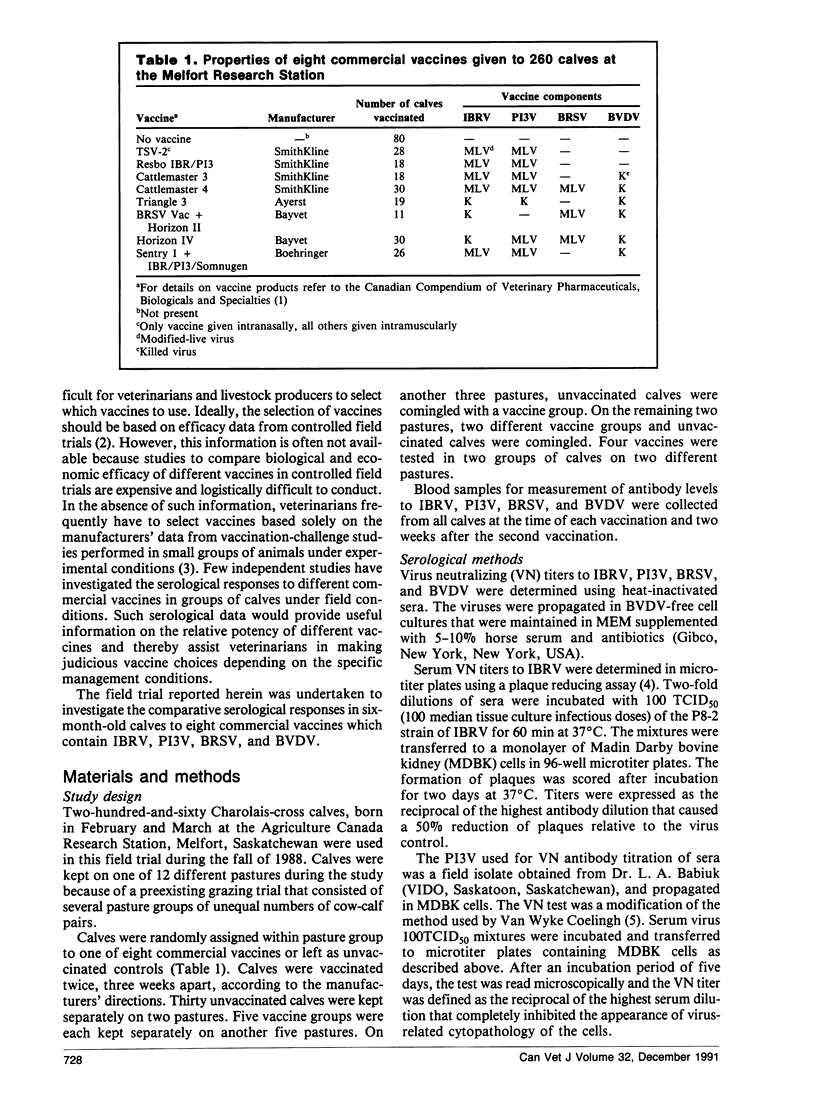
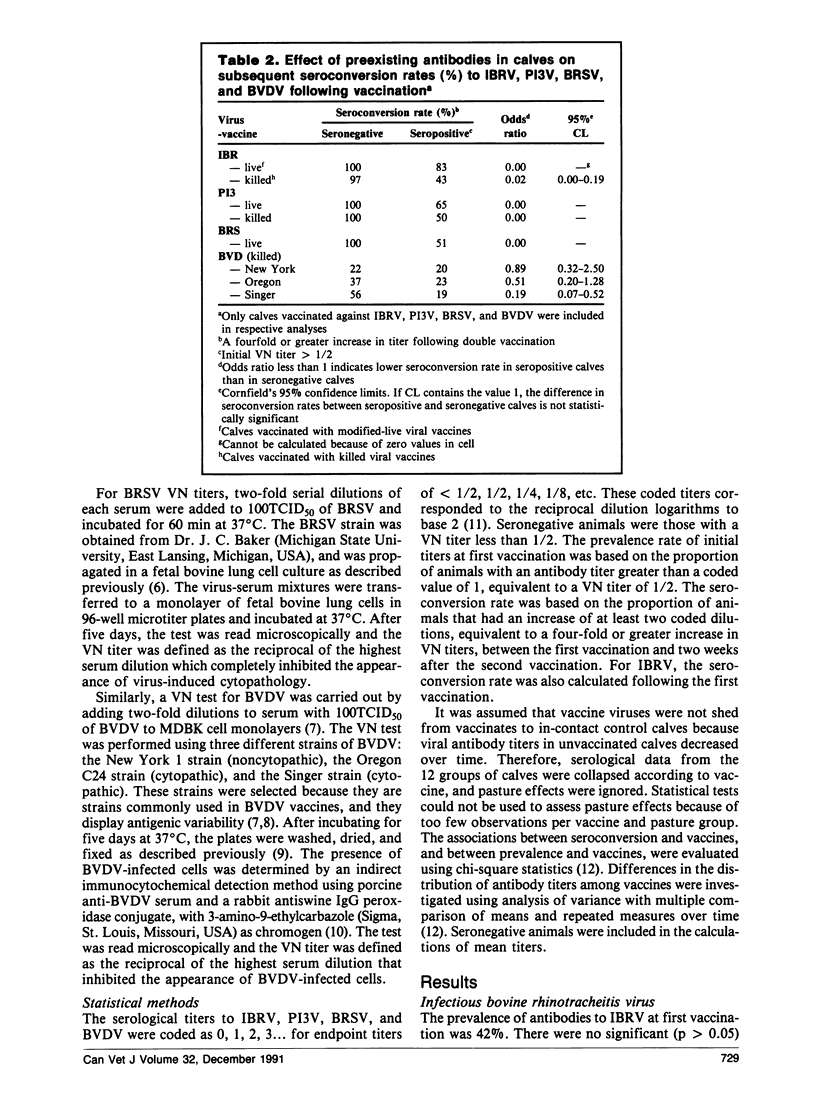
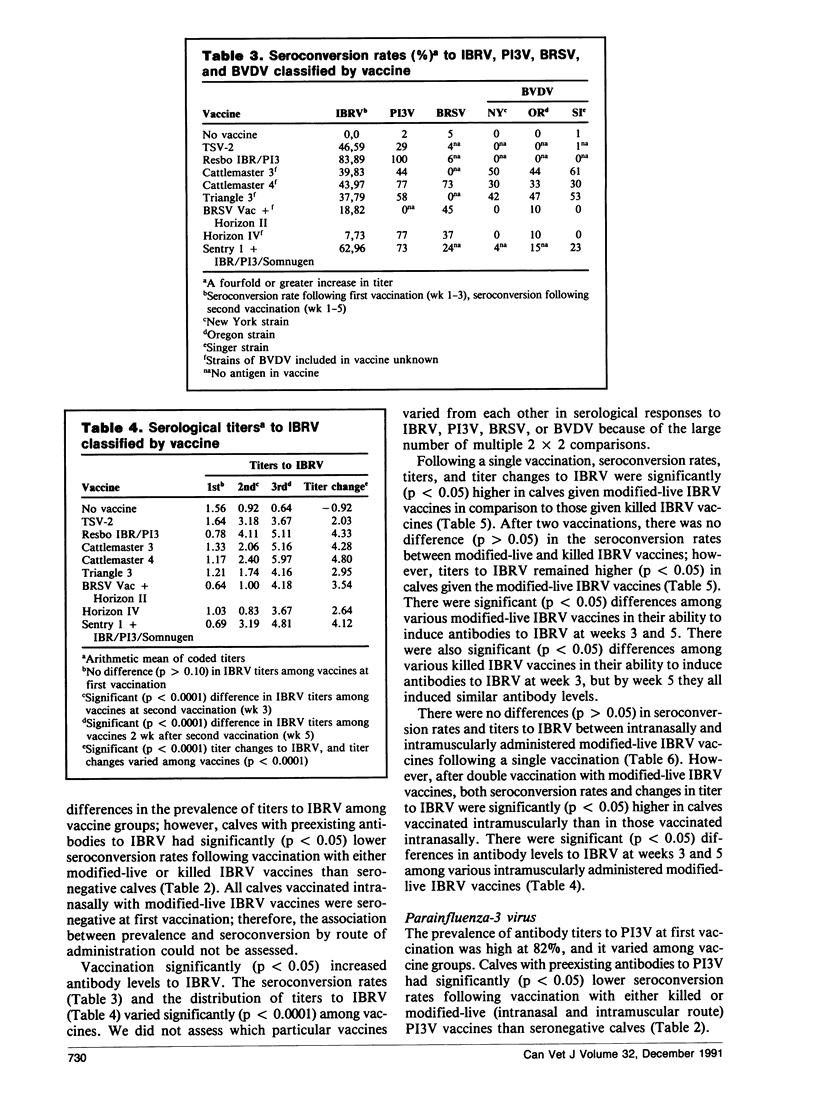
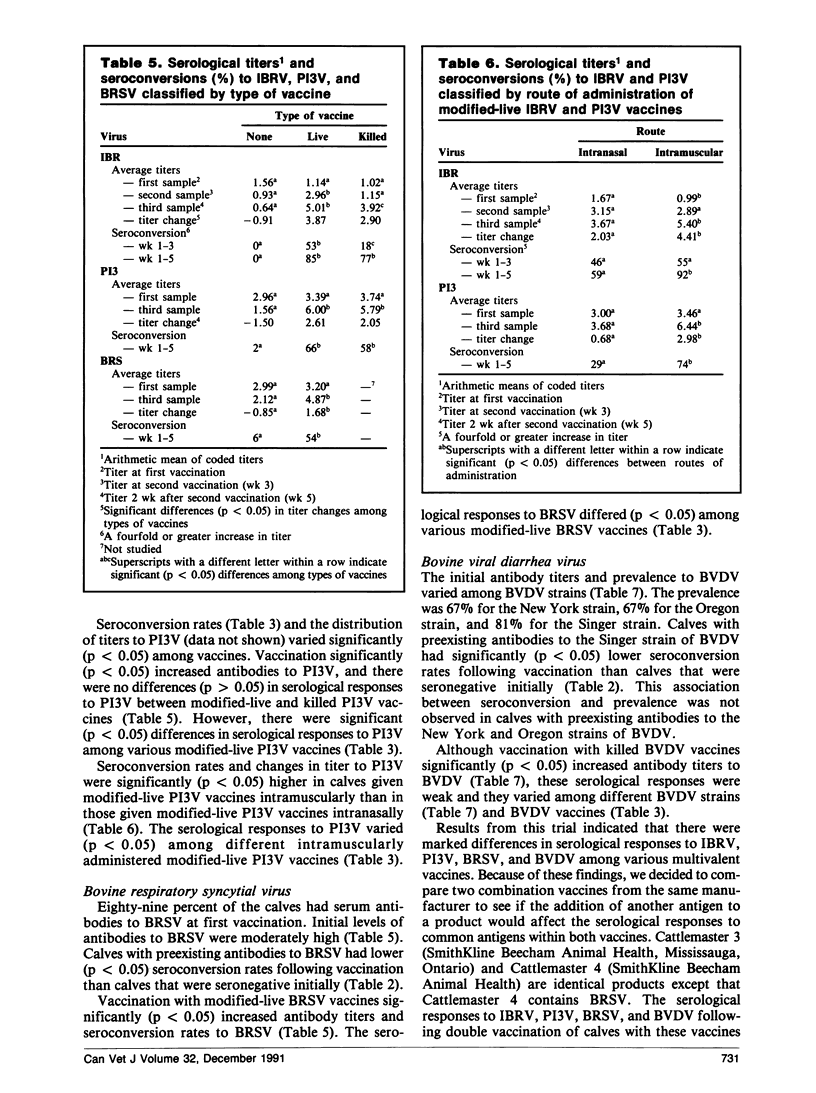
Abstract
A field trial was conducted to compare the serological responses in calves to eight commercial vaccines against infectious bovine rhinotracheitis virus (IBRV), parainfluenza-3 virus (PI3V), bovine respiratory syncytial virus (BRSV), and/or bovine viral diarrhea virus (BVDV). Calves given IBRV, P13V, BRSV, and BVDV vaccines had significantly higher antibodies to these viruses than unvaccinated controls; however, serological responses to killed BVDV vaccines were low. Calves with preexisting antibodies to IBRV, PI3V, BRSV, and the Singer strain of BVDV had lower seroconversion rates following vaccination than calves that were seronegative initially.
