Abstract
We studied the pattern of the cis-trans isomerization of unsaturated fatty acids in cells of Pseudomonas putida S12 grown in a medium supplemented with oleic acid which was deuterated at both of the C atoms of its double bond. Direct evidence that isomerization does not include a transient saturation of the double bond was obtained. In addition, analysis of the amino acid sequences of the seven known Cti proteins identified them as heme-containing proteins of the cytochrome c type.
Notwithstanding the toxicity of aromatic solvents to most microorganisms, bacteria that are able to tolerate high concentrations of these compounds in their environment do exist, and most of them belong to the genus Pseudomonas (11). One of the solvent adaptation mechanisms enabling these Pseudomonas strains to grow in the presence of membrane-disrupting compounds is the isomerization of cis- to trans-unsaturated fatty acids (for reviews, see references 1 and 13). The extent of the isomerization apparently correlates with the toxicity and the concentration of such organic compounds in the membrane (7, 8).
cis-trans isomerization of unsaturated fatty acids in the solvent-tolerant bacterium Pseudomonas putida S12.
The cis-trans isomerase activity is constitutively present in Pseudomonas, does not require ATP or other cofactors like NAD(P)H or glutathione, and works in the absence of de novo synthesis of lipids (2, 5, 7, 15). Its independence from ATP is consistent with the negative free energy of the cis-to-trans isomerization (19). The enzyme has been purified from the periplasmic fraction of Pseudomonas oleovorans (17) and Pseudomonas sp. strain E3 (16) and has been isolated as a His-tagged P. putida P8 protein heterologously expressed in Escherichia coli (9). The cis-trans isomerase gene cloned and sequenced from P. putida P8 (10, 21) and P. putida DOT-T1E (12) made evident that the isomerase has an N-terminal hydrophobic signal sequence, which is cleaved off after the enzyme has been targeted to the periplasmic space. Holtwick et al. (9) provided evidence that the enzyme is a cytochrome c-type protein, as they found CXXCH, a heme-binding site (14), in the predicted Cti polypeptide. For an enzyme preparation from Pseudomonas sp. strain E3, which is presumably homologous to the cti gene product of P. putida P8, it was suggested that iron (probably Fe3+) plays a crucial role in the catalytic reaction (16).
Despite the wealth of available information, the biochemical mechanism of the solvent-induced regulation of isomerase activity, in relation to membrane homeostasis (18), still remains obscure.
(Molecular biological investigations presented in this paper are part of the research conducted by Angelika von Wallbrunn in partial fulfillment of the requirements for a Ph.D. from the University of Münster, Münster, Germany.)
Cultivation of the strain and supplementation with deuterated oleic acid.
P. putida S12 (4) was cultivated in a mineral medium (3) with 15 mM glucose as the sole carbon source. Cells were grown in 100-ml shake cultures in a horizontally shaking water bath at 30°C. Growth was monitored by measuring the turbidity at 560 nm. When indicated, mineral medium was supplemented with 0.01% (wt/vol) [9,10-2H2]oleic acid and 0.01% (vol/vol) Triton X-100 as described by Diefenbach and Keweloh (2). After 4 h of exponential growth, cells were harvested by centrifugation and resuspended in the same volume of mineral medium without oleic acid and Triton X-100; prior to the addition of 1-octanol, the cells were incubated for 30 min. For activation of the cis-trans isomerase, 1-octanol at a concentration of 0.04% (vol/vol) was added to the cells. The cultures were incubated in the presence of octanol for 2 h in a shaking water bath at 30°C. The cells were then harvested, and the lipids were extracted and transferred to fatty acid methyl esters (FAME) (6). Analysis of FAME in hexane was performed with a quadropole gas chromatography-mass spectrometry (GC-MS) system (models HP6890 and HP5973; Hewlett-Packard, Palo Alto, Calif.) equipped with a split/splitless injector. A CP-Sil 88 capillary column (inside diameter, 0.32 mm; length, 30 m; film, 0.25 μm; Chrompack, Middelburg, The Netherlands) was used for the separation of the FAME. GC conditions were as follows. The injector temperature was held at 250°C. The split flow was 1:10, and the carrier gas was He. The temperature program was performed as follows: 80°C for 1 min isotherm, an increase of 15°C per min to 140°C, and an increase of 4°C per min to 280°C. The MS conditions were electron ionization mode with an ionization energy of 70 eV. The relative amounts of the carboxylic acids were determined by using their peak areas in total ion chromatograms. The fatty acids were identified by GC-MS and coinjection with authentic reference compounds obtained from Supelco (Bellefonte, Pa.).
