Abstract
We isolated menadione-resistant mutants of Xanthomonas campestris pv. phaseoli oxyR (oxyRXp). The oxyRR2Xp mutant was hyperresistant to the superoxide generators menadione and plumbagin and was moderately resistant to H2O2 and tert-butyl hydroperoxide. Analysis of enzymes involved in oxidative-stress protection in the oxyRR2Xp mutant revealed a >10-fold increase in AhpC and AhpF levels, while the levels of superoxide dismutase (SOD), catalase, and the organic hydroperoxide resistance protein (Ohr) were not significantly altered. Inactivation of ahpC in the oxyRR2Xp mutant resulted in increased sensitivity to menadione killing. Moreover, high levels of expression of cloned ahpC and ahpF in the oxyRXp mutant complemented the menadione hypersensitivity phenotype. High levels of other oxidant-scavenging enzymes such as catalase and SOD did not protect the cells from menadione toxicity. These data strongly suggest that the toxicity of superoxide generators could be mediated via organic peroxide production and that alkyl hydroperoxide reductase has an important novel function in the protection against the toxicity of these compounds in X. campestris.
Aerobic bacteria are always exposed to a variety of reactive oxygen species (ROS) that occur as by-products of normal aerobic metabolism and that arise from external sources. ROS such as superoxide anions, peroxides, and hydroxyl radicals are highly toxic to biological systems. To defend against oxidative stresses, bacteria have evolved inducible responses to protect themselves from oxidative damage. In Escherichia coli, two inducible defense regulons, oxyR and soxRS, have been characterized. OxyR and SoxS are transcription factors capable of activating other antioxidant genes in the regulon in response to sublethal concentrations of H2O2 and superoxide-generating compounds, respectively (19). Analyses of these mutants have facilitated the elucidation of resistance mechanisms to oxidants. Biochemical analysis of alkyl hydroperoxide reductase (AhpCF) reveals that it plays crucial roles in oxidative- and nitrosactive-stress protection. The enzyme consists of two subunits, a catalytic unit, AhpC, and a reductase, AhpF. AhpCF is involved in the detoxification of organic hydroperoxide, H2O2, and reactive nitrogen species (2, 3, 15, 16). In many bacteria, the expression of ahpC and ahpF is regulated by OxyR (15, 19).
Xanthomonads are gram-negative, aerobic, and plant-pathogenic bacteria. Exposure of the bacteria to low concentrations of H2O2 and the superoxide-generating compound menadione (2-methyl-1,4-naphthoquinone) highly induces expression of genes in the OxyR regulon, with menadione being 10-fold more potent. The oxyR gene from Xanthomonas campestris pv. phaseoli (oxyRXp) has been isolated and characterized (8, 10), and an oxyRXp mutant has been constructed (12). The mutant shows reduced aerobic plating efficiency and is highly sensitive to menadione and peroxide killing.
In this report, we describe the selection of suppressors of the menadione sensitivity phenotype of oxyRXp mutants. Analysis of these mutants suggested a new toxicity mechanism for menadione and a protective role of AhpCF in Xanthomonas.
Selection and physiological characterization of menadione-resistant oxyRXp mutants.
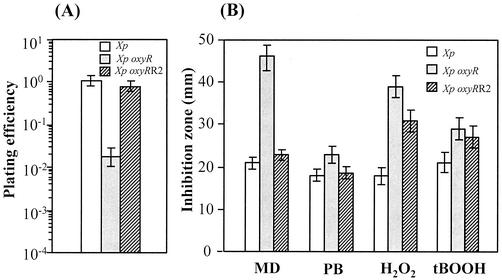
We assumed that the mechanism responsible for the menadione-hypersensitive phenotype in the oxyRXp mutant is due to an increase in superoxide production and its subsequent dismutation to H2O2. To better understand this mechanism, spontaneous mutation of the menadione hypersensitivity suppressor of the oxyRXp mutant was selected by plating stationary-phase cultures on Silva-Buddenhagen (SB) agar plates containing 200 μM menadione. These mutants were designated oxyRRXp mutants. The oxyRXp mutant shows a defect in aerobic plating efficiency that can be negated by the addition of 0.1% sodium pyruvate, a ROS-scavenging compound, to the medium (12). The plating efficiency was defined as the number of CFU obtained with cultures plated on SB medium divided by the number of CFU obtained with cultures plated on SB medium supplemented with pyruvate (0.1% [wt/vol]). This calculation was used as the basis for