Abstract
Yeast vacuoles undergo cycles of fragmentation and fusion as part of their transmission to the daughter cell and in response to changes of nutrients and the environment. Vacuole fusion can be reconstituted in a cell free system. We now show that the vacuoles synthesize phosphoinositides during in vitro fusion. Of these phosphoinositides, phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) are important for fusion. Monoclonal antibodies to PI(4,5)P2, neomycin (a phosphoinositide ligand), and phosphatidylinositol-specific phospholipase C interfere with the reaction. Readdition of PI(4,5)P2 restores fusion in each case. Phosphatidylinositol 3-phosphate and PI(3,5)P2 synthesis are not required. PI(4,5)P2 is necessary for priming, i.e., for the Sec18p (NSF)-driven release of Sec17p (α-SNAP), which activates the vacuoles for subsequent tethering and docking. Therefore, it represents the kinetically earliest requirement identified for vacuole fusion so far. Furthermore, PI(4,5)P2 is required at a step that can only occur after docking but before the BAPTA sensitive step in the latest stage of the reaction. We hence propose that PI(4,5)P2 controls two steps of vacuole fusion.
INTRODUCTION
Yeast vacuoles are very dynamic organelles. Their number, size, and shape change not only when vacuoles are transmitted to growing daughter cells, but also when the source of nutrient or other environmental factors (e.g., osmotic conditions) change (Wiemken et al., 1970; Weisman and Wickner, 1988, Conradt et al., 1992; Bone et al., 1998). The changes in number and size can readily be explained by cycles of fragmentation and fusion of vacuoles. A cell free system for the fusion of vacuoles from Saccharomyces cerevisiae has been developed, allowing both morphological and biochemical assays of the reaction (Conradt et al., 1992). Fusion can be quantified conveniently via the proteolytic cleavage and activation of proalkaline phosphatase (located at the luminal side of the vacuolar membrane) by the vacuolar proteinase A (Haas et al., 1994). Using this assay, vacuole fusion could be kinetically dissected into several steps: priming, tethering, docking, and fusion (Conradt et al., 1994; Mayer et al., 1996; Mayer and Wickner, 1997; Ungermann et al., 1998). Priming, tethering, and docking require the ATP-dependent activation of SNAREs through Sec17p (α-SNAP) and Sec18p (NSF) as well as the rab-like GTPase Ypt7p and LMA1 (Xu and Wickner, 1995; Mayer and Wickner, 1997; Xu et al., 1997, 1998; Ungermann et al., 1998). They lead to the formation of v/t-SNARE complexes in trans, i.e., between separate vacuoles, and to the stable association of the membranes.
Phosphatidylinositol phosphates have become a focus of research on intracellular trafficking (for review, see De Camilli et al., 1996; Shepherd et al., 1996; Martin, 1998). These phospholipids have been implicated in multiple trafficking steps and were shown to associate with and regulate different components required for these steps: e.g., the regulation of the activity of small GTPases and their cofactors in vesicle budding (Randazzo and Kahn, 1994; Terui et al., 1994; Roth, 1999) and endosome fusion (Jones and Clague, 1995; Li et al., 1995; Simonsen et al., 1998), regulation of the actin-based cytoskeleton (Fukami et al., 1992), Golgi-to-vacuole transport and formation of multivesicular bodies in yeast (Schu et al., 1993, Stack and Emr, 1994; Odorizzi et al., 1998; Fernandez-Borjaet al., 1999), endocytosis (Singer-Krüger et al., 1998; Wendland and Emr, 1998), and exocytosis (Eberhard et al., 1990; Hay et al., 1995). Biochemical approaches allowed the identification of enzymes for phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) synthesis and a mammalian PI transfer protein, which are required for Ca2+-activated secretion from PC12 cells (Hay and Martin, 1993; Hay et al., 1995). They account for at least part of the requirement for MgATP in the priming step of secretion (Holz et al., 1989; Eberhard et al., 1990). Thus far, the actual function of phosphoinositides in membrane fusion has remained largely enigmatic. PI(4,5)P2 binds specifically and stoichiometrically to the C2B domain of synaptotagmin 1, a protein necessary for fast neurotransmitter release (Schiavo et al., 1996). Moreover, the specific PI(4,5)P2 binding protein CAPS, which is required for the Ca2+-triggered phase of exocytosis (Ann et al., 1997; Loyet et al., 1998), has been isolated. Thus, PI(4,5)P2 may have a direct role in membrane fusion by binding and recruiting specific factors to the fusion site.
