Abstract
Numerous conventional vaccines for animal use are currently available, and many of these vaccines have been instrumental in the control of infectious diseases of major economic importance. A vaccine has even been instrumental in global eradication of smallpox, an important human disease. However, many of the current vaccines are deficient in efficiency, potency, or safety. It has been recognized that the conventional methodologies are a limitation to further vaccine development. Introduction of monoclonal antibodies, recombinant DNA, and protein engineering techniques has facilitated a rather rapid increase in the knowledge of pathogenetic mechanisms, as well as of protective antigens at the molecular level. This knowledge provides the basis for development of a new generation of vaccines. As a rule, these vaccines contain purified immunogens, or even isolated epitopes, identified and prepared by molecular biological techniques. The efforts to find better delivery systems and better adjuvants accompany the research on vaccines.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Ardman B., Khiroya R. H., Schwartz R. S. Recognition of a leukemia-related antigen by an antiidiotypic antiserum to an anti-gp70 monoclonal antibody. J Exp Med. 1985 Apr 1;161(4):669–686. doi: 10.1084/jem.161.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R., Maron E., Sela M., Anfinsen C. B. Antibodies reactive with native lysozyme elicited by a completely synthetic antigen. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1450–1455. doi: 10.1073/pnas.68.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Burdette S., Schwartz R. S. Current concepts: immunology. Idiotypes and idiotypic networks. N Engl J Med. 1987 Jul 23;317(4):219–224. doi: 10.1056/NEJM198707233170407. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Viral and bacterial vectors of immunogenes. Vaccine. 1985 Mar;3(1):45–48. doi: 10.1016/0264-410X(85)90011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. M., Kawaoka Y., Webster R. G. Protection of chickens from lethal influenza infection by vaccinia-expressed hemagglutinin. Virology. 1988 Dec;167(2):414–421. [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kennedy R. C. Monoclonal anti-idiotypic antibody mimics the CD4 receptor and binds human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3891–3895. doi: 10.1073/pnas.84.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Kennedy R. C. Anti-idiotypic antibodies as immunogens: idiotype-based vaccines. Vaccine. 1988 Jun;6(3):215–220. doi: 10.1016/0264-410x(88)90213-7. [DOI] [PubMed] [Google Scholar]
- De B. K., Shaw M. W., Rota P. A., Harmon M. W., Esposito J. J., Rott R., Cox N. J., Kendal A. P. Protection against virulent H5 avian influenza virus infection in chickens by an inactivated vaccine produced with recombinant vaccinia virus. Vaccine. 1988 Jun;6(3):257–261. doi: 10.1016/0264-410x(88)90221-6. [DOI] [PubMed] [Google Scholar]
- Dougan G., Highfield P. Molecular biology, the new genetics and vaccine development. Med Lab Sci. 1985 Oct;42(4):393–398. [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A., Giraudon P., Buckland R., Wild F., Lecocq J. P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Edelman R. Vaccine adjuvants. Rev Infect Dis. 1980 May-Jun;2(3):370–383. doi: 10.1093/clinids/2.3.370. [DOI] [PubMed] [Google Scholar]
