Abstract
During sporulation, Bacillus subtilis undergoes an asymmetric division that results in two cells with different fates, the larger mother cell and the smaller forespore. The protein phosphatase SpoIIE, which is required for activation of the forespore-specific transcription factor σF, is also required for optimal efficiency and timing of asymmetric division. We performed a genetic screen for spoIIE mutants that were impaired in sporulation but not σF activity and isolated a strain with the mutation spoIIEV697A. The mutant exhibited a 10- to 40-fold reduction in sporulation and a sixfold reduction in asymmetric division compared to the parent. Transcription of the σF-dependent spoIIQ promoter was increased more than 10-fold and was no longer confined to the forespore. The excessive σF activity persisted even when asymmetric division was prevented. Disruption of spoIIGB did not restore asymmetric division to the spoIIEV697A mutant, indicating that the deficiency is not a consequence of predivisional activation of the mother cell-specific transcription factor σE. Deletion of the gene encoding σF (spoIIAC) restored asymmetric division; however, a mutation that dramatically reduced the number of promoters responsive to σF, spoIIAC561 (spoIIACV233 M), failed to do so. This result suggests that the block is due to expression of one of the small subset of σF-dependent genes expressed in this background or to unregulated interaction of σF with some other factor. Our results indicate that regulation of SpoIIE plays a critical role in coupling asymmetric division to σF activation in order to ensure proper spatial and temporal expression of forespore-specific genes.
During sporulation, Bacillus subtilis undergoes a dramatic shift in the site of division from a medial site utilized during vegetative growth to a polar one. The result of this asymmetric division is two cells of unequal volumes with different fates, the larger mother cell and the smaller forespore (also known as the prespore). Immediately following asymmetric division, different programs of gene expression are initiated in the two cells by the cell-specific activation of the transcription factors σF in the forespore and σE in the mother cell (34). SpoIIE is a membrane-bound PP2C-like protein phosphatase (1, 38) that is essential for activation of the forespore-specific transcription factor σF. SpoIIE dephosphorylates, and thus activates, the anti-anti-sigma factor SpoIIAA. Activated SpoIIAA can release inhibition of σF by the anti-sigma factor SpoIIAB, resulting in transcription of forespore-specific genes (2, 9).
There is substantial evidence that SpoIIE has an additional role in asymmetric division that is independent of its phosphatase activity. Division in B. subtilis during growth and sporulation is preceded by the formation of a ring-like structure of the essential bacterial tubulin homologue FtsZ (43). FtsZ rings normally form at the midcell site during vegetative growth; however, during sporulation they are repositioned from this site to sites near both poles (22). It has recently been discovered that FtsZ ring switching occurs by the formation of a dynamic spiral-like intermediate. The spiral-like structures are a consequence of enhanced transcription of ftsZ as well as expression of spoIIE (6). B. subtilis strains bearing mutations in the phosphatase domain of SpoIIE that abolish σF activation still efficiently form asymmetric septa, whereas spoIIE null mutants do not (5). Deletion of spoIIE results in both a reduction and a delay in polar Z-ring formation (19).
SpoIIE colocalizes to the asymmetric division site with FtsZ (23) and has been shown biochemically to interact with this protein (26). Deletion of either the N-terminal transmembrane domain or the extreme C terminus (beyond the phosphatase domain) results in soluble SpoIIE protein that efficiently activates σF but poorly supports asymmetric division (3, 12). From this evidence, we speculated that SpoIIE plays a role in asymmetric division and performed a genetic screen to isolate spoIIE mutants that were deficient in this putative function but not in their ability to activate σF.
Below we describe the isolation and characterization of such a mutant having the mutation spoIIEV697A. However, the block in division is not caused by a specific loss of function of SpoIIE but rather is mediated by σF. Although it is not clear why σF impairs asymmetric division in this mutant, we determined that the effect does not depend on the mother cell-specific transcription factor σE. We have shown that a mutant version of σF that is unable to activate transcription of most known σF-dependent genes fails to restore asymmetric division in a spoIIEV697A mutant. This indicates that the block in division is caused either by expression of one of the small subset of σF-dependent genes expressed in this background or by unregulated interaction of σF with some other factor, possibly RNA polymerase or SpoIIAB. We observed that the spoIIEV697A mutant activates σF even when asymmetric division is prevented, thereby uncoupling the two events. In addition, spoIIEV697A in cis restores sporulation to a spoIIE48 mutant that normally cannot activate σF in response to asymmetric division.
Taken together, our results indicate that regulation of SpoIIE plays a crucial role in coupling asymmetric division to σF activation and that this regulation is disrupted by the spoIIEV697A mutation. Uncoupling the two events leads to a severe reduction in asymmetric division, compartmentalization of σF activity, and spore formation, reinforcing the concept that tight coordination between morphology and gene regulation is crucial for the developmental process.
MATERIALS AND METHODS
Media.
B. subtilis was grown in modified Schaffer's sporulation medium (MSSM) or on Schaeffer's sporulation agar (SSA) or Luria-Bertani (LB) agar (33, 37). When required, the medium contained 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 40 μg/ml, chloramphenicol at 5 μg/ml, erythromycin at 1.5 μg/ml, neomycin at 3.5 μg/ml, and spectinomycin at 100 μg/ml. Escherichia coli was grown on LB agar containing ampicillin at 100 μg/ml.
Strains and plasmids.
B. subtilis 168 strain BR151 (trpC2 metB10 lys-3) was used as the parent strain. Other B. subtilis strains and plasmids used are listed in Table 1. Escherichia coli strain DH5α (Gibco-BRL) was used to maintain plasmids. E. coli strain XL1-Red (Stratagene) was used for random mutagenesis of pDH3. E. coli strain XL-mutS (Stratagene) was used for site-directed mutagenesis of pDH2 to produce pDH4.
TABLE 1.