Yeast strains with mutations in a phosphatidylinositol 3-phosphate (PI(3)P) 5-kinase (Fab1p) or in a protein with homology to PI 4-kinases (Tor2p) have defects in vacuole inheritance and structure. fab1 mutant cells have enlarged and poorly acidified vacuoles, and the formation and transport of multivesicular bodies to the vacuole is disturbed (Yamamoto et al., 1995; Cooke et al., 1998; Gary et al., 1998; Odorizzi et al., 1998). Furthermore, inositol-(5)-phosphatase mutants, among other morphological abnormalities, have fragmented vacuoles (Srinivasan et al., 1997; Stolz et al., 1998). The Tor2 protein is necessary for cell cycle progression and therefore indispensable. In the tor2 mutant the order of inheritance of vacuoles and nucleus is invariably reversed, leading to a number of buds without vacuoles but with a nucleus (Cardenas and Heitman, 1995). Recent evidence (Schmidt et al., 1996; Helliwell et al., 1998) links this phenotype to the requirement for Tor2p for organization of the actin cytoskeleton, which is also needed for vacuole inheritance in vivo (Hill et al., 1996).
We have investigated the relevance of phosphoinositides for vacuole fusion. We have used the in vitro system for vacuole fusion to assay the synthesis of phosphoinositides on vacuoles and have dissected their requirement for vacuole fusion kinetically.
MATERIALS AND METHODS
The yeast strains BJ 3505 and DKY6281 were described in Haas et al. (1994) and RSY249 in Kaiser and Schekman (1990). FAB1 and fab1Δ strains were from Yamamoto et al., (1995). Deletions of PEP4 and PHO8 were made in these strains as described (Peters and Mayer, 1998). Reagents were described in Mayer et al. (1996), except for the following: Phosphatidylinositol-specific phospholipase C (PLC; Bacillus cereus, from Sigma; Eberhard et al., 1990) was dissolved in phosphate saline (PS) buffer (10 mM PIPES/KOH, pH 6.8, 200 mM sorbitol) with 150 mM KCl. The sample was repeatedly concentrated and diluted with this buffer in a microcon-10 (Amicon, Beverly, MA) device. Aliquots were frozen at −20°C and used within 2 weeks. [γ-32P]ATP was from Hartmann Analytic (Braunschweig, Germany). Neomycin sulfate was from Sigma (St. Louis, MO); PI(4)P and PI(4,5)P2 were from Boehringer Mannheim (Indianapolis, IN); monoclonal antibodies to PI(4)P and to PI(4,5)P2 were from PerSeptive Biosystems (Framingham, MA), monoclonal antibodies to phosphatidylserine (PS1G3) and phosphatidylcholine (JE-1) were generous gifts from Masato Umeda (Department of Inflammation Research, The Tokyo Metropolitan Institute of Medical Science; Nam et al., 1990; Reza et al., 1994; Schuurmanns Stekhoven et al., 1994). All antibodies were purified by chromatography on protein A- or protein G-Sepharose as described (Mayer et al., 1996) and stored in PS buffer with 150 mM KCl (anti-phosphatidylinositol 4-phosphate [PI(4)P] and anti-phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]) or with 750 mM KCl (PS1G3 and JE-1) at −20°C. The immunoblot assay for release of Sec17p was as in Mayer et al. (1996). All experiments were performed in silanized tubes.
Vacuole Fusion
Cytosol preparation, vacuole purification, the reaction buffer, and the standard fusion reaction were as in Mayer et al. (1996). Standard vacuole fusion reactions (Mayer et al.,1996) contained 3 μg of protein of each vacuole type (isolated from strains BJ3505 and DKY6281) in 30–35 μl of reaction mixture (10 mM PIPES/KOH, pH 6.8, 200 mM sorbitol, 150 mM KCl, 0.5 mM MgCl2, 0.5 mM MnCl2, 0.5 mM ATP, 1.5 mg/ml cytosol, 7.5 μM pefablock SC, 15 nM leupeptin, 3.75 μM o-phenanthroline, 50 nM pepstatin A, 20 mM creatine phosphate, and 35 U/ml creatine kinase). The standard reaction time was reduced to 70 min at 27°C. The background signal was considered by subtracting the values of a control sample that was kept at 0°C throughout the incubation. This entirely prevents fusion. Background signals were usually between 0.2 and 0.3 U. One unit of fusion activity is defined as 1 μmol p-nitrophenol developed per minute and per microgram of BJ3505 vacuoles.