comparing the plating efficiencies of the oxyRRXp isolates with those of the oxyRXp mutant and the wild-type parental strain. Since all of the oxyRRXp isolates examined showed similar properties, the typical results from one of the oxyRR2Xp mutants are shown (Fig. 1A). The aerobic plating efficiency of the oxyRR2Xp mutant was about 40-fold higher than that of the oxyRXp mutant and was comparable to that of the wild-type strain. In addition, the levels of resistance of the oxyRR2Xp and oxyRXp mutants and the wild-type parental strain to superoxide generators (menadione and plumbagin) and peroxides (H2O2 and organic hydroperoxide) were determined with a growth inhibition zone assay. As expected, the menadione resistance level of the oxyRR2Xp mutant was increased to almost the same level as that of the wild-type parental strain (Fig. 1B). Since it remains controversial whether menadione toxicity is due to superoxide anion production (18), we measured the resistance levels of the three strains to the superoxide generator plumbagin. The results showed resistance patterns similar to that of menadione, except that the relative difference in plumbagin sensitivities between the oxyRXp and the oxyRR2Xp mutants and the parental wild-type strain was much less than that for menadione sensitivities (Fig. 1B). This suggested that the oxyRR2Xp mutant had acquired mechanisms to overcome superoxide anion toxicity. We also determined the resistance levels of the three strains to H2O2 and to the organic peroxide tert-butyl hydroperoxide (tBOOH). The oxyRR2Xp mutant showed a moderate increase in H2O2 resistance compared to that of the oxyRXp strain; however, the level was significantly less than that of the wild type (Fig. 1B). By contrast, both the oxyRR2Xp and oxyRXp strains had similar levels of resistance to tBOOH that were lower than that displayed by the wild type (Fig. 1B).
FIG. 1.
Characteristics of the oxyRXp, oxyRR2Xp, and parental wild-type strains. (A) Abilities of the parental wild-type strain and the oxyRXp and oxyRR2Xp mutants to form colonies on SB agar plates. Exponential-phase cells were grown on SB agar and SB agar containing pyruvate. Plating efficiency is defined as the number of CFU on SB agar divided by the number of CFU on SB agar containing 0.1% pyruvate. (B) Determination of the level of resistance to oxidant killing in the oxyRXp, oxyRR2Xp, and parental strains. The growth inhibition zones developed in response to 1.0 M menadione (MD), 0.5 M plumbagin (PB), 0.5 M H2O2, and 0.5 M tBOOH by exponential-phase cultures of the parental wild-type strain and the oxyRXp and oxyRR2Xp mutants were determined as previously described (14).
There is a strong possibility that the menadione-resistant phenotype of the oxyRR2Xp strain could be due to a mutation leading to a defect in menadione uptake. To test this, we took advantage of the fact that, in Xanthomonas, there is an OxyR-independent, menadione-inducible protection against H2O2 killing (12). We reasoned that if a mutation resulted in the blocking of menadione uptake into the cell, then the menadione-inducible protective pathway against H2O2 toxicity should be abolished. Experimental results clearly showed that a low concentration of menadione (50 μM) induced cross-protection against H2O2 (10 mM) killing in both the oxyRR2Xp and oxyRXp strains (12; data not shown), indicating that the menadione-resistant phenotype of the oxyRR2Xp mutant was not due to a defect in menadione uptake.
The oxyRR2Xp mutant had high levels of the AhpCF subunits AhpC and AhpF.
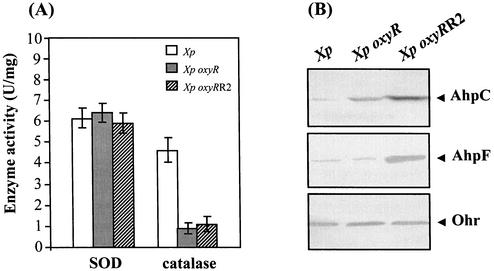
The levels of various enzymes involved in superoxide and peroxide protection were measured in the oxyRR2Xp mutant. Xanthomonas possesses a manganese (Mn) SOD capable of converting superoxide to H2O2 (17). Previous studies have shown that elevated levels of SOD make cells more resistant to superoxide generators (1). Thus, the levels of SOD in the oxyRR2Xp, oxyRXp, and the parental wild-type strains were determined spectrophotometrically. As shown in Fig. 2A, the SOD activities in all strains were not significantly different. This indicates that the menadione-resistant phenotype in the oxyRR2Xp mutant is independent of the SOD level.
FIG. 2.
