Abstract
The mouse SKD1 is an AAA-type ATPase homologous to the yeast Vps4p implicated in transport from endosomes to the vacuole. To elucidate a possible role of SKD1 in mammalian endocytosis, we generated a mutant SKD1, harboring a mutation (E235Q) that is equivalent to the dominant negative mutation (E233Q) in Vps4p. Overexpression of the mutant SKD1 in cultured mammalian cells caused defect in uptake of transferrin and low-density lipoprotein. This was due to loss of their receptors from the cell surface. The decrease of the surface transferrin receptor (TfR) was correlated with expression levels of the mutant protein. The mutant protein displayed a perinuclear punctate distribution in contrast to a diffuse pattern of the wild-type SKD1. TfR, the lysosomal protein lamp-1, endocytosed dextran, and epidermal growth factor but not markers for the secretory pathway were accumulated in the mutant SKD1–localized compartments. Degradation of epidermal growth factor was inhibited. Electron microscopy revealed that the compartments were exaggerated multivesicular vacuoles with numerous tubulo-vesicular extensions containing TfR and endocytosed horseradish peroxidase. The early endosome antigen EEA1 was also redistributed to these aberrant membranes. Taken together, our findings suggest that SKD1 regulates morphology of endosomes and membrane traffic through them.
INTRODUCTION
Biosynthetic and endocytic pathways join at endosomes, and multiple routes starting there are connected to different destinations such as lysosomes, the plasma membrane, and the trans-Golgi network (TGN) (reviewed in Gruenberg and Maxfield, 1995; Mellman, 1996; Mukherjee et al., 1997). Endosomes are structurally diverse but functionally and physically classified into two categories: early endosomes and late endosomes. The former can be subdivided into sorting endosomes and recycling endosomes, although the precise boundary between the two types remains uncertain (Gruenberg and Maxfield, 1995). After endocytosis, internalized macromolecules are first delivered to sorting endosomes and then sorted to each destination. Many of them, including recycling receptors such as transferrin receptor (TfR) and low-density lipoprotein (LDL) receptor, return to the cell surface via recycling endosomes (Goldstein et al., 1985). On the other hand, molecules destined for lysosomal degradation are routed to lysosomes via late endosomes. In addition, it has been recently discovered that a portion of newly synthesized TfR is transported from the TGN to the cell surface via early endosomes (Futter et al., 1995).
Great efforts have been made during the last decade in elucidating molecules involved in the endosomal functions, and a number of proteins that regulate membrane traffic via endosomes, including diverse members of the Rab-NSF-SNARE system, have been identified previously. A unique set of Rab GTPases, molecular switches controlling distinctive stages of membrane traffic, has been found to be associated with endosomes. For example, Rab5 and Rab7 are localized to early and late endosomes, respectively (Chavrier et al., 1990; Bucci et al., 1992). Growing lists of the syntaxin family and v-SNAREs, as well as the annexin family and ADP-ribosylation factors, in the endocytic pathway also provide seamarks on the traffic map. Moreover, it has been shown recently that functional linkage between the early-endosomal autoantigen EEA1, phosphatidyl-inositol-3-OH kinase, and Rab5 is required for fusion between early endosomes (Simonsen et al., 1998).
The yeast VPS4 is required for efficient transport of newly synthesized carboxy-peptidase Y from the TGN to the vacuole, the counterpart of the animal lysosome (Babst et al., 1997). The vps4 mutant strain accumulates carboxy-peptidase Y in a prevacuolar compartment, named the class E compartment, which is likely to be an aberrant endosome. The fact that VPS4 is allelic to END13 (Munn and Riezman, 1994) and GRD13 (Nothwehr et al., 1996) supports that VPS4 governs membrane traffic mediated by endosomes. Sequence analysis of VPS4 indicated that it belongs to the family of AAA (ATPase associated with a variety of cellular activities)-type ATPase. AAA-ATPases are implicated in diverse cellular functions and defined by a conserved ATPase domain (the AAA motif) that contains Walker homology sequences (Beyer, 1997; Patel and Latterich, 1998). N-Ethylmaleimide–sensitive factor (NSF), which is necessary to the most docking and fusion steps of vesicular transport, is the best-characterized AAA-ATPase (Patel and Latterich, 1998). The N-ethylmaleimide–sensitive ATPase activity of Vps4p is critical for its function on vacuolar protein transport (Babst et al., 1997). Upon analysis of Vps4p mutants that do not bind ATP or are defective in ATP hydrolysis, it has been suggested that there is a cycle of association and dissociation of a protein complex, including a Vps4p homo-oligomer with the endosomal membrane driven by ATP hydrolysis (Babst et al. 1998). Vps4p is found to be highly homologous to a mouse SKD1 protein (Babst et al., 1997), which shares 62% overall identity. SKD1 also contains the AAA motif sequence. The SKD1 gene was originally isolated from a mouse cDNA expression library in screening suppressors of the growth deficiency of a potassium transport mutant of yeast (Périer et al., 1994). The high homology predicts that SKD1 may be a functional counterpart of Vps4p in mammals. However, the function of SKD1 has remained to be investigated.
Although the outline of the endocytic pathway seems to be established and its molecular basis has become known in part, the mechanisms underlying the sorting and transport events in endosomes are still insufficiently understood. Thus, identification and characterization of novel regulatory components in the endocytic transport are of great importance for the further dissection of the mechanisms. In this study, we have assessed the possible role of SKD1 in mammalian endosomal functions by taking advantage of the fact that a mutation of Vps4p (Vps4pE233Q) results in a dominant negative phenotype (Finken-Eigen et al., 1997). By constructing a mutant SKD1E235Q protein, which is equivalent to Vps4pE233Q, and by analyzing cells transfected with SKD1E235Q, we provided evidence that SKD1 is involved in regulation of the morphology and the transport functions of endosomes.
MATERIALS AND METHODS
Materials
Enzymes and reagents used in DNA manipulations were purchased from Boehringer Mannheim (Indianapolis, IN), Promega (Madison, WI), and Takara (Otsu, Japan). Media and reagents for cell culture were from Life Technologies (Grand Island, NY). FuGENE6 transfection reagent was obtained from Boehringer Mannheim. Polyvinylidene difluoride membrane was from Millipore (Bedford, MA). An ECL system kit and Protein G-Sepharose 4 fast flow were from Amersham Pharmacia Biotech (Arlington Heights, IL). Human transferrin (Tf) conjugated with Texas Red, TRITC-conjugated dextran (Mr 7000), epidermal growth factor (EGF) biotinylated and complexed to Texas Red streptavidin (Texas Red-EGF), and a Slow Fade Light anti-fade kit were from Molecular Probes (Eugene, OR). R18-LDL was made as previously described by Ohashi et al. (1992). The cDNA encoding human TfR tagged with the myc epitope at the N-terminal end of TfR was a generous donation from Dr. Y. Takai (Osaka University, Osaka, Japan). Collagen-coated plastic coverslips (Cell tight C-1 Cell disk) were from Sumitomo Bakelite (Tokyo, Japan). Colloidal gold (Nanogold, 1.4 nm in diameter) and a silver enhancement kit (HQ silver) were obtained from Nanoprobes (Stony Brook, NY). Sulfo-NHS-biotin and immobilized streptavidin were from Pierce (Rockford, IL). [35S]Methionine/cysteine protein–labeling mix was from New England Nuclear (Boston, MA). An LSAB 2 kit containing biotinylated anti-mouse immunoglobulin G (IgG) and streptavidin conjugated with horseradish peroxidase (HRP) was from DAKO (Carpinteria, CA). All other reagents were purchased from Sigma (St. Louis, MO).
Antibodies
An anti-SKD1 antibody was prepared by immunization of rabbits with the 16-amino acid synthetic peptide corresponding to the C-terminal sequence of mouse SKD1, with one additional cysteine residue that was conjugated with bovine serum albumin (BSA) according to the method described elsewhere (Yoshimori et al., 1990). Rabbit sera against rat aldolase and against Rab7 were kind donations from Dr. E. Kominami (Juntendo University, Tokyo, Japan). Anti-Rab7 antiserum was prepared according to the method described by Chavrier et al. (1990). Generation of a mouse monoclonal antibody against human TfR (N-2) and rabbit antibodies against the endoplasmic reticulum (ER) retention signal KDEL sequence were described previously (Yoshimori et al., 1988, 1990). Rabbit polyclonal anti–green fluorescent protein (GFP) antibody was purchased from Clontech Laboratories (Palo Alto, CA). Mouse monoclonal antibodies against EEA1, TGN38, and c-myc (9E10) were from Transduction Laboratories (Lexington, KY), Affinity Bioreagents (Golden, CO), and Sigma, respectively. Rabbit serum against the rat GM130 95-kDa fragment was a generous donation from Dr. N. Nakamura (Kanazawa University, Kanazawa, Japan). Rabbit serum against rat Na,K-ATPase was a generous donation from Dr. K. Omori (Kansai Medical University, Osaka, Japan). A monoclonal antibody against human lamp-1 was a generous gift from Dr. T. August (Johns Hopkins University, Baltimore, MD). Fluorolink Cy5-labeled goat anti-rabbit and anti-mouse IgG antibodies used for indirect immunofluorescence were purchased from Amersham Pharmacia Biotech. Peroxidase-conjugated goat anti-rabbit and anti-mouse IgG antibodies used for immunoblot were from Jackson ImmunoResearch Laboratories (West Grove, PA). R-Phycoerythrin (PE)-conjugated F(ab′)2 fragment goat anti-mouse IgG used in flow cytometry was from Immunotech (Eugene, OR).