Microscopic Assay for Vacuole Docking
The microscopic assay for vacuole-to-vacuole docking was performed as in Mayer and Wickner (1997). Reactions for microscopic analysis were performed in 8 μM microcystin LR, 10 mM PIPES/KOH, pH 6.8, 200 mM sorbitol, 65 mM KCl, 0.25 mM MgCl2, 0.075 mM MnCl2, 0.25 mM ATP, 7.5 μM pefablock SC, 15 nM leupeptin, 3.75 μM o-phenanthroline, 50 nM pepstatin A, 10 mM creatine phosphate, and 17.5 U/ml creatine kinase. The composition of this buffer was chosen purposefully to minimize the ATP-independent formation of vacuole clusters, which occurs under standard fusion conditions. The extent of ATP-independent cluster formation was strain dependent. It is minimized by the reduction of the KCl, MgCl2, and MnCl2 concentrations. The optimized conditions permit fusion with slightly reduced efficiency but with similar kinetic properties as the standard buffer (our unpublished results). The samples were incubated at 27°C for 15 min and chilled on ice. Aliquots (12 μl) of each sample were mixed with the same volume of staining mixture (20 μM FM4–64 in 0.4% Seaplaque-agarose in PS buffer; kept liquid at 34°C), transferred immediately to a prechilled slide, and covered with a cover glass. After 5 min at 4°C, fluorescence pictures were taken on Kodak TMZ3200 film (Eastmann Kodak, Rochester, NY).
Labeling and Analysis of Phosphoinositides
Fusion reactions at 15× the standard volume were performed in the presence of [γ-32P]ATP (3 Ci/mmol; final concentration 0.2 Ci/l). The ATP-regenerating system (creatine phosphate and creatine kinase) was replaced by 8 mM ATP/MgCl2 to ensure a constant specific radioactivity throughout the experiment. At various times aliquots (90 μl) were removed, chilled on ice, and supplemented with 130 μl of 0.38 M HCl/2 M KCl. After 5 min on ice, 660 μl of 67% (vol/vol) chloroform and 33% (vol/vol) methanol were added, and the samples were mixed briefly by vortexing and shaken vigorously (2 min, room temperature). The phases were separated by centrifugation (1 min, 17,000 × g), and the lower phase was transferred to a new tube. Eighty-microliter aliquots were spotted onto high-performance, thin-layer chromatography (TLC) plates (NH2; Merck, Darmstadt, Germany) and chromatographed in 1-propyl acetate, 2-propanol, ethanol, 6% aqueous ammonia (3:9:3:9, by volume). Plates were dried, chromatographed in the same mixture again, and dried. The signal from the labeled products was detected via a phosphorimager.
Labeled standards of 3- and 4-phosphorylated phosphoinositides were produced in vitro by sonicating a mixture of 50% phosphatidylinositol or phosphatidylinositolmonophosphate/50% phosphatidylcholine (from Sigma; 1 mg/ml of lipid in 50 mM Tris/Cl, pH 7.2) for 6 min to obtain small unilamellar vesicles. Ten microliters of this lipid suspension were incubated for up to 2 h at 30 or 37°C with 12 μg/ml recombinant GST-Pik1p (PI 4-kinase from yeast) or human recombinant GST-PI 3-kinase γ (generous gift from R. Wetzker, Jena) in a reaction mixture of 10 mM HEPES/NaOH, pH 7.4, 8 mM MgCl2, 100 mM NaCl, 50 μM ATP in the presence of 1 μCi of [γ-32P]ATP (from NEN, Boston, MA) in a total volume of 50 μl. Reactions were stopped by adding 200 μl 0.4N HCl, 2 M KCl. Lipids were extracted by adding 1 ml hexane/isopropanol (24/16) and shaking vigorously. The upper phase was extracted again with an equal volume of 0.1N HCl and then subjected to TLC.
High-Performance Liquid Chromatography Analysis of Spots from TLC Plates
Individual spots were excised from the high-performance, TLC plates and placed in borosilicate glass tubes. Each spot was then extracted with 1 ml of CHCl3:CH3OH:conc. HCl (200:100:1 vol/vol/vol) for 10 min on ice with frequent agitation. Ten milligrams of bovine brain polyphosphoinositides (Sigma), 50 μl of water, and 200 μl of 0.6 M HCl were added, and the tubes were vortexed for 30 s. The phases were allowed to separate. The lower phase was removed to a fresh tube, and the upper phase containing the silica was reextracted as above with synthetic lower phase made by mixing CHCl3:CH3OH:0.6 M HCl in the ratios (8:4:3 vol/vol/vol). The lower phase from this second extraction was removed and pooled with the first lower phase and the extracted lipids dried in vacuo. Lipids were deacylated and analyzed by anion exchange high-performance liquid chromatography (HPLC) as described (Dove et al., 1997, Cooke et al., 1998) using pure yeast [3H]GroPInsPns as authentic standards.