- Ertl H. C., Finberg R. W. Sendai virus-specific T-cell clones: induction of cytolytic T cells by an anti-idiotypic antibody directed against a helper T-cell clone. Proc Natl Acad Sci U S A. 1984 May;81(9):2850–2854. doi: 10.1073/pnas.81.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Hoch S. P., McCormick J. B., Auperin D., Brown B. G., Castor M., Perez G., Ruo S., Conaty A., Brammer L., Bauer S. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc Natl Acad Sci U S A. 1989 Jan;86(1):317–321. doi: 10.1073/pnas.86.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francotte M., Urbain J. Induction of anti-tobacco mosaic virus antibodies in mice by rabbit antiidiotypic antibodies. J Exp Med. 1984 Nov 1;160(5):1485–1494. doi: 10.1084/jem.160.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell P. G., Moss P. A. Production of cell-mediated immune response to herpes simplex virus by immunization with anti-idiotypic heteroantisera. J Gen Virol. 1985 Aug;66(Pt 8):1801–1804. doi: 10.1099/0022-1317-66-8-1801. [DOI] [PubMed] [Google Scholar]
- Grzych J. M., Capron M., Lambert P. H., Dissous C., Torres S., Capron A. An anti-idiotype vaccine against experimental schistosomiasis. Nature. 1985 Jul 4;316(6023):74–76. doi: 10.1038/316074a0. [DOI] [PubMed] [Google Scholar]
- Hamblin C., Kitching R. P., Donaldson A. I., Crowther J. R., Barnett I. T. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus. III. Evaluation of antibodies after infection and vaccination. Epidemiol Infect. 1987 Dec;99(3):733–744. doi: 10.1017/s0950268800066590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzubai A., Maloney D. G., Levy R. The use of a monoclonal anti-idiotype antibody to study the biology of a human B cell lymphoma. J Immunol. 1981 Jun;126(6):2397–2402. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jolivet M. E., Audibert F. M., Gras-Masse H., Tartar A. L., Schlesinger D. H., Wirtz R., Chedid L. A. Induction of biologically active antibodies by a polyvalent synthetic vaccine constructed without carrier. Infect Immun. 1987 Jun;55(6):1498–1502. doi: 10.1128/iai.55.6.1498-1502.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Eichmann K., Müller I., Wrazel L. J. Vaccination against the intracellular bacterium Listeria monocytogenes with a clonotypic antiserum. J Immunol. 1985 Jun;134(6):4123–4127. [PubMed] [Google Scholar]
- Kennedy R. C., Adler-Storthz K., Henkel R. D., Sanchez Y., Melnick J. L., Dreesman G. R. Immune response to hepatitis B surface antigen: enhancement by prior injection of antibodies to the idiotype. Science. 1983 Aug 26;221(4613):853–855. doi: 10.1126/science.6603657. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R. Enhancement of the immune response to hepatitis B surface antigen. In vivo administration of antiidiotype induces anti-HBs that expresses a similar idiotype. J Exp Med. 1984 Mar 1;159(3):655–665. doi: 10.1084/jem.159.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Melnick J. L., Dreesman G. R. Antibody to hepatitis B virus induced by injecting antibodies to the idiotype. Science. 1984 Mar 2;223(4639):930–931. doi: 10.1126/science.6198721. [DOI] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Mannik M., Williams R. C. Individual Antigenic Specificity of Isolated Antibodies. Science. 1963 Jun 14;140(3572):1218–1219. doi: 10.1126/science.140.3572.1218. [DOI] [PubMed] [Google Scholar]
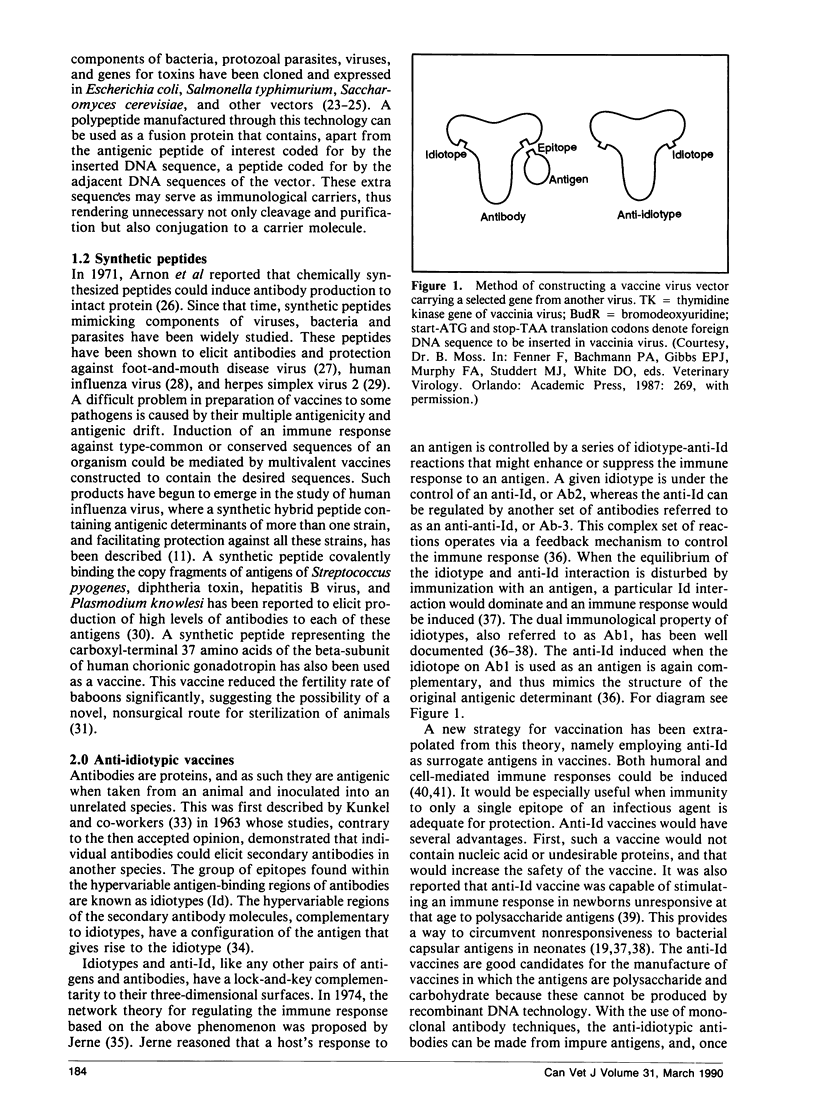
- McFarland M. D., Hill H. T. Vaccination of mice and swine with a pseudorabies virus mutant lacking thymidine kinase activity. Can J Vet Res. 1987 Jul;51(3):340–344. [PMC free article] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- Norrby E., Enders-Ruckle G., Meulen V. Differences in the appearance of antibodies to structural components of measles virus after immunization with inactivated and live virus. J Infect Dis. 1975 Sep;132(3):262–269. doi: 10.1093/infdis/132.3.262. [DOI] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Perez P., Hoffman R. W., Shaw S., Bluestone J. A., Segal D. M. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985 Jul 25;316(6026):354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- Piccini A., Paoletti E. The use of vaccinia virus for the construction of recombinant vaccines. Bioessays. 1986 Dec;5(6):248–252. doi: 10.1002/bies.950050604. [DOI] [PubMed] [Google Scholar]