Plasmid Construction
The SKD1 cDNA was amplified by reverse transcription-PCR from mouse kidney RNA with primers, SKD1-5s (5′-TCGGGAATTCACCATGGCGTCCACGAACAC-3′) and SKD1-3rc primer (5′-AAACGAATTCTTGCTGTCTTTGTGTTAGCC-3′). It was subcloned into the EcoRI site of pcDNA3 (Invitrogen, San Diego, CA) to produce pSKD1. pSKD1E235Q, which has a point mutation at amino acid 235 in the Walker B motif (from glutamic acid to glutamine), was created by PCR-based site-directed mutagenesis, and the mutation was confirmed by DNA sequencing. The SKD1 and SKD1E235Q cDNAs were also inserted to the BglII–SalI site of pEGFP-C1, a GFP-fusion protein expression vector (Clontech Laboratories) to obtain pGFP-SKD1 and pGFP-SKD1E235Q, respectively. These encode SKD1 proteins fused to the C-terminal end of GFP.
Cell Culture, Transfection, and Immunoblot Analysis
HeLa cells, human epidermoid carcinoma A-431 cells, normal rat kidney (NRK) cells, rat hepatoma H-4-II-E cells, and human embryonic kidney HEK293 cells were grown in DMEM containing 10% fetal calf serum (FCS). Chinese hamster ovary (CHO) cells were grown in Ham's F12 medium containing 5% FCS. All media were supplemented with 5 U/ml penicillin and 50 μg/ml streptomycin.
For transfection, cells were seeded on 35-mm dishes (in microscopic studies, coverslips had been placed previously in the bottom of these dishes), and the next day, the cells were transfected with a mixture of 1 μg of plasmid DNA and 3 μl of FuGENE6. At 18 h after transfection, cells were used for each experiment. In the case of NRK cells, the plasmid was microinjected.
For immunoblot, proteins were resolved by SDS-PAGE (Laemmli, 1970) and transferred to a polyvinylidene difluoride membrane, and the membrane was incubated with the primary antibodies and then with the secondary antibodies, essentially as described previously (Takemoto et al., 1992). Labeling was detected by the ECL system.
Assays for Endocytosis
For a Tf uptake assay, the transfected HeLa cells were incubated with DMEM for 1 h at 37°C and then with 100 nM Texas Red–conjugated Tf in 0.1% BSA/DMEM for 15 min at 37°C. R18-LDL binding (4°C for 1 h) and uptake (37°C for 10 min) were performed according to the method described previously (Ohashi et al., 1992). For dextran uptake, the transfected HeLa cells were incubated with 1 mg/ml TRITC-dextran in 10% FCS/DMEM for 8 h at 37°C. For an EGF uptake and degradation assay, the transfected HeLa or A-431 cells were incubated with DMEM medium for 1 h at 37°C and then with 3.3 μg/ml (for HeLa cells) or 0.1 μg/ml (for A-431 cells) Texas Red-EGF in 0.1% BSA/DMEM for 1 h at 4°C. The cells were washed and incubated for 30 min or 3 h at 37°C. After these treatments, cells were washed, fixed, and observed by immunofluorescence microscopy as described bellow.
For a HRP uptake experiment, HEK293 cells were incubated with 10 mg/ml HRP (type II; Sigma) dissolved in 10% FCS/DMEM at 37°C for 1 h. The cells were processed for electron microscopy as described below.
Quantitative Analysis of Cell Surface myc-Tagged TfR
The myc-tagged TfR cDNA, 0.5 μg, was transfected to the HeLa cells together with 0.5 μg of pGFP-SKD1, pGFP-SKD1E235Q, or only the vector as described above. The transfected cells were pulse labeled with 0.5 mCi of [35S]methionine/cysteine in 0.5 ml of methionine/cysteine-free DMEM for 15 min at 37°C and chased for 3 h at 37 or 4°C for negative control. Then the cells were surface-biotinylated and lysed as described previously (Yoshimori et al., 1996). The expressed myc-tagged TfR was immunoprecipitated using 0.3 μl of an anti-myc antibody 9E10 and 5 μl of Protein G-Sepharose as described previously (Brewer and Roth, 1991). The precipitated myc-tagged TfRs were eluted by boiling for 5 min with 100 μl of 2% SDS in phosphate-buffered saline (PBS). After centrifugation, a portion of supernatant was removed as total myc-tagged TfR, and the rest was diluted with 1.3 ml of 50 mM Tris-HCl, pH 7.5, 0.25 M NaCl, 5 mM EDTA, 1% NP-40, 1% BSA, and 0.5 mM phenylmethylsulfonyl fluoride. Then, biotinylated proteins in this (i.e., the surface myc-tagged TfR) were precipitated with streptavidin-agarose as described previously (Yoshimori et al., 1996). Both the total myc-tagged TfR and the surface myc-tagged TfR were analyzed by SDS-PAGE on a 7.5% acrylamide gel. Radioactivity in the individual bands was determined using a bioimage analyzer (BAS4000; Fuji Film, Tokyo, Japan), and the amount of the surface myc-tagged TfR was divided by the total amount for normalization.
Immunofluorescence Microscopy and Flow Cytometry
The transfected cells were washed with ice-cold PBS three times and fixed in 3% paraformaldehyde in PBS for 15 min. If indicated, prefix permeabilization with 50 μg/ml digitonin in PBS was carried out for 5 min. After fixation, the cells were permeabilized with 50 μg/ml digitonin in PBS for 5 min, quenched with 50 mM NH4Cl in PBS for 10 min, and then blocked with 0.1% gelatin in PBS (gelatin-PBS) for 5 min. The cells were incubated with first antibodies diluted in gelatin-PBS for 1 h. Concentration of the each first antibody was as follows: anti-TfR antibody, 4.7 μg/ml; anti-EEA1 antibody, 5 μg/ml; anti-Rab7 antibody, 10× dilution of a partially purified stock; anti-GM130 serum, 50× dilution; anti-KDEL antibody, 4.1 μg/ml; anti-Na,K-ATPase serum, 10× dilution. After washing the cells three times with gelatin-PBS, they were incubated with the second antibodies diluted in gelatin-PBS for 1 h and then washed three times with gelatin-PBS. Finally, they were mounted with Slow Fade and observed under a fluorescence laser scanning microscope (LSM510) with a plan-APOCHROMAT lens (63×) (Carl Zeiss, Thornwood, NY). All processes were done at room temperature. For cell surface labeling with the anti-TfR antibody, the transfected HeLa cells were incubated with the antibody at 7 μg/ml for 1 h at 4°C before fixation. Then, the cells were processed in the same manner as above except for no first antibody incubation.
For flow cytometric analysis, the transfected HeLa cells were incubated at 4°C with the anti-TfR antibody and with PE-conjugated anti-mouse IgG antibodies, each 1 h successively without fixation and then collected by treatment with 2 mM EDTA in PBS at 4°C for 1 h. The cells were examined using an EPICS Elite flow cytometer (Coulter, Hialeah, FL). Expression of the GFP-fused proteins and surface binding of the anti-TfR antibody were monitored by fluorescence measurement at 525 and 575 nm, respectively.
Electron Microscopy
Conventional Electron Microscopy.
HEK293 cells were cultured on collagen-coated plastic coverslips. After transfection with SKD1E235Q as described above, they were fixed in 2% glutaraldehyde in 0.1 M Na-phosphate buffer (PB), pH 7.4, for 1 h. The cells were washed in the same buffer three times and were postfixed in 1% OsO4 in 0.1 M cacodylate buffer, pH 7.4, for 1 h. After washing in distilled water, cells were incubated with 50% ethanol for 10 min and block stained with 2% uranyl acetate in 70% ethanol for 2 h. The cells were further dehydrated with a graded series of ethanol and were embedded in epoxy resin. Ultrathin sections were doubly stained with uranyl acetate and lead citrate and observed under an H7000 electron microscope (Hitachi, Tokyo, Japan).
To visualize the internalized HRP in HEK293 cells (see Assays for Endocytosis), the cells were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 30 min, washed three times in the same buffer, and incubated in diaminobenzidine (DAB) and H2O2 as described by Marsh et al. (1986). The cells were postfixed in 1% OsO4, washed in distilled water, incubated with 50% ethanol for 10 min, and stained with 2% uranyl acetate in 70% ethanol for 2 h. The cells were further dehydrated with a graded series of ethanol and were embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate.
Immunoelectron Microscopy.