Isomerization of deuterated oleic acid incorporated in the plasma membrane.
P. putida uses the anaerobic pathway to synthesize unsaturated fatty acids (13) and its membranes therefore contain cis-vaccenic acid (Δ11-cis-octadecenoic acid) as a C18 unsaturated fatty acid. Thus, in order to investigate the molecular mechanism of the cis-trans isomerase, cells of P. putida S12 were supplemented with oleic acid (Δ9-cis-octadecenoic acid), which does not occur naturally in the cells. For monitoring possible changes at the double bond during isomerization from the cis to the trans configuration, oleic acid with a deuteration at the two C atoms of the double bond ([9,10-2H2]oleic acid) was used in these experiments. Incorporation into the membrane lipids of the cells was found at levels of about 15% of total fatty acid; these findings are in accordance with observations for other bacteria (2, 6).
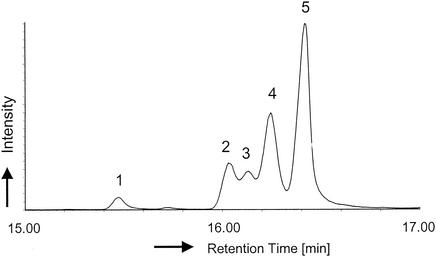
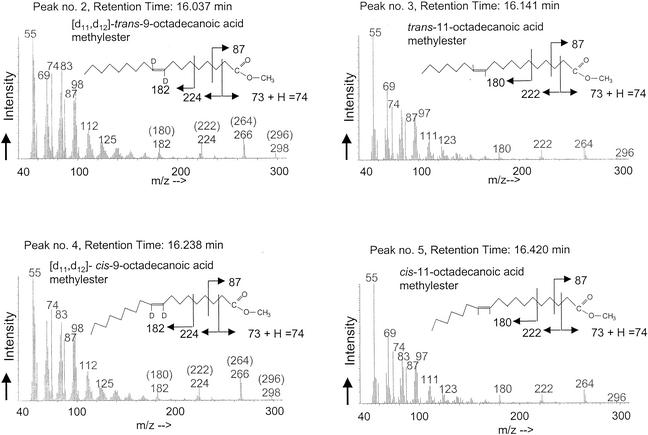
The addition of 1-octanol to cells previously grown exponentially in medium supplemented with [9,10-2H2]oleic acid for 4 h induced the formation of trans isomers of the native and supplemented fatty acids. Figures 1 and 2 show a representative GC together with the corresponding mass spectra of the extracted C18 FAME obtained from the membranes after incubation for 2 h in the presence of 0.04% (vol/vol) 1-octanol. The corresponding mass spectra of the C18:1 peaks are presented in Fig. 2. Next to the native C18 fatty acids of this bacterium, trans-vaccenic acid (Δ11-trans-octadecenoic acid methylester, peak no. 3, retention time 16.141 min, m/z 296, nondeuterated) and cis-vaccenic acid (Δ11-cis-octadecenoic acid methylester, peak no. 5, retention time 16.420 min, m/z 296, nondeuterated), the two nonnative fatty acids oleic acid (Δ9-cis-octadecenoic acid methylester, peak no. 4, retention time 16.238 min, m/z 298, doubly deuterated) and elaidic acid (Δ9-trans-octadecenoic acid methylester, peak no. 2, retention time 16.037 min, m/z 298, doubly deuterated) were detected. The occurrence of only one peak of elaidic acid with the molecular mass m/z 298 is most interesting, as it proves that the oleic acid was exclusively converted into the doubly deuterated derivative of elaidic acid. The mass fragmentogram of elaidic acid displays a completely labeled compound with two deuterium atoms and thus confirms that no deuterium was lost during the isomerization reaction (Fig. 2).
FIG. 1.
Capillary GC and peak assignment of methylated total lipid extracts in hexane, prepared from [9,10-2H2]oleic acid- and 1-octanol-treated cells of P. putida S12. The FAME were identified according to GC-MS analysis (see Fig. 2) and coinjection with the following authentic reference compounds (identified by number on the figure): 1, stearic acid (C18:0); 2, [9,10-2H2]elaidic acid (C18:1 Δ9-trans; retention time, 16.037 min); 3, trans-vaccenic acid (C18:1 Δ11-trans; retention time, 16.141 min); 4, [9,10-2H2]oleic acid (C18:1 Δ9-cis; retention time, 16.238 min); 5, cis-vaccenic acid (C18:1 Δ11-cis; retention time, 16.420 min)
FIG. 2.