RESULTS
Phosphoinositides Are Required for Vacuole Fusion
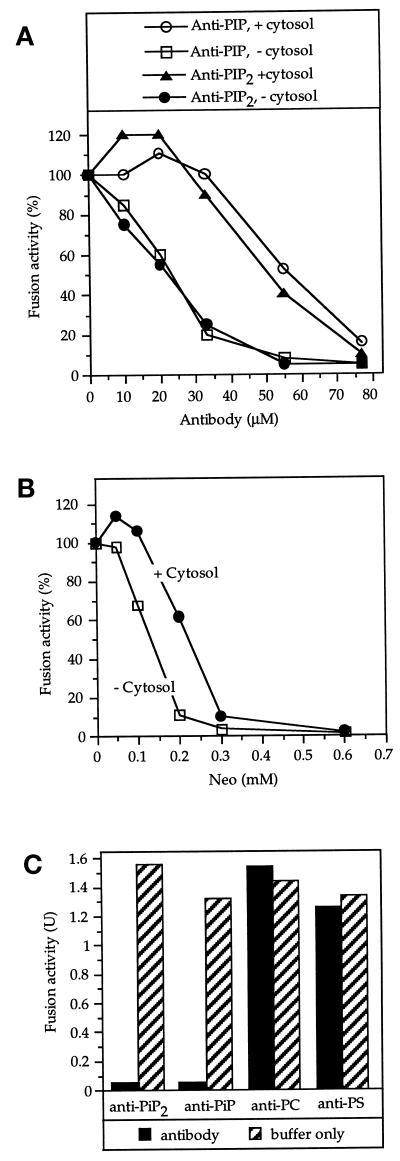
Monoclonal antibodies to PI(4)P or PI(4,5)P2 (Fukami et al., 1988) inhibited homotypic vacuole fusion (Figure 1A). Neomycin, which binds tightly to phosphatidylinositol phosphates (Schacht, 1978; Lodhi et al., 1979), also inhibited vacuole fusion (Figure 1B). In contrast, monoclonal antibodies to phosphatidylserine (Schuurmanns Stekhoven et al., 1994) and phosphatidylcholine (Nam et al., 1990) were not inhibitory (Figure 1C), showing the specificity of the inhibition by ligands of PI(4)P and PI(4,5)P2. The sensitivity to the antibodies as well as to neomycin was more pronounced in the absence of cytosol, possibly reflecting the involvement of cytosolic factors in the generation of PI(4)P and PI(4,5)P2.
Figure 1.
Inhibition of vacuole fusion by ligands of phosphatidylinositol phosphates. (A) Vacuoles (6 μg) were preincubated for 5 min on ice without inhibitor or with the indicated amounts of monoclonal antibodies to phosphatidylinositol 4-phosphate (PIP) or phosphatidylinositol 4,5-diphosphate (PIP2) in PS buffer with 50 mM KCl. The samples were then supplemented with salts and an ATP-regenerating system to yield standard fusion reactions. Cytosol was added as indicated. After 70 min at 27°C, fusion was assayed via alkaline phosphatase activity. (B) As in (A) but with neomycin as a phosphoinositide ligand. (C) The inhibitory effect is specific for PI(4)P and PI(4,5)P2 antibodies. Fusion reactions with cytosol were performed as in (A), in the absence of cytosol. Vacuoles were preincubated with monoclonal antibodies to PI(4)P, PI(4,5)P2, phosphatidylserine (PS), or phosphatidylcholine (PC; 35 μM each), or the respective control buffers.
The inhibition of fusion by neomycin, anti-PI(4)P or anti-PI(4,5)P2 could be overcome by readdition of PI(4)P or PI(4,5)P2, respectively (Figures 2, A and B). Approximately 300 μM externally added PI(4)P or PI(4,5)P2 is required to rescue fusion. Higher concentrations inhibit the reaction. This inhibition may be due to nonspecific, possibly detergent-like effects, because it also is seen with vacuoles that had not been exposed to antibodies (Figure 2). The PI(4,5)P2-rescued reaction was still ATP dependent and sensitive to inhibition by antibody to Sec18p (Figure 2C). Therefore, the rescued reaction followed the authentic pathway.