B. subtilis strains and plasmids used
| Plasmid or strain | Relevant characteristicsa | Origin or reference |
|---|---|---|
| Plasmids | ||
| pDH1 | pBluescript SK(−) spoIIE | This study |
| pDH2 | erm @ HincII site of pDH1 upstream of spoIIE ORF | This study |
| pDH3 | spc @ HincII site of pDH1 upstream of spoIIE ORF | This study |
| pDH4 | spoIIEV697A in pDH2 background isolated from SL8978 | This study |
| pDH5 | spoIIEV697A inserted by site-directed mutagenesis in pDH3 | This study |
| pDH6 | spoIIQ-gfp neo | This study |
| pDH7 | spoIIEV697A neo | This study |
| pEIA99 | amyE::spoIIQ-lacZ | Edward Amaya |
| pVK59 | Integrative vector with neo | Vasant Chary |
| pVK141 | thrC::spoIIQ-lacZ | Vasant Chary |
| PVK228 | spoIIAC::neo | Vasant Chary |
| Strains | ||
| BR151 | trpC2 metB10 lys-3 | Laboratory stock |
| SL1102 | trpC2 metB10 spoIIAC561 | Laboratory stock |
| SL8410 | trpC2 metB10 lys-3 spoIIGB::erm | This study |
| SL8603 | trpC2 metB10 lys-3 erm spoIIE amyE::spoIIQ-lacZ | This study |
| SL8625 | trpC2 metB10 lys-3 erm spoIIE::pSG1902 amyE::spoIIQ-lacZ | This study |
| SL8675 | trpC2 metB10 lys-3 spc spoIIE::pSG1902 amyE::spoIIQ-lacZ | This study |
| SL8978 | trpC2 metB10 lys-3 erm spoIIEV697A::pSG1902 amyE::spoIIQ-lacZ | This study |
| SL9055 | trpC2 metB10 spoIIAΔ4 | Laboratory stock |
| SL10002 | trpC2 metB10 lys-3 thrC::spoIIQ-lacZ | This study |
| SL10008 | trpC2 metB10 lys-3 erm spoIIEV697A thrC::spoIIQ-lacZ | This study |
| SL10174 | trpC2 metB10 lys-3 amyE::spoIIQ-lacZ | This study |
| SL10221 | trpC2 metB10 lys-3 spoIIQ::pDH6 | This study |
| SL10222 | trpC2 metB10 lys-3 erm spoIIEV697A spoIIQ::pDH6 | This study |
| SL10312 | trpC2 metB10 lys-3 erm spoIIE div-355 amyE::spoIIQ-lacZ | This study |
| SL10339 | trpC2 metB10 lys-3 erm spoIIEV697A amyE::spoIIQ-lacZ | This study |
| SL10423 | trpC2 metB10 lys-3 erm spoIIEV697A div-355 amyE::spoIIQ-lacZ | This study |
| SL10766 | trpC2 metB10 lys-3 erm spoIIEV697A | This study |
| SL10767 | trpC2 metB10 lys-3 erm spoIIE48 | This study |
| SL10905 | trpC2 metB10 spc spoIIEV697A spoIIAΔ4 | This study |
| SL10912 | trpC2 metB10 lys-3 spc spoIIEV697A | This study |
| SL11132 | trpC2 metB10 lys-3 spc spoIIEV697A spoIIGB::erm | This study |
| SL11142 | trpC2 metB10 spc spoIIEV697A spoIIAC561 | This study |
| SL11173 | trpC2 metB10 lys-3 spoIIE::pDH7 | This study |
| SL11201 | trpC2 metB10 lys-3 erm spoIIE48::pDH7 | This study |
| SL11274 | trpC2 metB10 lys-3 spoIIAC::neo | This study |
| SL11275 | trpC2 metB10 lys-3 spc spoIIEV697A spoIIAC::neo | This study |
ORF, open reading frame.
The spoIIE gene was cloned as a 3.3-kb fragment by PCR with Pfu polymerase (Stratagene) with the primers AGCGAAGATCGCTTGTCA and ACCGTAATCCCCTGCTCT and ligated into the HincII site of pBluescript SK−, destroying the HincII site and generating pDH1. The spoIIE gene encoded by pDH1 was able to restore sporulation to a strain in which spoIIE had been insertionally disrupted, indicating that no deleterious mutations were generated in the PCR amplification process (data not shown). To construct pDH2, pDH1 was linearized at the HincII site, located 418 bp upstream of the spoIIE start codon, and ligated with a 1.0-kb fragment derived from pIC177 Cmr::Err (40) containing an erythromycin resistance (erm) gene. In a separate ligation, a 1.2-kb fragment encoding a spectinomycin resistance gene (spc) isolated from pIC156 (40) was ligated with the HincII-digested pDH1 to generate pDH3. pDH4 is the name given to a derivative of pDH2 in which the spoIIEV697A mutation was introduced by passage through mutagenic E. coli XL-1 Red competent cells (Stratagene) (see Results). pDH5 is a derivative of pDH3 in which the spoIIEV697A mutation was introduced into pDH2 by site-directed mutagenesis with the Gene Editor kit (Promega) with the mutagenic primer TTTTTGAAGGCTGGATCGACG (base change in italic). pSG1902 (a gift from J. Errington, Oxford University, Oxford, United Kingdom) (44) was used to generate a spoIIE-green fluorescent protein gene (gfp) C-terminal translational fusion.
To generate a spoIIQ-gfp transcriptional fusion, the spoIIQ promoter region was cloned as a 0.5-kb fragment generated by PCR with Taq polymerase (Promega) with the primers GATGATGAATTCAATGAAGGCCATAAGTGA and GATGATGGATCCCACCACAGCAAGATTCGT. This PCR fragment was cloned into pGem T-Easy (Promega) by TA cloning. The resulting plasmid was digested with SalI and ligated with a 0.8-kb SalI fragment from pGreenTIR, which encodes the mut1 allele of the gfp gene associated with an enhanced ribosome-binding site (28). PCR was used to confirm that the gfp gene and the spoIIQ promoter were in the same orientation. This plasmid was then digested with ScaI, cutting at a unique site in the bla (ampicillin resistance) gene, and ligated with a 1.2-kb SmaI fragment from pBEST501 containing the neo (neomycin resistance) gene (16). The resulting plasmid, pDH6, was designed to integrate at the spoIIQ locus by single crossover (Campbell-like integration) and generate a spoIIQ-gfp transcriptional fusion.
To generate a vector that would introduce the spoIIEV697A mutation by Campbell-like integration at the 3′ end of the gene, we used pVK59 (kindly provided by V. Chary, Temple University, Philadelphia, Pa.), an integrative plasmid that has the neo gene. This plasmid was digested with ClaI and XhoI and ligated with a 1.1-kb ClaI-XhoI fragment of pDH4 encoding the C-terminal 292 residues of SpoIIEV697A. The resulting plasmid was named pDH7.
The div-355 mutant strain (kindly provided by R. Losick, Harvard University, Cambridge, Mass.) (21), which has the PY79 genetic background, was transformed with total DNA from SL8625, which has pDH3 integrated into the chromosome. The resulting strain, SL10242, has the erm gene in the chromosomal region between divIC and spoIIE, which are separated by less than 1 kb (21). Total DNA was prepared from one of these clones and transformed into SL10174, selecting for erythromycin resistance and screening for cotransformation of the div-355 phenotype (Spo− at 37°C, filamentation at 45°C), generating SL10312. This strain has div-355 in the BR151 genetic background.
β-Galactosidase assays.
β-Galactosidase assays were performed essentially as described previously (14), with lysozyme used to permeabilize the cells. Specific activity is expressed as nanomoles of ο-nitrophenyl-β-d-galactopyranoside (ONPG) hydrolyzed per minute per milligram of bacterial dry weight.
Other methods.