Fragmentation patterns of unsaturated fatty acids, whereby mass numbers in brackets show the mass fragments of nondeuterated species (peak no. 3 and 5). The shift of 2 atomic mass units indicates that no loss of deuterium was observed during the isomerization reaction. Peak numbers and retention times for the GC are indicated (see Fig. 1).
Alignment of the amino acid sequences of seven known Cti proteins.
The alignment of the amino acid sequences of seven known Cti proteins was carried out with the ClustalW program (20) and the Malign (Heidelberg Unix Sequence Analysis Resources [HUSAR] from DKFZ Heidelberg, Heidelberg, Germany) and Bio-Edit (version 5.09) programs.
Irrespective of the taxon, a heme group of the cytochrome c type is present as a highly conserved motif and as a functional domain in all the enzymes compared (9). The covalent binding site of the heme in cytochrome c proteins is located between the heme-vinyl groups and the two cysteines of the conserved heme-binding motif CXXCH (reference 14 and data not shown).
In all Cti sequences of the six Pseudomonas strains, an N-terminal signal sequence is present, indicative of the periplasmic localization of the cis-trans isomerase. Such localization was already proven for P. oleovorans and P. putida DOT-T1E (12, 17). However, a signal peptide characterization for sec-dependent secretion is not present in the Cti protein of Vibrio cholerae.
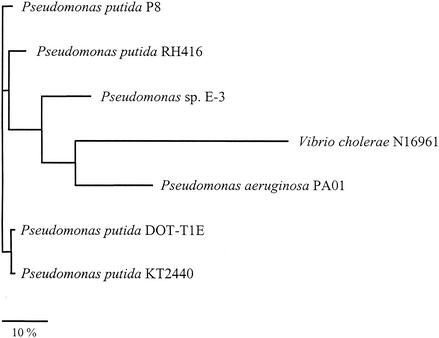
A multiple sequence alignment of the seven known Cti proteins revealed that proteins from Pseudomonas and Vibrio strains form a phylogenetic tree composed of three main branches (Fig. 3), suggesting a common ancestor of the enzyme. Interestingly, the predicted polypeptide from V. cholerae obviously does not constitute a separate group but rather emanates from the diverse groups of proteins from P. aeruginosa and Pseudomonas sp. strain E3.
FIG. 3.
Phylogenetic tree based on amino acid sequences of known fatty acid cis-trans-isomerases. Bar, 10 substitutions per 100 amino acids.
Though considerable research efforts have been carried out on the physiological, molecular biological, and biochemical mechanisms of the cis-trans isomerase of unsaturated fatty acids in Pseudomonas (and several Vibrio) strains, there are still at least two unanswered questions. One concerns the regulation of the activation of the constitutive protein which seems to be permanently present and potentially active in the periplasm. The regulation of enzyme activity may be brought about by giving the active center of the enzyme the ability to reach its substrate, the double bond, which in turn could depend on the fluidity of the membrane. Accordingly, the observed regiospecificity reflects penetration of the active site of the isomerase to a specific depth in the membrane (6). A periplasmic location of Cti supports the assumption that the enzyme is not an integral membrane protein; thus, only double bonds at a certain depth of the membrane would be within reach of the active site of the enzyme (6).
The second unanswered question concerns the molecular mechanism of the isomerization reaction. In principle, two mechanisms are conceivable. From a mechanistic point of view, cis-trans isomerization of carbon-carbon double bonds may be a coupled hydration-dehydration reaction, as was demonstrated for β-oxidation enzymes, which show 2-cis-enoyl- and 3-trans-enoyl-coenzyme A isomerase activities (9).
Since double-deuterated elaidic acid appears exclusively after cis-trans isomerization in supplementation experiments with the double-deuterated oleic acid, an intermediate saturation of the double bond, e.g., by a coupled hydration-dehydration reaction, is clearly excluded as this would cause the loss of the deuteration. Thus, our results strongly indicate a mechanism in which an enzyme-substrate complex is formed whereupon the electrophilic iron (probably Fe3+), provided by the heme domain present in the enzyme, removes an electron from the cis double bond, transferring the sp2 linking into an sp3. The double bond is then reconstituted after rotation to the trans configuration has occurred. cis-trans isomerization of unsaturated fatty acids can also be catalyzed nonenzymatically by iron, which is attached to the double bond during the reaction, suggesting a transformation of the double bond via sp2 to sp3 and a reconformation after rotation has taken place (19). These observations are in good accord with results of site-directed mutagenesis experiments carried out to destroy the heme-binding motif in Cti of P. putida P8 (9) that led to a loss of the enzyme activity and, therefore, indicated the presence of cytochrome c and heme, respectively, in the catalytic center of the enzyme. Cti activity is independent of additional factors such as ATP, NADPH, and O2 (2, 16); Cti differs in this respect from all other known heme-containing enzymes acting on fatty acids as substrates. There is, however, no need of a cofactor because no net electron power is consumed.