Serological responses in calves to IBRV, PI3V, BRSV, and BVDV differed among various commercial vaccines. Antibody titers to IBRV were higher in calves vaccinated with modified-live IBRV vaccines than in those vaccinated with killed IBRV vaccines. Following double vaccination with modified-live IBRV and PI3V vaccines, seroconversion rates and antibody titers to IBRV and PI3V were higher in calves vaccinated intramuscularly than in those vaccinated intranasally. Calves given Cattlemaster 4 had significantly higher titers to BRSV and PI3V, and lower titers to BVDV, than calves given Cattlemaster 3, suggesting that the addition of BRSV to Cattlemaster 4 caused some interaction among antigens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bielefeldt Ohmann H. Double-immunolabeling systems for phenotyping of immune cells harboring bovine viral diarrhea virus. J Histochem Cytochem. 1987 Jun;35(6):627–633. doi: 10.1177/35.6.3033062. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Rønsholt L., Bloch B. Demonstration of bovine viral diarrhoea virus in peripheral blood mononuclear cells of persistently infected, clinically normal cattle. J Gen Virol. 1987 Jul;68(Pt 7):1971–1982. doi: 10.1099/0022-1317-68-7-1971. [DOI] [PubMed] [Google Scholar]
- Durham P. J., Hassard L. E. Prevalence of antibodies to infectious bovine rhinotracheitis, parainfluenza 3, bovine respiratory syncytial, and bovine viral diarrhea viruses in cattle in Saskatchewan and Alberta. Can Vet J. 1990 Dec;31(12):815–820. [PMC free article] [PubMed] [Google Scholar]
- Frerichs G. N., Woods S. B., Lucas M. H., Sands J. J. Safety and efficacy of live and inactivated infectious bovine rhinotracheitis vaccines. Vet Rec. 1982 Aug 7;111(6):116–122. doi: 10.1136/vr.111.6.116. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Brownlie J., Clarke M. C. Comparison by the neutralisation assay of pairs of non-cytopathogenic and cytopathogenic strains of bovine virus diarrhoea virus isolated from cases of mucosal disease. Vet Microbiol. 1987 Apr;13(4):361–369. doi: 10.1016/0378-1135(87)90067-8. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., Babiuk L. A. The effect of dose, route and virulence of bovine herpesvirus 1 vaccine on experimental respiratory disease in cattle. Can J Comp Med. 1983 Apr;47(2):133–139. [PMC free article] [PubMed] [Google Scholar]
- Magar R., Minocha H. C., Montpetit C., Carman P. S., Lecomte J. Typing of cytopathic and noncytopathic bovine viral diarrhea virus reference and Canadian field strains using a neutralizing monoclonal antibody. Can J Vet Res. 1988 Jan;52(1):42–45. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Bateman K. G., Shewen P. E., Rosendal S., Bohac J. E. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res. 1989 Jul;53(3):355–362. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Bohac J. G. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can J Vet Res. 1986 Jul;50(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- McKercher D. G., Crenshaw G. L. Comparative efficacy of intranasally and parenterally administered infectious bovine rhinotracheitis vaccines. J Am Vet Med Assoc. 1971 Dec 1;159(11):1362–1369. [PubMed] [Google Scholar]
- Ribble C. S. Assessing vaccine efficacy. Can Vet J. 1990 Oct;31(10):679–681. [PMC free article] [PubMed] [Google Scholar]
- Schipper I. A., Kelling C. L. Evaluation of inactivated infectious bovine rhinotracheitis vaccines. Can J Comp Med. 1975 Oct;39(4):402–405. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. H., Williams J. M. Development and approval of new vaccines. Can Vet J. 1990 Sep;31(9):617–620. [PMC free article] [PubMed] [Google Scholar]
- Westenbrink F., Brinkhof J. M., Straver P. J., Quak J., De Leeuw P. W. Comparison of a newly developed enzyme-linked immunosorbent assay with complement fixation and neutralisation tests for serology of bovine respiratory syncytial virus infections. Res Vet Sci. 1985 May;38(3):334–340. [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Babiuk L. A. Topographical analysis of bovine herpesvirus type-1 glycoproteins: use of monoclonal antibodies to identify and characterize functional epitopes. Virology. 1985 Jul 15;144(1):216–227. doi: 10.1016/0042-6822(85)90319-8. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., London W. T., Murphy B. R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]