The preembedding silver enhancement immunogold method was performed as described by Mandai et al. (1997) with a slight modification. HEK293 cells cultured on collagen-coated plastic coverslips and transfected with GFP-SKD1E235Q were fixed in 4% paraformaldehyde and 0.1% glutaraldehyde (for GFP-SKD1E235Q and TfR) or in 4% paraformaldehyde (for EEA1) in PB for 30 min. The cells were washed in the buffer three times and were incubated in PB containing 0.25% saponin and 5% BSA for 30 min and then for 30 min for blocking in PB containing 0.005% saponin, 10% BSA, 10% normal goat serum, and 0.1% cold water fish skin gelatin (Hartmann et al., 1997). Cells were then treated with rabbit IgG against GFP (diluted ×500), the mouse monoclonal antibody against TfR (7.0 μg/ml), or a mouse monoclonal antibody against EEA1 (2.5 μg/ml) in the blocking solution, overnight. Then, the cells were washed in PB containing 0.005% saponin for 10 min six times and incubated with goat anti-rabbit IgG or anti-mouse IgG that was conjugated to colloidal gold (1.4 nm diameter) in the blocking solution for 2 h. Cells were washed with PB for 10 min six times and fixed with 1% glutaraldehyde in PB for 10 min. After washing, the gold labeling was intensified by using a silver enhancement kit for 7.5 min at 20°C in the dark. After washing in distilled water, cells were postfixed in 0.5% OsO4 for 90 min at 4°C, washed in distilled water, incubated with 50% ethanol for 10 min, and stained with 2% uranyl acetate in 70% ethanol for 2 h. The cells were further dehydrated with a graded series of ethanol and were embedded in epoxy resin. Ultrathin sections were doubly stained with uranyl acetate and lead citrate. For the endogenous SKD1, H-4-II-E cells were processed in the same way and stained with antibodies against SKD1.
For double-staining experiments, the GFP-SKD1E235Q-transfected HEK293 cells were treated with the blocking solution containing rabbit IgG against GFP and the mouse monoclonal antibody against TfR overnight. After washing in PB containing 0.005% saponin, the cells were incubated with goat anti-rabbit IgG labeled with colloidal gold (1.4 nm diameter) and biotinylated anti-mouse IgG in the blocking solution for 2 h. After washing in PB containing 0.005% saponin, the cells were treated with streptavidin conjugated with HRP for 30 min. The cells were washed with PB for 10 min six times and fixed with 1% glutaraldehyde in PB for 10 min. The gold labeling was intensified by using the silver enhancement method. After washing, HRP labeling was visualized by DAB reaction. Cells were postfixed in 0.5% OsO4 for 90 min, stained with 2% uranyl acetate in 70% ethanol for 2 h, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate.
Subcellular Fractionation
Fractionation of cell homogenates was carried out essentially as described by Ishihara et al. (1995). In brief, HeLa cells transfected with GFP-SKD1 or GFP-SKD1E235Q, or untransfected cells were washed, harvested, and homogenized with a sonicator (Ohtake Works, Tokyo, Japan) four times, for 5 s each in PBS containing 1 mM phenylmethylsulfonyl fluoride. After low-speed centrifugation of the homogenate, the supernatant was centrifuged for 30 min at 100,000 × g (a S45A rotor; Hitachi) at 4°C. The pellet was sonicated and centrifuged once more. Then, the resulting pellet (total membrane) was resuspended in the homogenization buffer. The supernatant (cytosol) in the first centrifugation was supplemented with concentrated lysis buffer for SDS-PAGE. The total membrane was also supplemented with the concentrated lysis buffer, and its volume was adjusted equal to the volume of the supernatant.
RESULTS
Construction of a Mutant SKD1
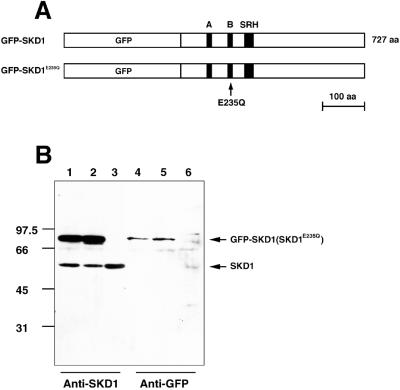
To define the possible function of SKD1 in the endocytic route, we constructed the mutant SKD1E235Q. The E235Q point mutation is situated within a Walker-type B motif in the AAA module conserved among AAA-ATPases (see Figure 1A) and is equivalent to the dominant mutation in Vps4p (E233Q), which causes a defect in vacuolar protein sorting (Finken-Eigen et al., 1997). In addition, the analogous mutant of NSF (E329Q) was shown to exert a dominant negative effect on the intra-Golgi transport in vitro (Whiteheart et al., 1994). Both Vps4pE233Q and NSF E329Q are inactive in ATP hydrolysis. We expected that overexpression of SKD1E235Q in cultured cells exerts a dominant negative effect on the cellular function(s) in which SKD1 is involved. To visualize the expressed wild-type and mutant SKD1, their cDNAs were fused to the C-terminal end of GFP (Figure 1A).
Figure 1.
Construction and expression of GFP-fused SKD1 and SKD1E235Q. (A) Schematic constructs of proteins used in this study. GFP tagged at N termini of SKD1 and SKD1E235Q, and Walker-type A, Walker-type B, and SRH motifs in the AAA domain are indicated. To construct SKD1E235Q, a single point mutation was introduced into SKD1, which leads to amino acid exchange from glutamic acid to glutamine at position 235 in Walker B motif (arrow). (B) HeLa cells were transfected with GFP-SKD1 (lanes 1 and 4), GFP-SKD1E235Q (lanes 2 and 5), or only the vector (lanes 3 and 6). Their extracts were analyzed by immunoblot using the antibody against SKD1 (lanes 1–3) or antibodies against GFP (lanes 4–6).
Using the antibody against the synthetic peptide corresponding to the carboxyl terminus of SKD1, we detected the endogenous SKD1 as a single band in the immunoblot of lysates of various cell lines, including HeLa cells, CHO cells, and HEK293 cells. When pGFP-SKD1 or pGFP-SKD1E235Q was transfected to HeLa cells, transient expression after 18 h was confirmed for each fusion protein by both antibodies to SKD1 and to GFP in the immunoblot (Figure 1B). Approximately equal amounts of GFP-SKD1 and GFP-SKD1E235Q, 2–3 times as much as that of the endogenous SKD1, were synthesized in transfected cells. We estimate that the fusion proteins were overexpressed in an individual transfected cell 5–30 times over the endogenous level with 10–40% transfection efficiency.
Overexpression of SKD1E235Q Causes Decrease of the Surface TfR
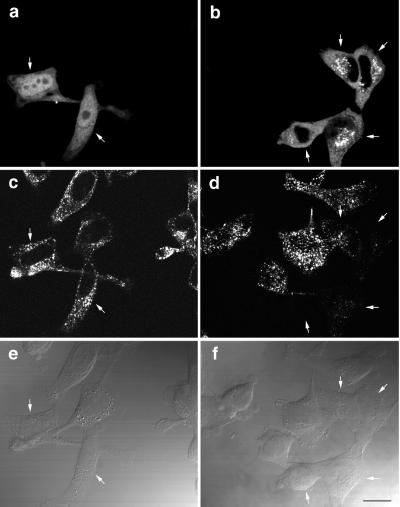
To assess the possibility that overexpression of SKD1E235Q has a dominant negative effect on endocytosis, HeLa cells transfected with GFP-SKD1 or GFP-SKD1E235Q were examined for their capacity to internalize Texas Red–conjugated Tf. After transfection, the cells were allowed to internalize Texas Red-Tf for 15 min at 37°C. The internalized Tf was visible as fine punctate fluorescence in the cells expressing GFP-SKD1 as well as in untransfected cells, as shown in Figure 2c. In contrast, the cells expressing GFP-SKD1E235Q apparently failed to internalize Texas Red-Tf (Figure 2d, arrows). The mutant SKD1-expressing cells showed not only defect in Tf internalization but also characteristic distribution of the mutant protein. Most of the endogenous SKD1 was diffusely distributed over the cytoplasm (see below), as is its yeast homologue, Vps4p (Babst et al., 1997). GFP-SKD1 expressed in HeLa cells also was spread over the cytoplasm and in the nucleoplasm (Figure 2a). (The reason for the nucleoplasmic staining is unknown. It was not, however, specific to SKD1, because it was seen even when GFP alone was expressed.) On the other hand, GFP-SKD1E235Q displayed a prominent punctate pattern concentrated in the perinuclear region in addition to the diffuse pattern (Figure 2b). Similar distribution of GFP-SKD1E235Q was observed in all other cell lines examined.
Figure 2.
HeLa cells overexpressing GFP-SKD1E235Q fail to internalize Tf. HeLa cells transfected with GFP-SKD1 (a, c, and e) or GFP-SKD1E235Q (b, d, and f) were incubated in the presence of 100 nM Texas Red-Tf at 37°C for 15 min. The cells were then fixed for fluorescence confocal microscopy. (a and b) Fluorescence micrographs showing the GFP-fused protein labeling; (c and d) fluorescence micrographs showing Texas Red-Tf labeling; (e and f) differential interference contrast micrographs. Each column (a, c, and e or b, d, and f) represents the same field. Cells expressing the GFP-fused proteins are indicated by arrows. Bar, 20 μm.
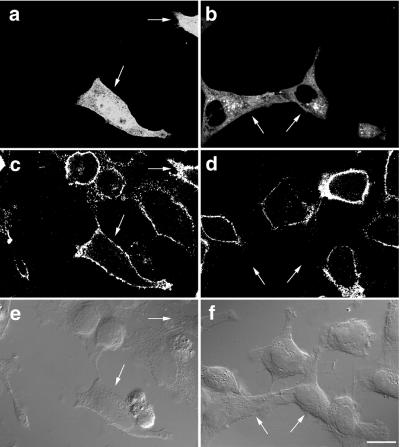
The effect on endocytosis was not limited to the Tf uptake in HeLa cells. Internalization of R18-labeled LDL for 10 min at 37°C in CHO cells also was impaired by overexpression of GFP-SKD1E235Q (Figure 3c, arrows). Again, GFP-SKD1 did not show any effect (our unpublished data).
Figure 3.