- Rankin E. M., Hekman A. Mouse monoclonal antibodies against the idiotype of human B cell non-Hodgkin lymphomas: production, characterization and use to monitor the progress of disease. Eur J Immunol. 1984 Dec;14(12):1119–1126. doi: 10.1002/eji.1830141211. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Wunner W. H., Wiktor T. J., Koprowski H. Anti-idiotypic antibodies induce neutralizing antibodies to rabies virus glycoprotein. J Virol. 1983 Dec;48(3):660–666. doi: 10.1128/jvi.48.3.660-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Warren J., Thuning C. A., Fanshaw M. S., Norrild B., Meignier B. Application of molecular genetics to the design of live herpes simplex virus vaccines. Dev Biol Stand. 1982;52:287–304. [PubMed] [Google Scholar]
- Rooney J. F., Wohlenberg C., Cremer K. J., Moss B., Notkins A. L. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988 May;62(5):1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht C. E., Wiktor T. J., Johnston D. H., Hamir A. N., Dietzschold B., Wunner W. H., Glickman L. T., Koprowski H. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7947–7950. doi: 10.1073/pnas.83.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Kirchhoff L. V., Hieny S., Sher A. Molecular mimicry of a carbohydrate epitope on a major surface glycoprotein of Trypanosoma cruzi by using anti-idiotypic antibodies. J Immunol. 1985 Dec;135(6):4155–4159. [PubMed] [Google Scholar]
- Shapira M., Jibson M., Muller G., Arnon R. Immunity and protection against influenza virus by synthetic peptide corresponding to antigenic sites of hemagglutinin. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2461–2465. doi: 10.1073/pnas.81.8.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons K. Anti-idiotype antibodies may do what vaccines don't. JAMA. 1986 Jan 24;255(4):447–448. [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Howard C. R. The potential of synthetic peptides for vaccines. Med Lab Sci. 1985 Oct;42(4):376–387. [PubMed] [Google Scholar]
- Takeda A., Tuazon C. U., Ennis F. A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988 Oct 28;242(4878):580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- Taylor J., Paoletti E. Fowlpox virus as a vector in non-avian species. Vaccine. 1988 Dec;6(6):466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Marchioli C. C., Yancey R. J., Jr, Post L. E. Replication and virulence of pseudorabies virus mutants lacking glycoprotein gX. J Virol. 1987 Jan;61(1):229–232. doi: 10.1128/jvi.61.1.229-232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uytdehaag F. G., Osterhaus A. D. Induction of neutralizing antibody in mice against poliovirus type II with monoclonal anti-idiotypic antibody. J Immunol. 1985 Feb;134(2):1225–1229. [PubMed] [Google Scholar]
- Wachsman M., Aurelian L., Smith C. C., Perkus M. E., Paoletti E. Regulation of expression of herpes simplex virus (HSV) glycoprotein D in vaccinia recombinants affects their ability to protect from cutaneous HSV-2 disease. J Infect Dis. 1989 Apr;159(4):625–634. doi: 10.1093/infdis/159.4.625. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Warren K. S. New scientific opportunities and old obstacles in vaccine development. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9275–9277. doi: 10.1073/pnas.83.24.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Stott E. J., Young K. K., Anderson K., Ball L. A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987 Feb;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilma T., Hsu D., Jones L., Owens S., Grubman M., Mebus C., Yamanaka M., Dale B. Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science. 1988 Nov 18;242(4881):1058–1061. doi: 10.1126/science.3194758. [DOI] [PubMed] [Google Scholar]
- Zhou E. M., Dreesman G. R., Kennedy R. C. Anti-idiotypic antibodies: a new generation of vaccines against infectious agents. Microbiol Sci. 1987 Feb;4(2):36–40. [PubMed] [Google Scholar]