Acknowledgments
This work was partially supported by contracts QLK3-CT-1999-00041 and QLRT-2001-00435 of the European Commission within its Fifth Framework Programme. Angelika von Wallbrunn thanks the Cusanuswerk (Bonn, Germany) for financial support.
REFERENCES
- 1.Cronan, J. E. 2002. Phospholipid modifications in bacteria. Curr. Opin. Microbiol. 5:202-205. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach, R., and H. Keweloh. 1994. Synthesis of trans unsaturated fatty acids in Pseudomonas putida P8 by direct isomerization of the double bond of lipids. Arch. Microbiol. 162:120-125. [DOI] [PubMed] [Google Scholar]
- 3.Hartmans, S., J. P. Smits, M. J. van der Werf, F. Volkering, and J. A. M. de Bont. 1989. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 55:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmans, S., M. J. van der Werf, and J. A. M. de Bont. 1990. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl. Environ. Microbiol. 56:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heipieper, H. J., and J. A. M. de Bont. 1994. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl. Environ. Microbiol. 60:4440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heipieper, H. J., P. de Waard, P. van der Meer, J. A. Killian, S. Isken, J. A. M. de Bont, G. Eggink, and F. A. de Wolf. 2001. Regiospecific effect of 1-octanol on cis-trans isomerization of unsaturated fatty acids in the solvent-tolerant strain Pseudomonas putida S12. Appl. Microbiol. Biotechnol. 57:541-547. [DOI] [PubMed] [Google Scholar]
- 7.Heipieper, H. J., R. Diefenbach, and H. Keweloh. 1992. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 58:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. de Bont. 1994. Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12:409-415. [Google Scholar]
- 9.Holtwick, R., H. Keweloh, and F. Meinhardt. 1999. cis/trans isomerase of unsaturated fatty acids of Pseudomonas putida P8: evidence for a heme protein of the cytochrome c type. Appl. Environ. Microbiol. 65:2644-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtwick, R., F. Meinhardt, and H. Keweloh. 1997. cis-trans isomerization of unsaturated fatty acids: cloning and sequencing of the cti gene from Pseudomonas putida P8. Appl. Environ. Microbiol. 63:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue, A., and K. Horikoshi. 1989. A Pseudomonas thrives in high concentrations of toluene. Nature 338:264-266. [Google Scholar]
- 12.Junker, F., and J. L. Ramos. 1999. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keweloh, H., and H. J. Heipieper. 1996. trans unsaturated fatty acids in bacteria. Lipids 31:129-137. [DOI] [PubMed] [Google Scholar]
- 14.Mathews, F. S. 1985. The structure, function and evolution of cytochromes. Prog. Biophys. Mol. Biol. 45:1-56. [DOI] [PubMed] [Google Scholar]
- 15.Morita, N., A. Shibahara, K. Yamamoto, K. Shinkai, G. Kajimoto, and H. Okuyama. 1993. Evidence for cis-trans isomerization of a double bond in the fatty acids of the psychrophilic bacterium Vibrio sp. strain ABE-1. J. Bacteriol. 175:916-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuyama, H., A. Ueno, D. Enari, N. Morita, and T. Kusano. 1998. Purification and characterization of 9-hexadecenoic acid cis-trans isomerase from Pseudomonas sp. strain E-3. Arch. Microbiol. 169:29-35. [DOI] [PubMed] [Google Scholar]
- 17.Pedrotta, V., and B. Witholt. 1999. Isolation and characterization of the cis-trans-unsaturated fatty acid isomerase of Pseudomonas oleovorans GPo12. J. Bacteriol. 181:3256-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos, J. L., E. Duque, J. J. Rodriguez-Herva, P. Gogoy, A. Haidour, F. Reyes, and F. Fernandez-Barrero. 1997. Mechanisms for solvent tolerance in bacteria. J. Biol. Chem. 272:3887-3890. [DOI] [PubMed] [Google Scholar]
- 19.Selzer, S. 1972. cis-trans, p. 381-406. In P. D. Boyer (ed.), The enzymes, vol. 6. Academic Press, Inc., New York, N.Y.
- 20.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Wallbrunn, A., H. J. Heipieper, and F. Meinhardt. 2002. cis/trans isomerization of unsaturated fatty acids in a cardiolipin synthase knock-out mutant of P. putida P8. Appl. Microbiol. Biotechnol. 60:179-185. [DOI] [PubMed] [Google Scholar]