CHO cells overexpressing GFP-SKD1E235Q neither bind nor internalize LDL. CHO cells transfected with GFP-SKD1E235Q were cultured in 5% LPDS/F12 medium. After 18 h, the cells were incubated in the presence of 10 μg/ml R18-LDL in the same medium at 37°C for 10 min (a and c) or at 4°C for 1 h (b and d). Then the cells were fixed for fluorescence confocal microscopy. (a and b) Fluorescence micrographs showing GFP-SKD1E235Q labeling; (c and d) fluorescence micrographs showing R18-LDL labeling. The pairs of a and c and b and d represent the same field. The cells expressing GFP-SKD1E235Q are indicated by arrows. Bar, 20 μm.
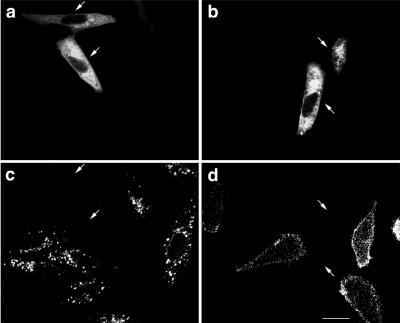
The loss of the ligand internalization led us to determine whether endocytosis of their receptors is blocked or they are absent on the cell surface. We first examined cell surface binding of LDL for 1 h at 4°C. HeLa cells expressing GFP-SKD1E235Q did not show LDL binding to their surfaces (Figure 3d, arrows). This suggests that its receptor disappeared from the cell surface. We next examined surface expression of TfR. HeLa cells transfected with GFP-SKD1E235Q were incubated for 1 h at 4°C in the presence of the monoclonal anti-TfR antibody. After washing out the excess unbound antibody, the cells were fixed and processed for immunofluorescence confocal microscopy. The surface expression of TfR was clearly observed in the untransfected cells as well as in the cells transfected with GFP-SKD1 (Figure 4c). Strikingly, no surface TfR staining was seen in the cells transfected with GFP-SKD1E235Q (Figure 4d, arrows). From these observations, we suppose that the impairment of the ligand internalization is due to reduction of the surface receptors. Expression of GFP alone did not exert any effect either on the internalization of Tf or LDL, or on the surface binding of LDL or the anti-TfR antibody (our unpublished data).
Figure 4.
TfR disappears from the surface of HeLa cells overexpressing GFP-SKD1E235Q. HeLa cells transfected with GFP-SKD1 or GFP-SKD1E235Q were incubated in the presence of the antibody against TfR at 4°C for 1 h. The cells were then fixed and incubated with a Cy5-conjugated second antibody for immunofluorescence confocal microscopy. (a and b) Fluorescence micrographs showing the GFP-fused protein labeling; (c and d) fluorescence micrographs showing the surface TfR staining; (e and f) differential interference contrast micrographs. Each column (a, c, and e or b, d, and f) represents the same field. Cells expressing the GFP-fused proteins are indicated by arrows. Bar, 20 μm.
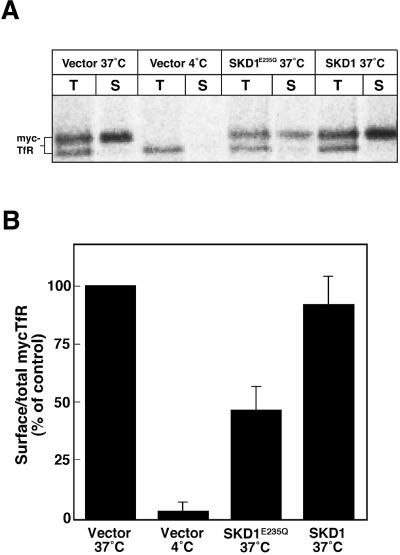
Furthermore, we quantified biochemically the fraction of TfR on the surface by a pulse–chase experiment. Under the given transfection efficiency (10–40%), the effect of the mutant SKD1 would not be detectable if the bulk TfR present in the all cells were measured. Therefore, we took advantage of the cotransfection of the mutant SKD1 with myc-tagged TfR, because the ectopic TfR was expected to be expressed together with the mutant SKD1 in the same population of the cell. By fluorescence microscopy, we confirmed that transfected cells coexpressed both ectopic proteins. After cotransfection, the cells were labeled with [35S]methionine/cysteine for 15 min and then chased for 3 h. The labeled myc-tagged TfR was detected by immunoprecipitation using an anti-myc antibody, and the amount of it on the cell surface was determined by surface biotinylation. Figure 5A shows a representative of results in three experiments done with duplicate dishes. Radioactivity in the individual bands was determined, and the ratio of the surface myc-tagged TfR to the total myc-tagged receptor was calculated (Figure 5B). As a result, we found that the ratio was significantly reduced in the cells cotransfected with GFP-SKD1E235Q, which was ∼50% of that in the control cells. GFP-SKD1 did not affect the ratio of the surface myc-tagged TfR. In the mutant-transfected cells, the myc-tagged TfR seemed to be processed from the high-mannose–type oligosaccharides form (the ER form; the lower bands seen in Figure 5A) to the complex type Golgi form (the upper bands) at the similar ratio to that in the control cells. Indeed, time course and level of the conversion from the ER form to the Golgi form in these cells were hardly distinct from those in the control cells (our unpublished data). Thus, we concluded that the mutant SKD1 affects neither glycosylation of TfR nor its transport through the Golgi complex. In addition, overexpression of the mutant SKD1 did not affect a stability of the pulse-labeled TfR during the chase period. A faint amount of the lower band was detected at the surface of the control cells and the mutant-transfected cells (Figure 5A). Although the reason for this is not clear, a small fraction of the ER form could be transported to the cell surface, consistent with an earlier study showing that some of the unglycosylated TfR is transported to the cell surface (Omary and Trowbridge, 1981).
Figure 5.
Overexpression of GFP-SKD1E235Q reduces the ratio of the cotransfected ectopic TfR on the cell surface to the total. HeLa cells were transfected with only the vector, GFP-SKD1E235Q, or GFP-SKD1, together with myc-tagged TfR. The cells were labeled with [35S]methionine/cysteine for 15 min, chased at 37°C or 4°C for 3 h, and surface biotinylated as described in MATERIALS AND METHODS. (A) Immunoprecipitated myc-tagged TfR (T) and the myc-tagged TfR bound to streptavidin-agarose (S) analyzed by SDS-PAGE. Total and surface myc-tagged TfR were quantified with a bioimage analyzer, and the surface expression was normalized by total amounts (B). The values are expressed relative to the surface myc-TfR in the cell cotransfected with only the vector and incubated at 37°C (Vector 37°C: 100%). Each column represents the mean ± SEM of three experiments done with duplicate dishes.
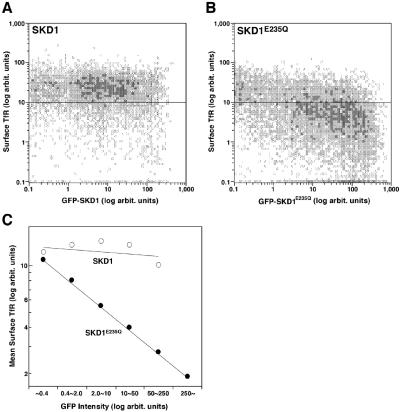
The above result strongly argued that overexpression of GFP-SKD1E235Q alters distribution of TfR. We could not, however, exclude a possibility that the distribution of the overexpressed ectopic TfR was affected by its overall expression level that varied among the samples, that is, its higher expression level could result in its longer retention on the cell surface. It was difficult to control or normalize the expression levels in the individual cells in the transient expression experiment. Therefore, to prove that the decrease of TfR depends on the expression of GFP-SKD1E235Q in the individual cells, we performed a flow cytometric analysis. To eliminate the possible effect of overexpression of TfR, we measured the endogenous TfR on the cell surface. HeLa cells transfected with GFP-SKD1E235Q or GFP-SKD1 were incubated for 1 h at 4°C with the anti-TfR antibody and then with PE-conjugated second antibodies. Fluorescence intensities of GFP and of PE in individual cells were measured by flow cytometry. Although the surface TfR level was almost constant regardless of the extent of the GFP-SKD1 expression in the cells transfected with GFP-SKD1 (Figure 6A), we found in the mutant-transfected cells a tendency that the higher the GFP-SKD1E235Q was expressed, the less TfR existed on the cell surface (Figure 6B). The linear correlation between the expression level of the mutant protein and the inhibitory effect is clear in a logarithmic plot of mean surface TfR against each range of the mutant SKD1 expression level (Figure 6C). The cell population exhibiting the highest range of GFP-SKD1E235Q fluorescence (>250 U) showed ∼85% mean decrease of the surface TfR compared with the GFP-SKD1–transfected cells or untransfected cells. This result clearly shows a correlation between expression levels of GFP-SKD1E235Q and the reduction of TfR on the cell surface, corroborating the morphological analysis of TfR and Tf uptake.
Figure 6.
Flow cytometric analysis correlates loss of the surface TfR and GFP-SKD1E235Q expression. HeLa cells transfected with GFP-SKD1 (A) or GFP-SKD1E235Q (B) were processed to stain the surface TfR as described in Figure 4, except that a PE-conjugated second antibody was used. Twenty thousand cells were analyzed for each transfection. Mean amounts of the surface TfR are plotted in C for each subpopulation of the cells expressing the GFP-fused proteins at the indicated range of intensity. Shown is a representative experiment repeated twice.