Cultures used for visualization of green fluorescent protein (GFP) were grown in MSSM at 33.5°C. Culture samples of 1 μl of unfixed cells were transferred to slides and examined by fluorescence microscopy essentially as described previously (46).
Cultures used for visualization of asymmetric septa were grown in MSSM at 37°C. Culture samples of 10 μl were mixed with an equal volume of the vital membrane stain FM4-64 (Molecular Probes) (previously diluted 100-fold in water) and incubated at 37°C without shaking for 10 min. One-microliter samples were transferred to slides and visualized essentially as described previously (35).
Sporulation was assayed 20 h after the end of exponential growth by diluting cultures and determining the heat-resistant count (80°C, 20 min) and the viable count in the diluted cultures. B. subtilis transformation, sporulation by exhaustion in MSSM, and all other methods were essentially as described previously (14, 31, 32, 46).
RESULTS
Isolation of a novel spoIIE mutation that reduces sporulation efficiency.
In order to isolate spoIIE mutants that were defective in asymmetric division, we performed a genetic screen. pDH2, encoding the full-length spoIIE gene on an integrative plasmid with the erm gene cloned upstream of the start codon of spoIIE, was randomly mutagenized by passage through E. coli XL1-Red mutagenic competent cells (Stratagene). The resulting transformants (greater than 10,000) were collected into pools of approximately 250 clones each for plasmid isolation. The plasmid pools were used to transform B. subtilis SL8625, selecting for erythromycin resistance. Strain SL8625 contains the spc gene at the same site upstream of spoIIE as erm in pDH2. Therefore, the resulting transformants could be screened for single-crossover (spectinomycin resistant) or double-crossover (spectinomycin sensitive) integration at the spoIIE locus. The presence of either the erm or spc gene at this site in the chromosome had no effect on growth or sporulation (data not shown). This strain also had a σF-dependent spoIIQ-lacZ transcriptional fusion (24) to avoid isolation of alleles that are defective in the phosphatase activity required for σF activation, a class that has already been described (5, 38). Lastly, the strain contained a C-terminal spoIIE-gfp translational fusion that would allow differentiation of nonsense (no GFP signal) versus missense (GFP signal) mutants (44)
Initial experiments indicated that potential mutants were unstable when selection for transformants with SL8625 was done on a medium (SSA) that supported sporulation. Subsequently, SL8625 transformants were selected on LB agar, which does not support sporulation. Two thousand such transformants were patched individually onto SSA plates containing X-Gal to screen for the desired phenotype, revealing one mutant that appeared to sporulate poorly but strongly activated σF. This mutant was the product of a single-crossover recombination event at the spoIIE locus. In order to generate a strain containing a double-crossover integration of the mutant allele presumed to be responsible for the phenotype, total DNA from bacteria from the same clone maintained on LB agar (designated SL8978) was used to transform E. coli DH5α, selecting for ampicillin resistance. The resulting E. coli transformants contained a plasmid designated pDH4, a mutant version of pDH3 that was capable of introducing the spoIIE allele responsible for the reduced sporulation, hyper-σF activity phenotype into B. subtilis (data not shown). Subsequent double-crossover integration of the mutant allele encoded by this plasmid resulted in a stable B. subtilis strain that exhibited the same phenotype (SL10008).
We subsequently observed that integration of pSG1902, a plasmid encoding the C-terminal 184 residues of SpoIIE (44), was capable of restoring sporulation to SL10008 (data not shown). Sequencing of this region of pDH4 revealed a G-to-C transition at nucleic acid position 2090 of the coding strand of the spoIIE open reading frame (4), indicating a change of valine to alanine at residue 697. In order to verify that this mutation was causing the observed phenotype, it was generated in vitro with site-directed mutagenesis of pDH2 (see Materials and Methods) to produce pDH5. Introduction of the mutant spoIIE allele from this plasmid into B. subtilis resulted in a phenotype identical to that of SL10008 (data not shown).
The effect of spoIIEV697A on sporulation was determined by heat survival (see Materials and Methods). The following data are the average of three independent experiments plus and minus the standard deviation. BR151, the spo+ parent, produced 3.1 × 108 ± 1.1 × 108 spores per ml. In contrast, SL10766, containing the spoIIEV697A mutation, only produced 1.5 × 107 ± 0.5 × 107 spores per ml. Therefore, the spoIIEV697A mutation caused a 10- to 40-fold reduction in sporulation.
spoIIEV697A severely impairs asymmetric division.
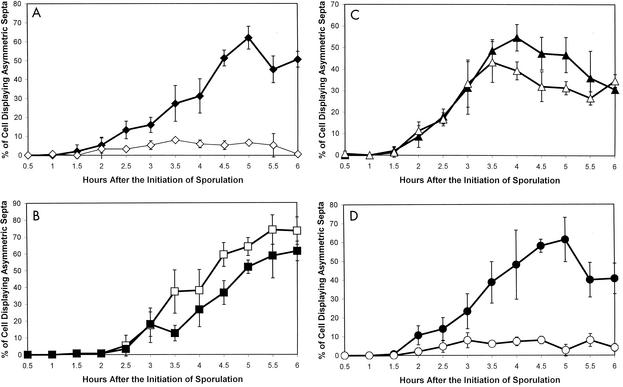
Our rationale for screening for spoIIE mutants that were impaired in sporulation but continued to activate σF was that these mutants would be deficient in some other putative function of SpoIIE, potentially its role in asymmetric division. To analyze asymmetric division, BR151 and the isogenic mutant SL10912 were induced to sporulate in MSSM, and samples were stained with the vital membrane stain FM4-64 and scored for the presence of asymmetric septa by fluorescence microscopy (35). The proportion of the BR151 population undergoing asymmetric division rose to 40% by T4.5 and eventually reached a peak of greater than 60% by T6 (T0 indicates the end of exponential growth and the initiation of sporulation) (Fig. 1). In contrast, for strain SL10912 bearing the spoIIEV697A mutation, the proportion with asymmetric septa rarely exceeded 10% (Fig. 1). In all of the samples analyzed after T4, the mutant displayed at least a sixfold reduction in frequency of asymmetric division compared to BR151, indicating a severe defect in the morphological progression of sporulation in the spoIIEV697A mutant (Fig. 1).
FIG. 1.
Frequency of asymmetric division during sporulation in the parent strain BR151 (solid squares) and in the spoIIEV697A mutant SL10766 (open squares). Fifty cells were scored at each time. The results are the averages of three independent experiments plus and minus the standard deviation.
spoIIEV697A causes excessive, uncompartmentalized σF activity.
