B. González. 2002. The copy number of the catabolic plasmid pJP4 affects growth of Ralstonia eutropha JMP134(pJP4) on 3-chlorobenzoate. FEMS Microbiol. Lett. 212:95-100. [DOI] [PubMed] [Google Scholar]
- 38.van der Meer, J. R. 1994. Genetic adaptation of bacteria to chlorinated aromatic compounds. FEMS Microbiol. Rev. 15:239-249. [DOI] [PubMed] [Google Scholar]
- 39.van der Meer, J. R., A. R. W. ven Neerven, E. J. de Vries, V. M. de Vos, and A. J. B. Zehnder. 1991. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J. Bacteriol. 173:6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Meer, J. R., W. M. de Vos, S. Harayama, and A. J. B. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmer, M. D., U. Schell, V. Seibert, S. Lakner, and M. Schlömann. 1999. Substrate specificities of the chloromuconate cycloisomerases from Pseudomonas sp. B13, Ralstonia eutropha JMP134 and Pseudomonas sp. P51. Appl. Microbiol. Biotechnol. 51:598-605. [DOI] [PubMed] [Google Scholar]