TfR and Other Endocytic Cargoes Are Accumulated in the SKD1E235Q-positive Compartments
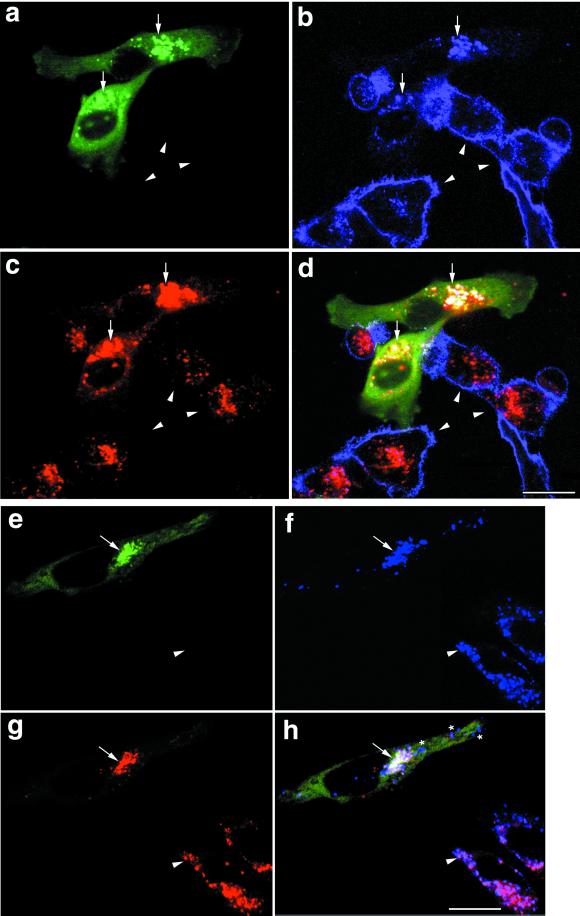
The preceding results suggested that overexpression of GFP-SKD1E235Q may perturb intracellular transport of TfR. This explanation gives rise to a possibility that TfR would be accumulated in the particular compartment where the mutant SKD1 inhibits traffic. To address this possibility, we next examined distribution of the receptor in the interior of the GFP-SKD1E235Q–transfected cells. HeLa cells were permeabilized after fixation to make the anti-TfR antibody accessible to the antigen both outside and inside of the cells. As indicated by arrowheads in Figure 7b, in the untransfected HeLa cells, a large fraction of TfR existed on the plasma membrane, and the rest was on punctate structures inside, representing early endosomes. A distinct distribution of TfR was, on the other hand, observed in the transfected cells (Figure 7b, arrows). TfR was clearly absent from the cell surface again and was accumulated inside the cells. The TfR inside was notably colocalized with GFP-SKD1E235Q (Figure 7a, arrows).
Figure 7.
TfR, the endocytosed dextran, and lamp-1 are accumulated in the E235Q compartments. HeLa cells transfected with GFP-SKD1E235Q were incubated in the presence of 1 mg/ml TRITC-dextran at 37°C for 8 h. The cells were then fixed, permeabilized, and subjected to immunofluorescence confocal microscopy using a Cy5-conjugated second antibody. Each set of a–d and e–h shows fluorescence micrographs of the same field. GFP-SKD1E235Q labeling (a and e), TfR staining (b), lamp-1 staining (f), TRITC-dextran labeling (c and g), and a merged image (d and h) are shown. Arrowsheads, arrows, and asterisks indicate the untransfected cells, the E235Q compartments in the GFP-SKD1E235Q-transfected cells, and lamp-1, whose distribution is distinct from the E235Q compartments, respectively. Bar, 20 μm.
Dextran can be internalized into cells by fluid-phase endocytosis and delivered to lysosomes via endosomes (Ohkuma and Poole, 1978; Dunn et al., 1994). Dextran is finally accumulated in lysosomes because it is resistant to lysosomal digestion. To determine whether the mutant-transfected HeLa cells are still able to internalize rhodamine-conjugated dextran and wether the compartment accumulating GFP-SKD1E235Q and TfR is accessible to the endocytic marker, the cells were loaded with rhodamine-dextran for 8 h at 37°C. In cells without transfection, we confirmed distribution of rhodamine-dextran in punctate lysosomes that could be labeled with antibodies against a lysosomal membrane marker, lamp-1 (Figure 7, e–g, arrowheads). The transfected cells (indicated by arrows) could internalize rhodamine dextran (Figure 7c), and the internalized dextran mostly overlapped with the punctate structures in which both the mutant SKD1 and TfR were located (Figure 7d). We, therefore, reason that the overexpression of GFP-SKD1E235Q does not inhibit endocytosis of dextran but causes its accumulation in the GFP-SKD1E235Q/TfR–positive compartments (E235Q compartments). Moreover, majority of lamp-1 was also colocalized in these compartments (Figure 7, e–h, arrows), although some were distributed in granules distinct from the E235Q compartments (Figure 7h, stars).
Accumulation of the cargoes transported via endosomes, such as TfR, dextran, and lamp-1 in the E235Q compartments, suggests that overexpression of the mutant SKD1 may affect some steps of the endosomal membrane traffic. To provide functional data supporting this, we monitored endocytosis of EGF, which is bound to its receptor on the cell surface, then internalized into endosomes, and delivered into lysosomes to be degraded (Yoshimori et al., 1991). HeLa or A-431 cells transfected with the mutant SKD1 were incubated with Texas Red-EGF at 4°C for 1 h, washed, and then allowed to uptake EGF at 37°C for 30 min or 3 h. In both cell lines, the internalized EGF was visible as bright spots at 30 min in both the untransfected cells and the cells expressing the mutant SKD1 (Figure 8, a–d and i–l), confirming that the mutant expression does not affect the surface distribution and endocytosis of the nonrecycling receptor such as EGF receptor. The prolonged incubation of 3 h resulted in significant decrease of the EGF fluorescence in the untransfected cells, indicating degradation of the internalized EGF in lysosomes (Figure 8, e–h and m–p). Both the HeLa and A-431 cells expressing the mutant SKD1, however, retained a substantial amount of EGF (Figure 8, e–h and m–p, arrows). It was again accumulated in the E235Q compartments. The result advocated that expression of GFP-SKD1E235Q causes inhibition of transport from endosomes to lysosomes.
Figure 8.
Degradation of the internalized EGF is inhibited in the cells expressing GFP-SKD1E235Q. HeLa (a–h) and A-431 (i–p) cells transfected with GFP-SKD1E235Q were incubated at 4°C for 1 h in the presence of 3.3 and 0.1 μg/ml Texas Red-EGF, respectively. After washing, the cells were incubated at 37°C for 30 min (a–d and i–l) or for 3 h (e–h and m–p). Then the cells were fixed for fluorescence confocal microscopy. GFP-SKD1E235Q labeling (a, e, i, and m), Texas Red-EGF labeling (b, f, j, and n), a merged image (c, g, k, and o), and a differential interference contrast image (d, h, i, and p) are shown. Each row represents the same field. The cells expressing GFP-SKD1E235Q are indicated by arrows. Bar, 20 μm.