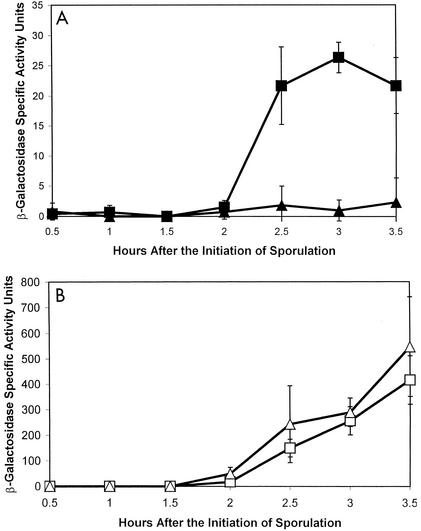
Colonies of the spoIIEV697A mutant containing the σF-dependent spoIIQ-lacZ transcriptional fusion gave an intensely blue color on SSA plates containing X-Gal, suggesting that σF activity was higher than in the parent (data not shown). Since it has been postulated that SpoIIE may play a role in the spatial and temporal regulation of σF (3, 11, 19), we wanted to characterize further the effect of spoIIEV697A on its activity. In order to quantitatively analyze σF activity in this mutant, the strain containing the spoIIQ-lacZ fusion and the spoIIEV697A mutation (SL10008) was induced to sporulate in MSSM, and samples were taken for analysis of β-galactosidase activity (see Materials and Methods). In the spo+ parent, the spoIIQ-lacZ fusion became active at T2.5, and β-galactosidase activity reached a peak specific activity of 30 nmol of ONPG hydrolyzed per min per mg at T3.5 (Fig. 2). In contrast, the fusion in the spoIIEV697A mutant became active slightly earlier, at T1.5, and β-galactosidase specific activity rose to over 275 nmol of ONPG hydrolyzed per min per mg by T4, indicating at least a ninefold increase in expression of spoIIQ-lacZ (Fig. 2).
FIG. 2.
Expression of spoIIQ-lacZ in the parent strain SL10002 (solid squares) and in the spoIIEV697A mutant SL10008 (open squares) in MSSM. Specific activity units are nanomoles of ONPG hydrolyzed per minute per milligram of bacterial dry weight. The results are the averages of three independent experiments plus and minus the standard deviation.
We also tested the effect of spoIIEV697A on expression of two other σF-dependent promoters, spoIIR (17) and spoIIIG (42). In both cases, the mutation caused at least a threefold to fourfold increase in peak activity (data not shown). These results suggest that in addition to impairing asymmetric division, spoIIEV697A causes hyperactivation of σF, a phenotype that is consistent with the role of SpoIIE as a phosphatase in triggering σF activation (2, 9).
It had been previously demonstrated that overexpression of SpoIIE can cause abnormally high levels of σF activation and severely impair sporulation (2), a phenotype reminiscent of that observed in the spoIIEV697A mutant. In order to determine if the level of SpoIIE protein was disturbed by this mutation, whole-cell lysates were isolated at various times during sporulation from strains containing C-terminal translational fusions of gfp to either wild-type spoIIE or spoIIEV697A. The presence of the C-terminal fusion did not affect sporulation of the parent or the mutant. Western blots of the lysates with anti-GFP antibodies revealed little difference in SpoIIE-GFP protein concentrations between the parent and the mutant (data not shown).
The activity of σF is normally confined to the forespore after asymmetric division occurs, and mutations that block asymmetric division prevent σF activation (12, 19, 21). This coupling of asymmetric division to σF activation establishes a developmental checkpoint that ensures proper spatial and temporal transcription of forespore-specific genes (34). However, in the spoIIEV697A mutant, we separately observed low levels of asymmetric division (Fig. 1) yet very high levels of σF activity (Fig. 2). This led us to speculate that the checkpoint had been disrupted and that in some cells in the spoIIEV697A population, σF activity would be uncompartmentalized. In order to address this, we generated strains that had the spoIIQ promoter transcriptionally fused to gfp, the gene encoding GFP, with pDH6 (see Materials and Methods). A spoIIQ-gfp transcriptional fusion had previously been shown to be expressed exclusively in the forespore (24).
Strains SL10221 (spo+) and SL10222 (spoIIEV697A) were induced to sporulate in MSSM, and samples were examined by fluorescence microscopy and scored as either having no signal, signal confined to the forespore, or signal throughout the cell. In the parent, 96% of the fluorescent cells had signal present only in the forespore at T6 (Table 2). In contrast, only 2.2% of the mutant cells exhibiting signal at this time showed forespore-specific expression (Table 2). The rest of the fluorescent cells had a pattern of whole-cell fluorescence (Table 2). Therefore, spoIIEV697A largely abolished compartmentalization of σF activity. Similar results were observed with a spoIIIG-gfp transcriptional fusion located at an ectopic locus (data not shown). The fact that greater than 45% of the spoIIEV697A mutant cells exhibited whole-cell spoIIQ-gfp activity (Table 3) when less than 10% of these cells had undergone asymmetric division at a similar time in a previous assay (Fig. 1) strongly suggested that spoIIEV697A enables B. subtilis to activate σF in the absence of asymmetric division. We proceeded to test this possibility in the following experiment.
TABLE 2.
Pattern of spoIIQ-gfp expression in the parent strain and spoIIEV697A mutant
| Strain | No. of cells showing indicated fluorescence patterna:
|
% Compartment- alizationb | ||
|---|---|---|---|---|
| Forespore | Whole cell | No signal | ||
| SL10221 (parent) | 102 | 4 | 219 | 96 |
| SL10222 (mutant) | 4 | 178 | 145 | 2.2 |
These values are the sum of three independent experiments in which cultures were grown in MSSM at 33.5°C and samples were taken at T6. At least 100 cells were scored in each experiment.
The number of cells expressing GFP in the forespore was determined as a percentage of the total number of cells expressing GFP.
TABLE 3.
Sporulation frequency of parent strain and spoIIE mutants
| Strain | Relevant genotype | Avg no. heat survivors, CFU (±SD)/ml | Heat survivors as % of parent value |
|---|---|---|---|
| BR151 | spo+ | 7.4 (± 0.4) × 108 | 100 |
| SL10767 | spoIIE48 | 5.3 (± 4.0) × 101 | 0.0000074 ± 0.0000057 |
| SL11173 | spoIIEV697A | 4.5 (± 0.8) × 107 | 6.2 ± 1.5 |
| SL11201 | spoIIE48 spoIIEV697A | 5.9 (± 1.0) × 108 | 71 ± 18 |
spoIIEV697A uncouples σF activation from polar division.
div-355 is a conditional mutation in the essential cell division gene divIC. This mutation is very useful because it allows vegetative division to occur at 37°C but blocks asymmetric division during sporulation at that temperature (21). Therefore, it would allow us to test our hypothesis that spoIIEV697A activates σF in the absence of polar division without the complication of the approximately 10% of cells that undergo asymmetric division in the population (Fig. 1). We constructed a double mutant strain with div-355 and spoIIEV697A as well as a spoIIQ-lacZ transcriptional fusion (SL10423). In addition, we also generated isogenic strains with the mutations singly (SL10312 with div-355 and SL10339 with spoIIEV697A). These three strains, along with the spo+ parent also containing the spoIIQ-lacZ fusion (SL8603), were induced to sporulate in MSSM, and samples were taken for analysis of β-galactosidase activity. This experiment was performed at 37°C, a temperature that we had previously determined was permissive for vegetative division but nonpermissive for asymmetric division for the strain with the div-355 mutation in the BR151 background (data not shown).