Figure 2.
Inhibition by neomycin or antibodies is reversible. Vacuoles were preincubated as in Figure 1 with (A) neomycin (300 μM), (B) antibodies to PI(4)P or to PI(4,5)P2 (35 μM), or with buffer only. The samples were then supplemented to yield complete fusion reactions, mixed with the indicated amounts of PI(4)P or PI(4,5)P2, incubated for 70 min at 27°C, and assayed for fusion. (C) The rescued reaction is ATP and Sec18p dependent. Preincubations were performed with anti-PI(4,5)P2 as in (B). Where indicated, PI(4,5)P2 was added to reactivate the vacuoles. Affinity-purified antibodies to Sec18p and the ATP-regenerating system were added as indicated, and the samples were incubated for 70 min at 27°C. Then fusion was assayed.
Phosphoinositides Are Synthesized during Vacuole Fusion
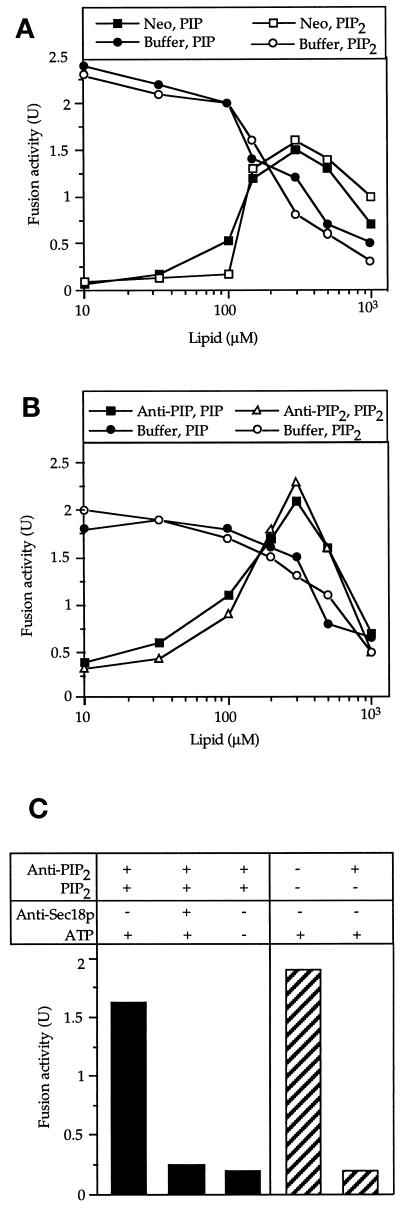
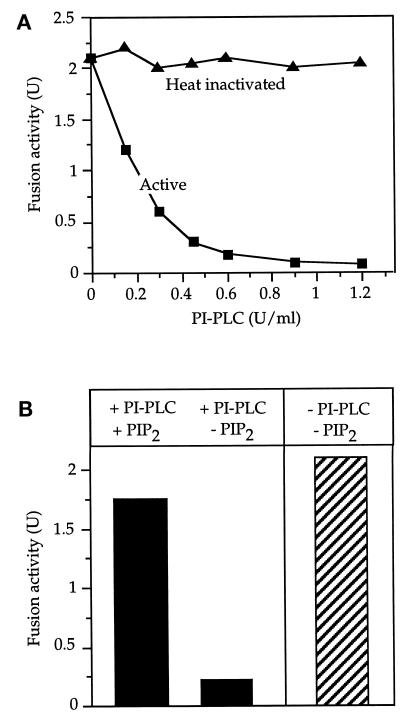
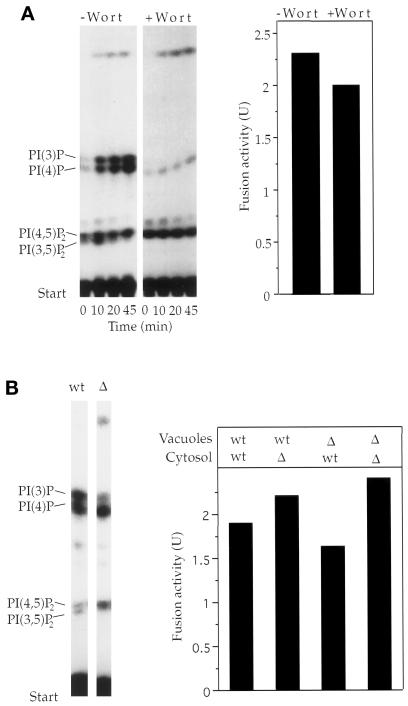
Fusion reactions also were inhibited by the addition of phosphatidylinositol (PI)-PLC, a bacterial phosphatidylinositol-specific phospholipase C. This enzyme hydrolyzes PI into inositol-(1)-phosphate and diacylglycerol but cleaves neither PI(4)P nor PI(4,5)P2 and does not hydrolyze phosphatidylcholine or phosphatidylethanolamine (Eberhard et al., 1990) or any of several other phospholipids (Sundler et al., 1978). If phosphoinositide synthesis during the reaction was crucial, PI-PLC should inhibit fusion by removing the source material for phosphorylation. In fact, addition of PI-PLC did inhibit fusion (Figure 3A). Inhibition could be reversed by adding PI(4,5)P2 to the fusion reaction (Figure 3B), confirming that inhibition was due to a reduction of the PI(4,5)P2 pool. To monitor the synthesis of phosphoinositides directly, we performed fusion reactions in the presence of [γ-32P]ATP, which radiolabels newly synthesized phosphoinositides. The lipids were extracted at different times after the start of a fusion reaction and separated by TLC. Radioactive spots were scraped off the plate and deacylated, and the resulting lipid headgroups were identified by HPLC (Dove et al., 1997). This, as well as comigration with radiolabeled lipid standards, confirmed the identity of the indicated phosphoinositides. We detected rapid accumulation of PI(3)P, PI(4)P, PI(4,5)P2 and—transiently and to varying degrees—phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) (Figure 4A). PIP3 synthesis could not be detected (using other TLC systems that gave clear separation of PIP3; our unpublished