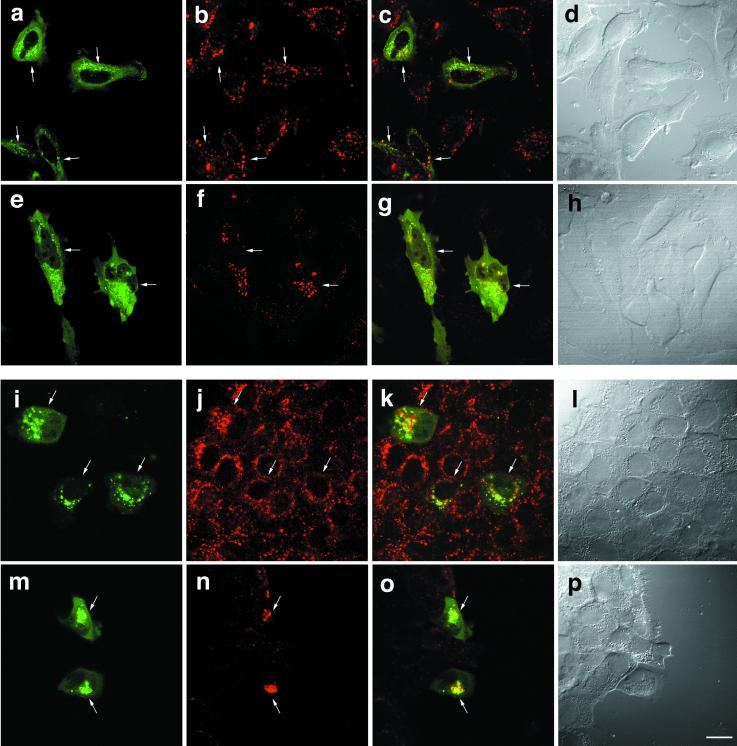
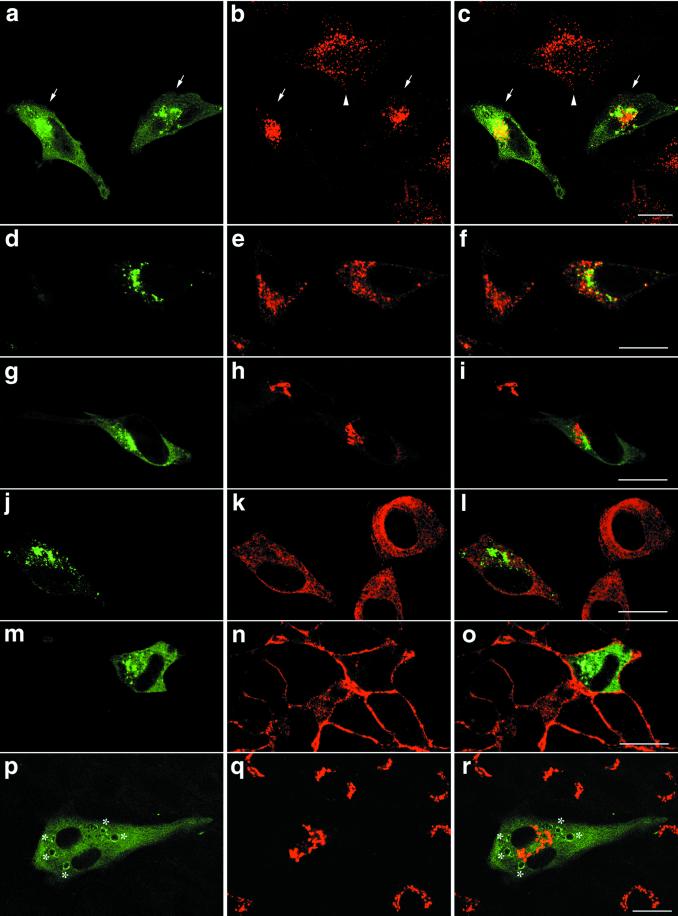
We next examined distribution of various organelle markers in the cells transfected with GFP-SKD1E235Q by immunofluorescence microscopy. Figure 9, a–c, shows the distribution of EEA1, an established marker for early endosomes (Mu et al., 1995). As expected, we observed that punctate staining of EEA1 spread in the cytoplasm of the untransfected cells (Figure 9, arrowhead). Remarkably, in the cells expressing GFP-SKD1E235Q, the EEA1 staining shifted toward a perinuclear region in which GFP-SKD1E235Q was distributed (Figure 9, arrows). Thus, we suggest that expression of the mutant SKD1 affects distribution of early endosomes. We also stained the cells with antibodies against Rab7, another endosomal marker that is localized mainly in late endosomes. Although distribution of Rab7 did not seem to be changed dramatically by overexpression of GFP-SKD1E235Q, some colocalization with the mutant protein was observed (Figure 9, d and e). In contrast to these endosome markers, in immunofluorescence microscopy for ER luminal resident proteins (Figure 9, j–l), a cis-Golgi matrix protein GM130 (Figure 9, g–i) and Na,K-ATPase (Figure 9, m–o), we found neither colocalization with the mutant SKD1 nor noticeable change in distribution of the secretory pathway markers in HeLa cells expressing GFP-SKD1E235Q compared with the untransfected cells. Distribution of TGN38, which is known to be localized predominantly to the TGN in NRK cells, also was distinct from that of GFP-SKD1E235Q in the transfected NRK cells (Figure 9, p–r). Interestingly, overexpression of GFP-SKD1E235Q in NRK cells caused to formation of large vacuoles edged with the mutant SKD1 (indicated by an asterisk). Altogether, it is very conceivable that the E235Q compartments are derived from the endocytic pathway rather than the secretory pathway.
Figure 9.
Overexpression of GFP-SKD1E235Q changes distribution of the early endosome antigen EEA1 but not that of markers for the secretory pathway. HeLa cells transfected with GFP-SKD1E235Q were subjected to immunofluorescence confocal microscopy using antibodies against EEA1 for early endosomes (a–c), Rab7 for late endosomes (d–f), GM130 for the Golgi apparatus (g–i), the KDEL sequence for the ER (j–l), and Na,K-ATPase for the plasma membranes (m–o). For Rab7 staining, permeabilization was done before fixation. The transfected NRK cells were also examined for TGN38 (p–r). Left column, fluorescence micrographs of GFP-SKDE235Q labeling; middle column, fluorescence micrographs of the each marker staining using a Cy5-conjugated second antibody; right column, merged images. Each row represents the same field. Arrows and arrowheads in a–c indicate the cells transfected with GFP-SKD1E235Q and the untransfected cells, respectively. Asterisks indicate the mutant SKD1-associated aberrant vacuoles formed in NRK cells. Bar, 20 μm.
The E235Q Compartments Are Exaggerated Vacuoles with Tubulo-vesicular Membranes
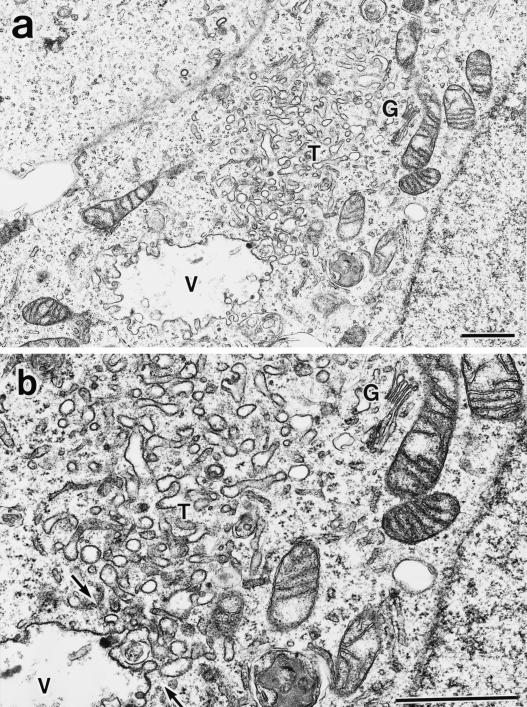
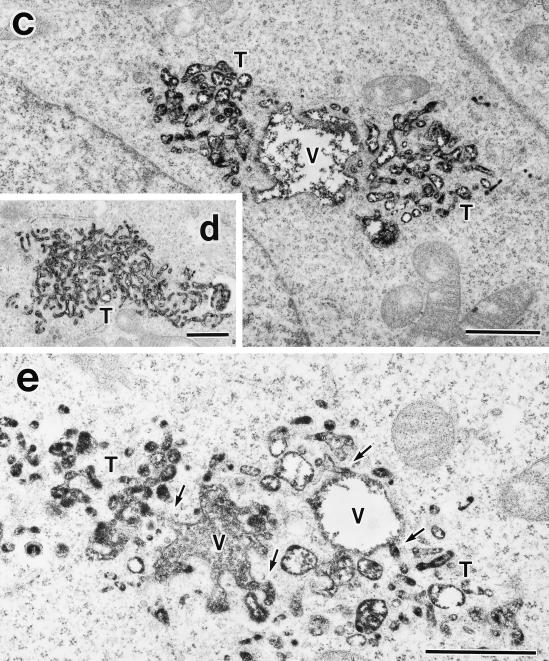
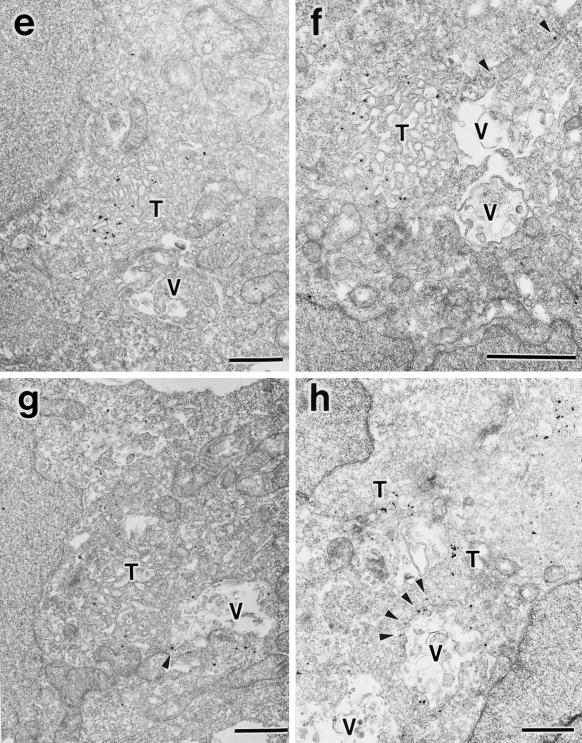
Having established formation of the perinuclear E235Q compartments accumulating the transport cargoes, we characterized morphology of the compartments at the ultrastructural level by electron microscopy. For this purpose, we used HEK293 cells because the high transfection efficiency (>50%) would increase the chance of finding the transfected cells in the sections. We confirmed that overexpression of SKD1E235Q caused a defect in ligand internalization also in this cell line. We detected large membrane-bound compartments in the cytoplasm, which were never seen in control cells (Figure 10a). These aberrant vacuoles included internal membranes. It is noteworthy that they always associate numerous tubulo-vesicular structures. Actually the tubulo-vesicular membranes seemed to be continuous with the vacuoles (Figure 10b, arrows). Size of the tubules was 50–80 nm in diameter. The morphology of the Golgi stack (Figure 10a) and the ER appeared intact. We definitively confirmed both the vacuoles and the tubulo-vesicular membranes as endocytic by prior uptake of an endocytic marker, HRP. They were heavily stained with the endocytosed HRP (Figure 10, c–e). The HRP staining provided more convincing pictures, supporting interconnection between these two structures (Figure 10e, arrows).
Figure 10.