As in a previous experiment (Fig. 2), we observed that the spoIIQ-lacZ fusion became active at T2.5 and specific β-galactosidase activity reached a peak of between 15 and 25 nmol of ONPG hydrolyzed per min per mg in the spo+ parent (Fig. 3A). This stands in stark contrast to the div-355 mutant, in which β-galactosidase activity was completely abolished (Fig. 3A). The spoIIEV697A mutation in the wild-type divIC background displayed the same pattern as described in a previous experiment (Fig. 2), becoming active slightly earlier (at T2) and with β-galactosidase activity reaching a far higher level (at least 300 nmol of ONPG hydrolyzed per min per mg at T3.5) than with the spo+ parent (Fig. 3B). The div-355 spoIIEV697A double mutant had a pattern of spoIIQ-lacZ activity that was not significantly different from that of the spoIIEV697A single mutant (Fig. 3B). From these results, we can conclude that the spoIIEV697A mutation uncouples σF activation from asymmetric division, allowing σF to become active even when division is blocked.
FIG. 3.
Expression of spoIIQ-lacZ in strains containing mutations in spoIIE and/or divIC. (A) Expression in the spoIIE+ divIC+ parent strain SL10174 (solid squares) and the spoIIEV697A div-355 mutant SL10312 (solid triangles). (B) Expression in the spoIIEV697A divIC+ mutant SL10339 (open squares) and in the spoIIEV697A div-355 mutant SL10423 (open triangles). Specific activity units are nanomoles of ONPG hydrolyzed per minute per milligram of bacterial dry weight. The results are the averages of three independent experiments plus and minus the standard deviation.
spoIIEV697A is an intragenic suppressor of spoIIE48.
Based on the previous experiments, we strongly suspected that spoIIEV697A bypassed the developmental checkpoint coupling σF activation to asymmetric division (Fig. 3B). As an independent test to confirm this, we wanted to determine if this mutation could restore sporulation to a mutant that is normally incapable of passing this checkpoint. Such a mutant, carrying spoIIE48 (spoIIES361F), has been described as being capable of substantially dephosphorylating SpoIIAA but unable to activate σF and as a consequence is severely impaired in spore formation (19).
To test this possibility, the spo+ parent, the spoIIE48 mutant (SL10767), the spoIIEV697A mutant (SL11173), and the spoIIE48 spoIIEV697A double mutant (SL11201) were induced to sporulate in MSSM, and their ability to form spores was analyzed by heat survival. It is important to note that SL11201 contains a single copy of the spoIIE gene harboring both mutations. Whereas the spoIIE48 mutant was severely impaired in sporulation (53 ± 40 spores per ml [Table 3]), the spoIIE48 spoIIEV697A double mutant was Spo+, producing almost as many spores as the spo+ parent (5.9 × 108 ± 1.0 × 108, compared to 7.4 × 108 ± 0.4 × 108 spores per ml [Table 3]). In turn, the double mutant produced nearly 10 times as many spores as the spoIIEV697A single mutant (5.9 × 108 ± 1.0 × 108 compared to 4.5 × 107 ± 0.8 × 107 spores per ml [Table 3]). These results indicate that spoIIEV697A can function as an intragenic suppressor of spoIIE48. Since spoIIE48 is normally incapable of activating σF in response to asymmetric division, this provides further evidence that spoIIEV697A severely interferes with the checkpoint coupling the two events.
Reduction in asymmetric division in the spoIIEV697A mutant is dependent upon the spoIIA operon but independent of σE activity.
We had separately observed uncompartmentalized σF activity (Table 2) and a severe reduction in asymmetric division (Fig. 2) in the spoIIEV697A mutant. We wanted to investigate the possibility that these two phenotypes were related. It had been determined previously that activation of the mother cell-specific transcription factor σE is required to block a second asymmetric division from occurring in the mother cell (15). σE is synthesized as an inactive precursor, pro-σE, which is proteolytically cleaved into the active form by the inferred aspartyl protease SpoIIGA (20, 39). This activation is coupled to asymmetric division because expression of spoIIR directed by σF is required for proteolysis to occur (17, 25). However, uncoupling spoIIR expression from asymmetric division causes uncompartmentalized activation of σE in some cells (41, 46). A different study has demonstrated that predivisional expression of three σE-dependent genes, spoIID, spoIIM, and spoIIP, impairs asymmetric division (10).
Taken together, these results suggest that one possible cause of the defect in asymmetric division observed in the spoIIEV697A mutant would be predivisional transcription of spoIIR leading to premature activation of σE. In order to test this possibility, we constructed a spoIIGB::erm (13) spoIIEV697A double mutant (SL11132). This strain was induced to sporulate in MSSM along with the isogenic single mutant carrying spoIIGB::erm (SL8410). Samples were stained with FM4-64 and scored for the presence of asymmetric septa. Whereas 55% of the spoIIGB::erm single-mutant cells exhibited asymmetric septa by T5, they were present in less than 12% of the spoIIGB::erm spoIIEV697A double-mutant cells at all times analyzed (Fig. 4A). Comparison of the frequency of asymmetric division in the spoIIEV697A mutant with that in the spoIIGB::erm spoIIEV697A double mutant revealed no significant differences at any time analyzed (data not shown). We conclude that predivisional expression of σE-dependent genes inhibitory to asymmetric division is not responsible for the deficiency in asymmetric division observed in the spoIIEV697A mutant. Consistent with this conclusion, we have observed that transcription from the σE-dependent cotEP1 (8) and spoIID (36) promoters is drastically reduced in the spoIIEV697A mutant, making it unlikely that any aspect of the spoIIEV697A phenotype is due to inappropriate σE activity (data not shown).
FIG. 4.
Frequency of asymmetric division during sporulation in strains containing mutations in spoIIE, spoIIGB, and/or spoIIA. (A) Frequency of asymmetric division in the spoIIGB::erm mutant SL8410 (solid diamonds) and in the spoIIEV697A spoIIGB::erm mutant SL11132 (open diamonds). (B) Frequency of asymmetric division in the spoIIAΔ4 mutant SL9055 (solid squares) and in the spoIIEV697A spoIIAΔ4 mutant SL10905 (open squares). (C) Frequency of asymmetric division in the spoIIAC::neo mutant SL11274 (solid triangles) and the spoIIEV697A spoIIAC::neo mutant SL11275 (open triangles). (D) Frequency of asymmetric division in the spoIIAC561 mutant SL1102 (solid circles) and in the spoIIEV697A spoIIAC561 mutant SL11142 (open circles). Fifty cells were scored at each time. The results are the averages of three independent experiments plus and minus the standard deviation.