Levels of antioxidant enzymes in the oxyRXp, oxyRR2Xp, and the parental wild-type strains. (A) SOD and catalase activities in bacterial lysates prepared from exponential-phase cultures were assayed as previously described (14). (B) Western immunoblot analysis of AphC, AhpF, and Ohr. Crude protein (20 μg) was loaded, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane as previously described (23). Immunodetection was performed as previously described (23).
Next, the levels of enzymes involved in peroxide metabolism, such as catalase, AhpC, and Ohr, were determined. Previously, we showed a direct correlation between catalase activity and resistance to H2O2 killing in Xanthomonas (13). Moreover, the hypersensitivity to H2O2 of the exponential-phase oxyRXp mutant is due to a decrease in the catalase level (12). Since we have shown that the catalase activities in exponentially growing cultures of the oxyRXp and oxyRR2Xp strains are similar and significantly lower than that of the wild-type parental strain (Fig. 2A), the data suggest that the increased H2O2 resistance level in the oxyRR2Xp mutant is not a result of an increase in the level of catalase activity.
There are at least two systems for the detoxification of organic hydroperoxides in Xanthomonas. One is the well-known mechanism mediated by AhpCF (15), and another is the recently reported Ohr system (11). The levels of Ohr and the AhpCF subunits AhpC and AhpF were measured by Western immunoblotting with polyclonal antibodies raised against Salmonella AhpC and AhpF (20) and Xanthomonas Ohr. The Western blot analysis showed that the levels of AhpC and AhpF in the oxyRR2Xp mutant were 10-fold higher than those of both the oxyRXp and parental wild-type strains, while the Ohrs level in all strains were not significantly different from each other (Fig. 2B). These data strongly suggest that the elevation of AhpCF levels in the oxyRR2Xp mutant was responsible for the suppression of the menadione-hypersensitive phenotype in the oxyRXp strain. Additional evidence supporting this assumption came from the observation that an ahpC1Xp mutant was hypersensitive to menadione killing (14).
AhpCF plays an important role in the menadione-resistant phenotype.
In order to evaluate the role of AhpCF in the menadione-resistant phenotype of the oxyRR2Xp mutant, an insertional inactivation of the gene encoding the catalytic subunit AhpC was performed as previously described (14). The resultant oxyRR2Xp ahpC mutant was confirmed by both Southern and Western blotting (data not shown). Furthermore, the resistance levels of the mutant to menadione, H2O2, and organic hydroperoxide were determined with an inhibition zone assay. The results showed that the increased resistance of the oxyRR2Xp mutant to menadione was eliminated by the inactivation of ahpC, since the oxyRR2Xp ahpC mutant had a resistance level to menadione that was similar to that of the oxyRXp strain (data not shown).
It is possible that the increase in the level of AhpCF is a coincidental event and not the cause of the menadione-resistant phenotype. However, the fact that inactivation of ahpC in the oxyRR2Xp mutant returns the level of menadione resistance to that of the parental wild-type strain strongly suggests that AhpCF plays a critical role in the increased menadione resistance of the oxyRR2Xp mutant. Additional experiments were then performed to confirm that the increased level of AhpCF is indeed responsible for the increased menadione resistance of the oxyRR2Xp mutant. We reasoned that if no other factors were involved, artificially high levels of AhpCF, attained through the introduction of the ahpC and ahpF expression plasmid pahpCF (8, 10), should confer increased resistance to menadione in the oxyRXp mutant. pahpCF was electroporated into the oxyRXp strain, and the resistance level of the resulting oxyRXp(pahpCF) strain to both menadione and H2O2 was determined (Table 1). The presence of the ahpC and ahpF expression plasmid in the oxyRXp(pahpCF) strain conferred increased menadione resistance to a level similar to that of the parental wild-type strain. In addition, the oxyRXp(pahpCF) strain displayed a sensitivity to H2O2 that was slightly higher than that of the parental wild-type strain (Table 1). These results prove that high levels of AhpCF protect Xanthomonas against menadione killing. The experiments were then extended to investigate whether the overexpression of other Xanthomonas enzymes involved in the detoxification of ROS, such as the monofunctional catalase encoded by katE on the plasmid pkat (22) and the MnSOD encoded by sodA on the plasmid psod (17), could confer increased menadione and H2O2 resistance in the oxyRXp strain. The plasmids were transferred into the oxyRXp strain, and the resulting strains harboring each plasmid were then checked for their sensitivities to menadione and H2O2 with the inhibition zone assay. The results in Table 1 show that high expression of SOD in the oxyRXp(psod) strain and of catalase in the oxyRXp(pkat) strain had no appreciable affect on menadione sensitivity. As expected, the oxyRXp(pkat) strain showed a significant decrease in sensitivity to H2O2.