Aberrant endocytic membranes are observed by electron microscopy in the SKD1E235Q-transfected cells. (a) HEK293 cells transfected with SKD1E235Q were fixed and observed by electron microscopy. (b) Higher-magnification image of a. (c–e) The SKD1E235Q-transfected HEK293 cells were incubated at 37°C for 1 h in the presence of 10 mg/ml HRP. The cells were fixed and stained with DAB to detect the endocytosed HRP in electron microscopy. An exaggerated aberrant multivesicular vacuole (V) accompanied by many tubulo-vesicular structures (T) emerging in the cytoplasm was observed. The morphology of the Golgi apparatus (G) appeared to be normal. The tubulo-vesicular membranes seemed to be continuous to the vacuole (arrows). Bar, 1 μm..
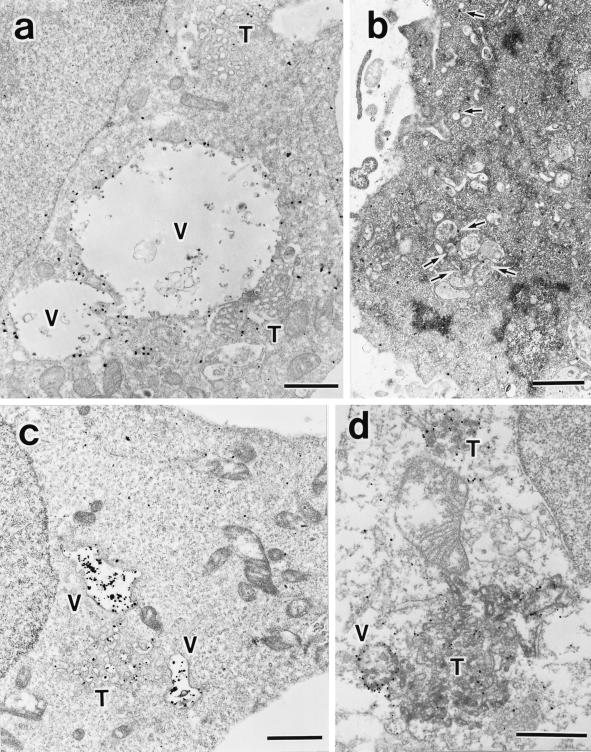
To assess whether these aberrant membranes correspond to the E235Q compartments seen in fluorescence microscopy, we investigated localization of GFP-SKD1E235Q expressed in HEK293 cells by an immunogold and silver-enhancement method using anti-GFP antibody. The silver-enhanced gold particles showing the presence of GFP-SKD1E235Q were extensively associated with the cytoplasmic side of the aberrant membranes, especially in the vacuolar part (Figure 11a). Little binding of the silver-enhanced gold particles to the section was observed in the untransfected control cells (our unpublished data). In marked contrast to the mutant SKD1, the endogenous SKD1 was located mostly in the cytoplasm (Figure 11b). Some of them were, however, associated occasionally with the membranes of vesicles or some endosome-like compartments localized beneath the plasma membrane (arrows).
Figure 11.
Immunoelectron microscopy for GFP-SKD1E235Q, the endogenous SKD1, TfR, and EEA1. HEK293 cells transfected with GFP-SKD1E235Q were fixed, and the localization of GFP-SKD1E235Q (a), TfR (c), and EEA1 (e–h) was examined by silver-enhanced immunogold electron microscopy using antibodies against GFP, TfR, and EEA1, respectively. Distribution of the endogenous SKD1 in H-4-II-E cells was also examined using antibodies against SKD1 (b). (d) Double stain of GFP-SKD1E235Q (silver-enhanced gold particles) and TfR (HRP-DAB staining). V, exaggerated aberrant multivesicular vacuole; T, tubulo-vesicular structures. Arrows in b, endogenous SKD1 associated with membrane compartments/vesicles; arrowheads in f–h, EEA1 associated with protrusions of the vacuoles. Bar, 1 μm..
TfR also was found to be accumulated in similar compartments by immunogold electron microscopy (Figure 11c). In agreement with the results of fluorescence microscopy, in control cells, many gold particles were found on the plasma membrane (our unpublished data), whereas only very few were there in the transfected cells (Figure 11c). To confirm that the same compartments include both TfR and GFP-SKD1E235Q, we performed a double-labeling experiment using the antibody against GFP together with the antibody against TfR. Figure 11d shows exact distribution of TfR (represented by HRP-DAB staining) in the aberrant membranes associated with GFP-SKD1E235Q (represented by silver-enhanced gold particles). Finally, we found a significant amount of EEA1 on membranes of the aberrant compartments (Figure 11, e–h). The gold particles indicating EEA1 localization were detected in both the vacuolar and tubulo-vesicular parts in some cases (Figure 11, g and h). The gold particles were, however, often distributed preferentially in the tubulo-vesicular part (Figure 11, e and f). Protrusions of the vacuoles were also frequently labeled (Figure 11, f–h, arrowheads). We often observed in immunofluorescence that EEA1 and GFP-SKD1E235Q overlapped only partially but were tangled together compactly (e.g., Figure 9c, right arrow). On the basis of observations by immunogold electron microscopy, we assume that the unique double-staining pattern might reflect the preferential distribution of EEA1 to the tubulo-vesicular part and the protrusions of the vacuoles in the E235Q compartments.
These observations establish that overexpression of SKD1E235Q causes the formation of the exaggerated multivesicular vacuoles with numerous tubulo-vesicular extensions, containing the endocytosed HRP, GFP-SKD1E235Q, TfR, and EEA1.
Some of the Endogenous SKD1 Becomes Pelletable by Overexpression of the Mutant SKD1
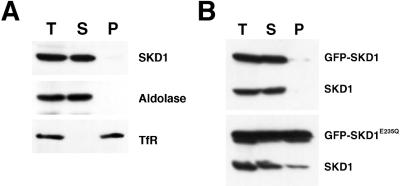
Finally, to better understand the molecular basis of the effect of the mutant SKD1, we examined its influence on the subcellular localization of the endogenous SKD1. HeLa cell homogenates were centrifuged at 100,000 × g, and the resulting supernatant and pellet were analyzed by immunoblot analysis. The endogenous SKD1 in untransfected cells was found mostly in the supernatant (Figure 12A). This is quite consistent with the morphological study. As expected, a considerable amount of the ectopic GFP-SKD1E235Q was recovered in the pellet (Figure 12B). Intriguingly, transfection of GFP-SKD1E235Q led to an increase in the amount of the endogenous SKD1 present in the pellet fraction. Because only a small population of the cells was expressing the mutant SKD1 in the transient transfection experiment, the data indicated that a significant fraction of the endogenous SKD1 moved to the pellet in the transfected cells. In contrast, the ectopic GFP-SKD1 was recovered mostly in the supernatant, and it did not affect the localization of the endogenous SKD1 (Figure 12B). These results imply that the effect of the mutant SKD1 is mediated by affecting the normal function of the endogenous SKD1.
Figure 12.
Subcellular localization of the endogenous and the ectopic SKD1. (A) A cell homogenate prepared from HeLa cells (T) was fractionated into supernatant (S) and pellet (P) by centrifugation at 100,000 × g. These fractions were analyzed by immunoblot using antibodies against SKD1, TfR (as a membrane protein marker), and aldolase (as a cytosolic marker). (B) HeLa cells transfected with GFP-SKD1 or GFP-SKD1E235Q were subjected to cell fractionation as described in A. The fractions were analyzed by immunoblot using the antibody against SKD1.
DISCUSSION
Using a dominant negative mutant protein, we have provided here the first evidence that SKD1 is involved in the endosomal functions in mammalian cultured cells.
In contrast to the normal surface distribution of Na,K-ATPase, TfR seemed to be disappear from the cell surface by overexpression of GFP-SKD1E235Q. The decrease of the surface TfR also was shown biochemically by the cotransfection of the mutant SKD1 and the ectopic TfR. Quantitative analysis by flow cytometry confirmed that severity of the surface TfR reduction depended on expression level of the mutant protein. Moreover, TfR was accumulated in the perinuclear compartments, which were specifically decorated with GFP-SKD1E235Q. These compartments, termed E235Q compartments, were accessible by the endocytic tracers such as dextran and HRP. EEA1, the marker for early endosomes, came to be distributed in the compartments (or at least in part of them), whereas the markers for the ER and the Golgi complex were not colocalized. These observations advocate that TfR accumulated in compartments belonging to the endocytic pathway rather than in those belonging to the secretory pathway in SKD1E235Q-transfected cells. We suggest, therefore, the redistribution of TfR reflects interference with its transport to the cell surface via endosomes, implying inhibition of the TfR recycling and/or inhibition of the newly synthesized TfR transport to the cell surface via endosomes (Futter et al., 1995). The result in the experiment of LDL indicates that the effect is not limited to TfR but common to the recycling receptors. In addition, accumulation of dextran, EGF, and HRP, which are destined for lysosomes, in the E235Q compartments, possibly denotes that transport to lysosomes is also abrogated as well as the transport to the cell surface, yet the internalization machinery is not hindered, because the SKD1E235Q-transfected cells could take up dextran, HRP, and EGF. Inhibition of degradation of EGF strongly supports this scenario. Overexpression of the mutant SKD1 also seems to affect biosynthetic transport of lamp-1 mediated by endosomes (Akasaki et al., 1995). The observation that some of lamp-1 showed the distinct distribution from the E235Q compartments (Figure 7h) may imply existence of other route bypassing the E235Q compartments or of the remains of lamp-1 in lysosomes. It is notable that the distribution of the endocytosed dextran was limited to the E235Q compartments and that the marker did not reach such satellite lamp-1 spots, which probably represent lysosomes. This is also consistent with inhibition of the endosome-to-lysosome transport.