From previous studies, we had speculated that SpoIIE had a role in promoting asymmetric division (5, 18, 23, 26). The mutation that we isolated (spoIIEV697A), caused deficiency in asymmetric division but also excessive, uncompartmentalized σF activity (Fig. 1 and 2, Table 3). We wanted to determine whether the asymmetric division phenotype was directly attributable to the loss of a specific division function encoded by spoIIE or if it was a consequence of the hyper-σF activity. In order to do this, we used a spoIIAΔ4 mutant (33), carrying a deletion of the spoIIA operon encoding the structural gene for σF, spoIIAC, as well as the spoIIAA and spoIIAB genes, encoding regulators of σF (34). The spoIIEV697A spoIIAΔ4 double mutant (SL10905) and the single spoIIAΔ4 mutant (SL9055) were induced to sporulate in MSSM, and samples were stained with FM4-64 and scored for the presence of asymmetric septa.
Compared to the spoIIAΔ4 single mutant, the spoIIEV697A spoIIAΔ4 double mutant exhibited no significant reduction in frequency of asymmetric division and actually demonstrated a modest increase at several times after T3 (Fig. 4B). Whereas rarely more than 10% of the cells in the spoIIEV697A single-mutant population exhibited asymmetric septa at the times analyzed (Fig. 1), the spoIIEV697A spoIIAΔ4 double-mutant cells underwent asymmetric division at a much higher frequency, reaching 50% by T4.5 and greater than 65% by T6 (Fig. 4B). There was at least a fivefold increase in the frequency of asymmetric division in the spoIIEV697A spoIIAΔ4 double mutant compared to the spoIIEV697A single mutant at all times analyzed after T4 (Fig. 1 and 4B). These results indicate that the deficiency in asymmetric division observed in the spoIIEV697A mutant is dependent upon some product of the spoIIA operon.
Deletion of spoIIAC but not a point mutation that largely abolishes promoter recognition by σF restores asymmetric division in a spoIIEV697A mutant.
We thought it was most likely that the asymmetric division phenotype in the spoIIEV697A mutant was being mediated by σF because the major role for the other products of the spoIIA operon, SpoIIAA and SpoIIAB, is to regulate its activity (34). In order to test this possibility, we utilized a spoIIAC::neo deletion, which does not interfere with either the spoIIAA or the spoIIAB open reading frame. We constructed a spoIIAC::neo single mutant (SL11274) and a spoIIEV697A spoIIAC::neo double mutant (SL11275) and induced them to sporulate in MSSM. Samples were stained with FM4-64 and scored for the presence of asymmetric septa. The frequency of asymmetric septation in both strains was very similar, reaching a peak of about 40% (Fig. 4C). This was a dramatic increase over that of the spoIIEV697A single mutant, in which the frequency rarely exceeded 10% of the population (Fig. 1). At all times analyzed after T4, the frequency of asymmetric septation in the spoIIEV697A spoIIAC::neo double mutant was at least threefold higher than that in the spoIIEV697A single mutant (Fig. 1 and 4C). This indicates that the block in division is indeed mediated by σF.
We next wished to address the question of why hyperactivation of σF might inhibit asymmetric division. The most likely explanation seemed that its activity as a sigma factor would lead to hyperexpression of some gene whose product inhibits division. We refined the analysis further by testing the effect of the spoIIAC561 mutation (15). This mutation causes a V233M change in the 4.2 promoter recognition region of σF (45). As a consequence, most σF-directed promoters are not recognized, although at least one σF-controlled gene, spoIIR, becomes hyperexpressed (17).
We used a spoIIAC561 mutant (SL1102) as well as a spoIIEV697A spoIIAC561 double mutant (SL11142) and induced these strains to sporulate in MSSM. Samples were stained with FM4-64 and scored for the presence of asymmetric septa. Whereas the spoIIAC561 mutant formed asymmetric septa at a high frequency, reaching nearly 50% by T5, the frequency in the spoIIEV697A spoIIAC561 double mutant population rarely exceeded 10% (Fig. 4D). Compared to the spoIIAC561 mutant, the spoIIEV697A spoIIAC561 double mutant exhibited a reduction of between three- and eightfold in asymmetric division between T3 and T5. The frequency of asymmetric division in the spoIIEV697A single mutant and the spoIIEV697A spoIIAC561 double mutant was not significantly different at any time analyzed (Fig. 1 and 4D). This indicates that the block in division observed in the spoIIEV697A mutant results either from expression of one of the σF-dependent genes whose promoter is still recognized by σF561 or from interaction of σF with some other factor.
DISCUSSION
The exact mechanism by which B. subtilis switches from a medial division site to a polar one during sporulation remains unclear. There is genetic evidence that both a burst of ftsZ transcription and the expression of SpoIIE are required for this switch to occur (6, 18). However, the mechanism by which SpoIIE facilitates asymmetric division remains unknown. Here we report the isolation and characterization of a mutation, spoIIEV697A, that causes a severe impairment in the formation of polar septa during sporulation. However, it appears that this impairment is indirect, mediated via σF in the spoIIEV697A mutant. This is consistent with previous observations that deletion of the gene encoding the anti-sigma factor SpoIIAB caused unregulated σF activity and a concomitant block in asymmetric division (7). Although our findings do not provide additional evidence for a direct role of SpoIIE in promoting asymmetric division, they suggest that one function of SpoIIE may be to delay σF activation until after polar division is complete, thereby tightly coupling these two events to ensure the proper spatial and temporal expression of forespore-specific genes.
SpoIIE has a C-terminal PP2C-like phosphatase domain that has a well-characterized role in activation of the forespore-specific transcription factor σF (1, 2, 9, 38). SpoIIE dephosphorylates, and thus activates the anti-anti-sigma factor SpoIIAA, which can then relieve inhibition of σF by the anti-sigma factor SpoIIAB (2, 9). In turn, SpoIIAB is a serine kinase that can phosphorylate, and thus inactivate, SpoIIAA (29). Although the biochemistry underlying these interactions is reasonably well understood, it is not yet clear why σF becomes active only after asymmetric division and only in the forespore (34). It has been proposed that SpoIIE itself is regulated by a number of different mechanisms in order to explain the temporal and spatial regulation of σF activity.
Two broad models have emerged. In the first, SpoIIE is inactive in its default state and is activated by interaction with cell division proteins (19). In the second, SpoIIE is active in the default state and is negatively regulated to prevent inappropriate σF activation (3). The first, activator, model was developed from three lines of research (19). It was demonstrated that the early cell division protein FtsZ was required for SpoIIE to efficiently dephosphorylate SpoIIAA. Next, it was demonstrated that the div-355 mutation of divIC allows efficient dephosphorylation of SpoIIAA by SpoIIE but not activation of σF. Lastly, the spoIIE48 (spoIIES361F) mutant was found to have the same phenotype with regards to σF activation as the div-355 mutant (i.e., dephosphorylation of SpoIIAA but no σF activation) (19). These results suggested a regulatory step in which SpoIIE prevents dephosphorylated SpoIIAA from attacking the SpoIIAB-σF complex and activating σF until asymmetric division is complete (19).