TABLE 1.
Assays of inhibition zones against various oxidants
| Strain | Inhibition zone (mm)a
|
|
|---|---|---|
| Menadione | H2O2 | |
| Wild-type parent | 20.7 ± 1.5 | 22.5 ± 1.5 |
| oxyRXp (pUFR047) mutant | 45.8 ± 2.7 | 39.5 ± 3.2 |
| oxyRXp (psod) mutant | 43.0 ± 2.5 | 40.2 ± 2.1 |
| oxyRXp (pkat) mutant | 45.0 ± 2.5 | 19.0 ± 1.4 |
| oxyRXp (pahpCF) mutant | 23.8 ± 2.4 | 29.1 ± 2.7 |
The growth inhibition zones developed in response to menadione (1.0 M) and H2O2 (0.5 M) of exponential-phase cultures of the indicated strains were determined as previously described (15). The data represented are the means ± standard deviations of results from three independent experiments.
The data described above led to the conclusion that AhpCF has a novel role in protection against menadione in Xanthomonas. Exactly how AhpCF protects against the lethal effects of menadione is not known. Menadione is a synthetic derivative of ubiquinone, a membrane-associated compound (4). The most toxic effect of menadione may be due to lipid peroxidation of membrane fatty acids generated via superoxide radicals (21). The organic peroxides that are produced may be substrates of AhpCF. Supporting evidence for this hypothesis comes from the observation that the antioxidant vitamin E (α-tocopherol), a membrane-associated compound, could protect E. coli from menadione toxicity (5). Moreover, high expression of cytosolic MnSOD could only partially relieve the menadione hypersensitivity in the oxyRXp strain by the removal of superoxide anions generated cytosolically (17). The enzyme could not fully dismutate superoxide radicals that occurred in the membrane. In an E. coli oxyR mutant, overexpression of catalase (both KatG bifunctional catalase-peroxidase and KatE monofunctional catalase) could suppress hypersensitivity to superoxide generators (6, 7), possibly by the removal of H2O2 generated by the dismutation of superoxide anions. In addition, increased levels of AhpCF in a revertant of an E. coli oxyR mutant has been observed; however, there was no evidence linking this to increased menadione resistance (6). Our results with Xanthomonas show that a high level of monofunctional catalase does not suppress the menadione-hypersensitive phenotype of the oxyRXp strain. These results suggest that the lethal toxicity of menadione in Xanthomonas is likely a result of organic peroxide production and is not due to H2O2 production via the dismutation of superoxide anions.
Although the oxyRR2Xp mutant produced high levels of AhpCF, the level of tBOOH sensitivity was not fully restored to the wild-type level. This evidence suggests that other oxyR-dependent mechanisms are required in order to fully protect Xanthomonas from tBOOH toxicity. In addition, the moderate increase in resistance of the oxyRR2Xp mutant to H2O2 compared to that of the oxyRXp mutant might be due to the increase in AhpCF, since the purified enzyme can use both organic hydroperoxide and H2O2 as substrates (15). Alkyl hydroperoxide reductase has also been shown to protect E. coli from H2O2 toxicity (16). Thus, elevated levels of AhpCF could increase resistance to H2O2 in Xanthomonas.
In Xanthomonas, the expression of ahpC and ahpF is under the regulation of OxyR. The increased level of AhpC and AhpF in the oxyRR2Xp mutant, in the absence of a functional oxyR gene is quite surprising. In Xanthomonas, ahpC is transcribed as a monocistronic mRNA, while ahpF and oxyR are transcribed as a polycistronic mRNA. The ahpC gene has a strong promoter which is differentially regulated by OxyR. Reduced OxyR represses the promoter, while oxidized OxyR activates it (9). Since the oxyRR2Xp mutant resulted from strong selection pressure, rare mutations might have occurred in the ahpC and ahpF promoters, thereby leading to constitutive high-level expression of these genes without the requirement of OxyR. Alternatively, an increase in ahpC and ahpF mRNA stability or a decrease in AhpCF degradation could be responsible for the observed high levels of these proteins. The molecular mechanism responsible for the increase in ahpC and ahpF expression is currently under investigation.
Acknowledgments
We thank J. M. Dubbs for critical reading of the manuscript and Piyapol Munpiyamit for manuscript preparation.