How are the E235Q compartments formed? Although the basic architecture of the E235Q compartments consisting of a multivesicular part and tubulo-vesicular extensions is reminiscent of sorting endosome morphology (Griffiths et al., 1989), the E235Q compartments are characterized by the enlargement of the vacuolar part and the increased number of tubulo-vesicular extensions. A blockade of membrane efflux from sorting endosomes but not of membrane influx into the compartments might increase their surface area. Homotypic endosome–endosome fusion is also likely to contribute to the enlargement. The diameter of the tubule (50–80 nm) of the E235Q compartments was similar to that of the tubular portion (∼60 nm) of sorting endosomes. Sometimes we observed highly developed tubulo-vesicular extensions in the transfected cells (e.g., Figure 10d), which resemble aberrant early endosomes induced by treatment of cells with bafilomycin A1, which is an inhibitor of vacuolar-type H+-ATPase and prevents transport from early endosomes to late endosomes (Clague et al., 1994).
The E235Q compartments seem to possess also some features of late endosomes/lysosomes. Rab7 showed partial colocalization with the mutant SKD1, and the localization of the E235Q compartments resemble that of late endosomes/lysosomes. Sorting endosomes are usually dispersed in the cytoplasm (Mukherjee et al., 1997; this study), whereas late endosomes/lysosomes are near the nucleus probably because of cytoplasmic dynein–microtubule interaction (Mukherjee et al., 1997). Components of the transport intermediates including Rab7 and cytoplasmic dynein may assemble on early endosomes despite inhibition of their budding off, because the membrane components required for the assembly may also accumulate in the aberrant endosomes because of the blockade of their transport to late endosomes. Other possibilities, however, still remain: 1) fusion between preexisting early endosomes and late endosomes/lysosomes is induced; 2) maturation of early endosomes into late endosomes/lysosomes is prevented; that is, the former can acquire components of the latter but cannot sort out their own elements; and 3) components of late endosomes that otherwise bypass early endosomes are mistargeted to early endosomes. Further analysis of the E235Q compartment will reveal which is the reality.
Most of the endogenous SKD1 as well as GFP-SKD1 was diffusely distributed over the cytoplasm, although part of it was distributed in vesicles and some endosome-like compartments under the plasma membrane (Figure 11b). Consistent with this, the fractionation experiment indicates that the endogenous SKD1 and the expressed GFP-SKD1 exist mostly in the cytoplasm, but a small portion is localized in some membranes. These results may imply that the wild-type SKD1 is transiently associated with the endosome-like compartments. The amount of the endogenous SKD1 in the pellet fraction was apparently increased by overexpression of GFP-SKD1E235Q, which was constantly bound to the endosomal membrane. Moreover, two-hybrid analysis revealed binding of the mutant SKD1 to the wild-type or mutant SKD1 (our unpublished results). Taken together, we suggest the formation of an oligomer containing a mixture of wild-type and the mutant SKD1, which cannot be dissociated from endosomes and causes perturbation of their structures and their functions: outgoing transport to the cell surface and to lysosomes. If it is the case, it is most likely that transient association of oligomer of the wild-type SKD1 with endosomes is required for maintaining morphology of endosomes and for sorting and/or transport in the compartments. Results described here, however, are phenotypes associated with the mutant SKD1 and do not necessarily imply that the wild-type SKD1 functions at exactly the same stage. We cannot exclude another possibility, that SKD1 might function in another place, for example, transport from the plasma membrane to endosomes, and might influence secondarily the endosome activity. Further studies on SKD1 would unravel the involvement of an SKD1-containing protein complex, similar to that of the Vps protein complex (Babst et al., 1998), in activities of endosomes.
VPS4 is identical to CSC1, which is involved in autophagy in yeast (Shirahama et al., 1997). Autophagy is the major route of delivery of cytoplasmic macromolecules and organelles into the lysosome/vacuole for degradation (Dunn, 1994). Under starvation conditions, cells induce autophagy to supply nutrients by digesting the delivered contents. Intriguingly, it has been documented that csc1E291K (csc1-1) mutation of Vps4p in the SRH region of the AAA module results in induction of autophagy even in the presence of nutrients (Shirahama et al., 1997). csc1E291K is a gain-of-function allele. Together with an electron microscopic observation showing convergence of autophagy and the endocytic pathway in mammals (Liou et al., 1997), the results upon analysis of csc1E291K suggest that SKD1 may regulate a putative pathway from endosomes to autophagic compartments, which is necessary for formation or maturation of autophagosomes.
In summary, the data presented in this report document that the dominant negative mutant SKD1E235Q protein leads to formation of the aberrant endosomes. The effects of the mutant protein raise the possibility that the mammalian counterpart of Vps4p is involved in the function of endosomes and in maintenance of their structure. Future experiments will be directed toward the understanding of the molecular machinery including SKD1. SKD1E235Q would become a useful tool to define a tree of complex and highly dynamic endocytic compartments.
ACKNOWLEDGMENTS
We are grateful to Drs. Y. Takai and T. Sasaki for providing the myc-tagged TfR cDNA and to Drs. E. Kominami, N. Nakamura, T. Ueno, K. Omori, and T. August for providing antibodies. We thank Dr. N. Ishihara in our laboratory and the National Institute for Basic Biology Center for Analytical Instruments for excellent technical assistance with subcellular fractionation and for synthesizing peptides, respectively. We are grateful to Drs. W. Hong (Institute of Molecular and Cell Biology, Singapore), T. Kobayashi, and J. Gruenberg (University of Geneva, Geneva, Switzerland) for their helpful comments and critical reading of the manuscript. We also thank Dr. T. Noda in our laboratory for giving a hint to start this work. This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Culture, and Sports of Japan.
Abbreviations used:
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- DAB
diaminobenzidine
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- FCS
fetal calf serum
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- LDL
low-density lipoprotein
- NRK
normal rat kidney
- NSF
N-ethylmaleimide–sensitive factor
- PE
R-phycoerythrin
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- Tf
transferrin
- TfR
transferrin receptor
- TGN
trans-Golgi network
REFERENCES
- Akasaki K, Michihara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. Sequence analysis of the AAA protein family. Protein Sci. 1997;6:2043–2058. doi: 10.1002/pro.5560061001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Dunn KW, Mayor S, Myers JN, Maxfield FR. Applications of ratio fluorescence microscopy in the study of cell physiology. FASEB J. 1994;8:573–582. doi: 10.1096/fasebj.8.9.8005385. [DOI] [PubMed] [Google Scholar]
- Dunn WAJ. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Finken-Eigen M, Rohricht RA, Kohrer K. The VPS4 gene is involved in protein transport out of a yeast prevacuolar endosome-like compartment. Curr Genet. 1997;31:469–480. doi: 10.1007/s002940050232. [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Back R, Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hartmann T, et al. Distinct sites of intracellular production for Alzheimer's disease A β40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Yamashina S, Sakaguchi M, Mihara K, Omura T. Malfolded cytochrome P-450 (M1) localized in unusual membrane structures of the endoplasmic reticulum in cultured animal cells. J Biochem. 1995;118:397–404. doi: 10.1093/oxfordjournals.jbchem.a124920. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Griffiths G, Dean GE, Mellman I, Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci USA. 1986;83:2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mu FT, et al. EEA1, an early endosome-associated protein. EEA1 is a conserved α-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Munn AL, Riezman H. Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Bryant NJ, Stevens TH. The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol Cell Biol. 1996;16:2700–2707. doi: 10.1128/mcb.16.6.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M, Murata M, Ohnishi S. A novel fluorescence method to monitor the lysosomal disintegration of low density lipoprotein. Eur J Cell Biol. 1992;59:116–126. [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Trowbridge IS. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981;256:12888–12892. [PubMed] [Google Scholar]
- Patel S, Latterich M. The AAA team: related ATPases with diverse functions. Trends Cell Biol. 1998;8:65–71. [PubMed] [Google Scholar]
- Périer F, Coulter KL, Liang H, Radeke CM, Gaber RF, Vandenberg CA. Identification of a novel mammalian member of the NSF/CDC48p/Pas1p/TBP- 1 family through heterologous expression in yeast. FEBS Lett. 1994;351:286–290. doi: 10.1016/0014-5793(94)00879-5. [DOI] [PubMed] [Google Scholar]
- Shirahama K, Noda T, Ohsumi Y. Mutational analysis of Csc1/Vps4p: involvement of endosome in regulation of autophagy in yeast. Cell Struct Funct. 1997;22:501–509. doi: 10.1247/csf.22.501. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Takemoto H, Yoshimori T, Yamamoto A, Miyata Y, Yahara I, Inoue K, Tashiro Y. Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch Biochem Biophys. 1992;296:129–136. doi: 10.1016/0003-9861(92)90554-a. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. N-Ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Semba T, Takemoto H, Akagi S, Yamamoto A, Tashiro Y. Protein disulfide-isomerase in rat exocrine pancreatic cells is exported from the endoplasmic reticulum despite possessing the retention signal. J Biol Chem. 1990;265:15984–15990. [PubMed] [Google Scholar]
- Yoshimori T, Shimonishi Y, Uchida T. Binding properties of monoclonal antibody to the cytoplasmic domain of transferrin receptor. Cell Struct Funct. 1988;13:311–324. doi: 10.1247/csf.13.311. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]