The second, repressor, model is based on studies utilizing a spoIIE mutant, in which the region encoding the N-terminal transmembrane domains has been deleted, rendering the protein cytoplasmic. Surprisingly, the mutant protein supported relatively high levels of asymmetric division, compartmentalization of σF activity, and sporulation (3). The authors concluded that there is a cytoplasmic inhibitor of SpoIIE that is capable of interacting with and regulating (albeit less efficiently) the cytoplasmic form of SpoIIE. Indeed, the in vitro phosphatase activity of SpoIIE, which activates SpoIIAA, is 100 times stronger than the kinase activity of SpoIIAB, which inactivates it; since the levels of the SpoIIE and SpoIIAB proteins are very similar during the early part of sporulation, an additional factor may be involved in negatively regulating the phosphatase activity of SpoIIE (27).
The spoIIEV697A mutation does not help us to distinguish between the two models. Consistent with the first, the mutant protein no longer needs activation by a division component and activates σF in the div-355 background. This mutant is the converse of another phosphatase-competent mutant, SpoIIE48, which cannot activate σF even when division occurs. The SpoIIEV697A phenotype is reminiscent of a hybrid MalF′-′SpoIIE protein in which the SpoIIE transmembrane domains are replaced by the two MalF transmembrane domains. The hybrid protein strongly activated σF and could do so in a div-355 background (19). However, consistent with the second model, the mutant protein could be thought of as an inhibitor-resistant form of SpoIIE. Furthermore, a combination of the two models is also possible, with SpoIIE being subject to both positive and negative regulation and SpoIIEV697A being insensitive to just one type of regulation. This might explain why spoIIEV697A causes a milder phenotype than an in-frame spoIIAB deletion (which also causes hyper-σF activity) (7). Consistent with the idea that the spoIIEV697A mutant is less sensitive to some regulator, we found that the spoIIEV697A allele is trans-dominant to wild-type spoIIE as well as to the loss-of-function alleles spoIIE48 and spoIIE64 (unpublished observations).
The effect of the spoIIEV697A mutation on sporulation division is mediated by σF. This is indicated by our observation that deletion of the spoIIA operon and of spoIIAC, the structural gene for σF, largely overcame the effect of the spoIIEV697A mutation on sporulation division. We showed that the effect was not a secondary consequence of σE activation. We also determined that if the effect was caused by expression of a σF-dependent gene, it must be one that continues to be expressed in a spoIIAC561 mutant, in which expression of most known σF-dependent genes is abolished. The σF protein interacts with core RNA polymerase and also with the SpoIIAB protein. Interaction of σF with either (or with some unknown protein), rather than its role as a sigma factor, could somehow affect septation. We saw no impairment of transcription of the key division gene ftsZ (unpublished observations) as a possible consequence of an increase in σF sequestering core polymerase but cannot exclude an effect on transcription elsewhere. SpoIIAB has a pivotal role in σF activation (34), and it may be part of the link between activation and septation. The presence of σF can affect SpoIIAB stability (30). Although speculative, the presence of the mutant σF protein might affect septation through its interaction with SpoIIAB.
Feucht et al. (11) recently described mutations in a different region of spoIIE, the hinge region mutations spoIIEG334R and spoIIEQ344P, which give rise to a phenotype similar to that of the spoIIEV697A mutant in that they dissociate σF activation from septation, cause uncompartmentalized hyper-σF activity, and impair spore formation. The purified mutant proteins had in vitro phosphatase activity similar to that of wild-type SpoIIE, and it was concluded that the mutant proteins were refractory to a regulatory step unrelated to their phosphatase activity (11). The hinge region is involved in oligomerization and in the interaction with FtsZ (26), but the mutants retained the ability to localize to a septum (11). It is possible that the hinge region, located close to the membrane, is subject to one type of regulation, whereas the phosphatase domain, which is thought to project into the cytoplasm (4), is subject to a distinct cytoplasmic regulator. Loss of response to either type of regulator may result in an effect similar to that seen here with the phosphatase domain V697A mutation and by Feucht et al. (11) with the hinge domain G334R and Q344P mutations. Consistent with the idea of interdependence of regulation of the domains, we observed that a strain with a single copy of spoIIE containing both the spoIIE48 mutation (hinge domain, no σF activation) and the spoIIEV697A mutation was Spo+. In addition, the spoIIEV697A mutation has been independently isolated as a spontaneous suppressor of a hinge spoIIE mutation associated with a phenotype identical to that of the spoIIE48 mutant (K. Carniol and R. Losick, personal communication).
Our data, and those of Feucht et al. (11) and Carniol and Losick, strongly support the general theme that regulation of SpoIIE plays a crucial role in both coupling σF activation to asymmetric division and confining σF activity to the forespore. These studies reinforce the concept that precise coupling of asymmetric division to σF activation is critical for efficient spore formation.
Acknowledgments
We thank Karen Carniol, Vasant Chary, Nicole King, and Rich Losick for helpful discussions. We thank Karen Carniol and Rich Losick for communicating results prior to publication and for kindly providing the div-355 mutant. We also thank Edward Amaya, Vasant Chary, and Jeff Errington for kindly providing plasmids.
This work was supported by Public Health Service grant GM43577 (to P.J.P.) and training grant T32AI07101 (to D.W.H.) from the National Institutes of Health.