The research was supported by a grant from the Thailand Research Fund (PDF/65/2543) to P.V. and a Research Team Strengthening Grant from the National Center for Genetic Engineering and Biotechnology (BIOTEC) to S.M.
REFERENCES
- 1.Balzan, R., D. R. Agius, and W. H. Bannister. 1999. Cloned prokaryotic iron superoxide dismutase protects yeast cells against oxidative stress depending on mitochondrial location. Biochem. Biophys. Res. Commun. 256:63-67. [DOI] [PubMed] [Google Scholar]
- 2.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407:211-215. [DOI] [PubMed] [Google Scholar]
- 3.Chen, L., Q. W. Xie, and C. Nathan. 1998. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol. Cell 1:795-805. [DOI] [PubMed] [Google Scholar]
- 4.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuentes, A. M., and C. F. Amabile-Cuevas. 1998. Antioxidant vitamins C and E affect the superoxide-mediated induction of the soxRS regulon of Escherichia coli. Microbiology 144:1731-1736. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg, J. T., and B. Demple. 1988. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 7:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanova, A. B., G. V. Glinsky, and A. Eisenstark. 1997. Role of rpoS regulon in resistance to oxidative stress and near-UV radiation in delta oxyR suppressor mutants of Escherichia coli. Free Radic. Biol. Med. 23:627-636. [DOI] [PubMed] [Google Scholar]
- 8.Loprasert, S., S. Atichartpongkun, W. Whangsuk, and S. Mongkolsuk. 1997. Isolation and analysis of the Xanthomonas alkyl hydroperoxide reductase gene and the peroxide sensor regulator genes ahpC and ahpF-oxyR-orfX. J. Bacteriol. 179:3944-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loprasert, S., M. Fuangthong, W. Whangsuk, S. Atichartpongkun, and S. Mongkolsuk. 2000. Molecular and physiological analysis of an OxyR-regulated ahpC promoter in Xanthomonas campestris pv. phaseoli. Mol. Microbiol. 37:1504-1514. [DOI] [PubMed] [Google Scholar]
- 10.Mongkolsuk, S., S. Loprasert, W. Whangsuk, M. Fuangthong, and S. Atichartpongkun. 1997. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 179:3950-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongkolsuk, S., W. Praituan, S. Loprasert, M. Fuangthong, and S. Chamnongpol. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongkolsuk, S., R. Sukchawalit, S. Loprasert, W. Praituan, and A. Upaichit. 1998. Construction and physiological analysis of a Xanthomonas mutant to examine the role of the oxyR gene in oxidant-induced protection against peroxide killing. J. Bacteriol. 180:3988-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mongkolsuk, S., P. Vattanaviboon, and W. Praituan. 1997. Induced adaptive and cross-protection responses against oxidative stress killing in a bacterial phytopathogen. Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 146:217-222. [Google Scholar]
- 14.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 182:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole, L. B., and H. R. Ellis. 1996. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry 35:56-64. [DOI] [PubMed] [Google Scholar]
- 16.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, S. G., T. J. G. Wilson, J. M. Dow, and M. J. Daniels. 1996. A gene for superoxide dismutase from Xanthomonas campestris pv. campestris and its expression during bacterial-plant interactions. Mol. Plant-Microbe Interact. 9:584-593. [DOI] [PubMed] [Google Scholar]
- 18.Soballe, B., and R. K. Poole. 2000. Ubiquinone limits oxidative stress in Escherichia coli. Microbiology 146:787-796. [DOI] [PubMed] [Google Scholar]
- 19.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 20.Storz, G., F. S. Jacobson, L. A. Tartaglia, R. W. Morgan, L. A. Silveira, and B. N. Ames. 1989. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J. Bacteriol. 171:2049-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzeng, W. F., J. L. Lee, and T. J. Chiou. 1995. The role of lipid peroxidation in menadione-mediated toxicity in cardiomyocytes. J. Mol. Cell Cardiol. 27:1999-2008. [DOI] [PubMed] [Google Scholar]
- 22.Vattanaviboon, P., and S. Mongkolsuk. 2000. Expression analysis and characterization of the mutant of a growth-phase- and starvation-regulated monofunctional catalase gene from Xanthomonas campestris pv. phaseoli. Gene 241:259-265. [DOI] [PubMed] [Google Scholar]
- 23.Vattanaviboon, P., T. Varaluksit, and S. Mongkolsuk. 1999. Modulation of peroxide stress response by thiol reagents and the role of redox sensor-transcription regulator, OxyR, in mediating the response in Xanthomonas. FEMS Microbiol. Lett. 176:471-476. [Google Scholar]