REFERENCES
- 1.Adler, E., A. Donella-Deana, F. Arigoni, L. A. Pinna, and P. Stragier. 1997. Structural relationship between a bacterial developmental protein and eukaryotic PP2C protein phosphatases. Mol. Microbiol. 23:57-62. [DOI] [PubMed] [Google Scholar]
- 2.Arigoni, F., L. Duncan, S. Alper, R. Losick, and P. Stragier. 1996. SpoIIE governs the phosphorylation state of a protein regulating transcription factor sigma F during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arigoni, F., A. Guérout-Fleury, I. Barák, and P. Stragier. 1999. The SpoIIE phosphatase, the sporulation septum and the establishment of forespore-specific transcription in Bacillus subtilis: a reassessment. Mol. Microbiol. 31:1407-1415. [DOI] [PubMed] [Google Scholar]
- 4.Barak, I., J. Behari, G. Olmedo, P. Guzman, D. P. Brown, E. Castro, D. Walker, J. Westpheling, and P. Youngman. 1996. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol. Microbiol. 19:1047-1060. [DOI] [PubMed] [Google Scholar]
- 5.Barák, I., and P. Youngman. 1996. SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual function for the SpoIIE protein. J. Bacteriol. 178:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Yehuda, S., and R. Losick. 2002. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell 109:257-266. [DOI] [PubMed] [Google Scholar]
- 7.Coppolecchia, R., H. DeGrazia, and C. P. Moran, Jr. 1991. Deletion of spoIIAB blocks endospore formation in Bacillus subtilis at an early stage. J. Bacteriol. 173:6678-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diederich, B., K. M. Tatti, C. H. Jones, B. Beall, and C. P. Moran, Jr. 1992. Genetic suppression analysis of σE interaction with three promoters in sporulating Bacillus subtilis. Gene 121:63-69. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, L., S. Alper, F. Arigoni, R. Losick, and P. Stragier. 1995. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science 270:641-644. [DOI] [PubMed] [Google Scholar]
- 10.Eichenberger, P., P. Fawcett, and R. Losick. 2001. A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol. Microbiol. 42:1147-1162. [DOI] [PubMed] [Google Scholar]
- 11.Feucht, A., L. Abbotts, and J. Errington. 2002. The cell differentation protein SpoIIE contains a regulatory site that controls its phosphatase activity in response to asymmetric septation. Mol. Microbiol. 45:1119-1130. [DOI] [PubMed] [Google Scholar]
- 12.Feucht, A., R. A. Daniel, and J. Errington. 1999. Characterization of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis. Mol. Microbiol. 33:1015-1026. [DOI] [PubMed] [Google Scholar]
- 13.Henriques, A. O., E. M. Bryan, B. W. Beall, and C. P. Moran, Jr. 1997. cse15, cse60, and csk22 are new members of mother cell-specific sporulation regulons in Bacillus subtilis. J. Bacteriol. 179:389-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch, J. A. 1991. Genetic analysis in Bacillus subtilis. Methods Enzymol. 204:305-320. [DOI] [PubMed] [Google Scholar]
- 15.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of σE and σF in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 17:4410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karow, M. L., P. Glaser, and P. J. Piggot. 1995. Identification of a gene, spoIIR, that links the activation of σE to the transcriptional activity of σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 92:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khvorova, A., L. Zhang, M. L. Higgins, and P. J. Piggot. 1998. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J. Bacteriol. 180:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King, N., O. Dreesen, P. Stragier, K. Pogliano, and R. Losick. 1999. Septation, dephosphorylation, and the activation of σF during sporulation in Bacillus subtilis. Genes Dev. 13:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBell, T. L., J. E. Trempy, and W. G. Haldenwang. 1987. Sporulation specific σ factor σ29 of Bacillus subtilis is synthesized from precursor protein, P31. Proc. Natl. Acad. Sci. USA 43:1784-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin, P. A., and R. Losick. 1994. Characterization of a cell division gene from Bacillus subtilis that is required for vegetative and sporulation septum formation. J. Bacteriol. 176:1451-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin, P. A., and R. Losick. 1996. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 10:478-488. [DOI] [PubMed] [Google Scholar]
- 23.Levin, P. A., R. Losick, P. Stragier, and F. Arigoni. 1997. Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol. Microbiol. 25:839-846. [DOI] [PubMed] [Google Scholar]
- 24.Londõno-Vallejo, J. A., C. Fréhel, and P. Stragier. 1997. spoIIQ, a forespore expressed gene required for engulfment in Bacillus subtilis. Mol. Microbiol. 24:29-39. [DOI] [PubMed] [Google Scholar]
- 25.Londõno-Vallejo, J. A., and P. Stragier. 1995. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 9:503-508. [DOI] [PubMed] [Google Scholar]
- 26.Lucet, I., A. Feucht, M. D. Yudkin, and J. Errington. 2000. Direct interaction between the cell division protein FtsZ and the differentiation protein SpoIIE. EMBO J. 19:1467-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucet, I., R. Borriss, and M. D. Yudkin. 1999. Purification, kinetic properties, and intracellular concentration of SpoIIE, an integral membrane protein that regulates sporulation in Bacillus subtilis. J. Bacteriol. 181:3242-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, W. G., and S. E. Lindow. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149-153. [DOI] [PubMed] [Google Scholar]
- 29.Min, K. T., C. M. Hilditch, B. Diederich, J. Errington, and M. D. Yudkin. 1993. σF, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-σ factor that is also a protein kinase. Cell 74:735-742. [DOI] [PubMed] [Google Scholar]
- 30.Pan, Q., D. A. Garsin, and R. Losick. 2001. Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-sigma factor in B. subtilis. Mol. Cell 8:873-883. [DOI] [PubMed] [Google Scholar]
- 31.Piggot, P. J. 1973. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporulation operons. J. Bacteriol. 114:1241-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piggot, P. J., and C. A. M. Curtis. 1987. Analysis of the regulation of gene expression during Bacillus subtilis sporulation by manipulation of the copy number of spo-lacZ fusions. J. Bacteriol. 169:1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piggot, P. J., C. A. M. Curtis, and H. DeLencastre. 1984. Use of integrational plasmid vectors to demonstrate the polycistronic nature of a transcriptional unit (spoIIA) required for sporulation of Bacillus subtilis. J. Gen. Microbiol. 130:2123-2136. [DOI] [PubMed] [Google Scholar]
- 34.Piggot, P., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A.L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 35.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-DeMello, A. Perez, Y.L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong, S., M. S. Rosenkrantz, and A. L. Sonenshein. 1986. Transcriptional control of the Bacillus subtilis spoIID gene. J. Bacteriol. 165:771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeter, R., S. Schlisio, I. Lucet, M. D. Yudkin, and R. Borriss. 1999. The Bacillus subtilis regulator protein SpoIIE shares functional and structural similarities with eukaryotic protein phosphatases 2C. FEMS Microbiol. Lett. 174:117-123. [DOI] [PubMed] [Google Scholar]
- 39.Shazand, K., Frandsen, N., and P. Stragier. 1995. Cell-type specificity during development in Bacillus subtilis: the molecular and morphological requirements for σE activation. EMBO J. 14:1439-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 41.Stragier,P., C., Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 43:697-704. [DOI] [PubMed] [Google Scholar]
- 42.Sun, D. X., R. M. Cabrera-Martinez, and P. Setlow. 1991. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma G. J. Bacteriol. 173:2977-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, X., and J. Lutkenhaus. 1991. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol. Microbiol. 9:435-442. [DOI] [PubMed] [Google Scholar]
- 44.Wu, L. J., A. Feucht, and J. Errington. 1998. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev. 12:1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yudkin, M. D. 1987. Structure and function in Bacillus subtillis sporulation-specific sigma factor: molecular nature of mutations in spoIIAC. J. Gen. Microbiol. 133:475-481. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, L., M. L. Higgins, P. J. Piggot and M.L. Karow. 1996. Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J. Bacteriol. 178:2813-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]