results). We investigated whether PI(3)P synthesis would be relevant to vacuole fusion by using the PI-kinase inhibitor wortmannin. This inhibitor abrogated synthesis of PI(3)P and PI(3,5)P2 but not that of PI(4)P and PI(4,5)P2 (Figure 4A, right panel). Wortmannin did not inhibit fusion. Vacuoles from fab1 mutant cells were defective in the synthesis of PI(3,5)P2 and PI(3)P and—like wortmannin-treated vacuoles—showed an increased steady-state level of PI(4,5)P2. They were fusion competent (Figure 4B). Therefore, PI(3)P and PI(3,5)P2 are not essential for vacuole fusion, although a regulatory influence cannot be excluded. The relevant phosphoinositides appear to be PI(4)P and PI(4,5)P2. Both are synthesized from PI in the course of the reaction.
Figure 3.
Inhibition by phosphatidylinositol-specific phospholipase C (PI-PLC). (A) PI-PLC was added at the indicated concentrations to the fusion reaction either in active form or after heat denaturation (5 min, 95°C) of the enzyme. The samples contained 150 mM KCl, but no cytosol, and were kept on ice without ATP for 5 min. The reaction was started by adding the ATP-regenerating system and transferring to 27°C for 70 min. (B) Preincubation with or without PI-PLC (0.6 U/ml) was performed as in (A). PI(4,5)P2 (200 μM) was added where indicated, and fusion was assayed after 70 min at 27°C.
Figure 4.
Synthesis of phosphoinositides during the fusion reaction. (A) Fusion reactions (300 μl each), containing 300 μM wortmannin or the respective control buffer only, were performed in the presence of [γ-32P]ATP. At the indicated times, aliquots (90 μl each) were withdrawn and subjected to lipid extraction, TLC, and autoradiography. Labeled spots were scraped off the plate and deacylated. The headgroups were identified by HPLC. The identity of the indicated phosphoinositides was deduced from this analysis and confirmed by comigration of the indicated lipids with radiolabeled standards for PI(3)P, PI(4)P, and PI(4,5)P2. Equivalent parallel incubations were performed and assayed for fusion after 70 min at 27°C. (B) Influence of a fab1 mutation on fusion and phosphoinositide synthesis on the vacuoles. After 15 min of fusion, lipid synthesis was assayed as in (A) using vacuoles from FAB1 (wt) and fab1 deletion (Δ) strains. In parallel, the vacuoles were tested in standard fusion reactions for 70 min at 27°C, using cytosol from FAB1 wt or fab1 deletion cells, respectively.
Phosphatidylinositol Phosphates Are Required after the Docking Step
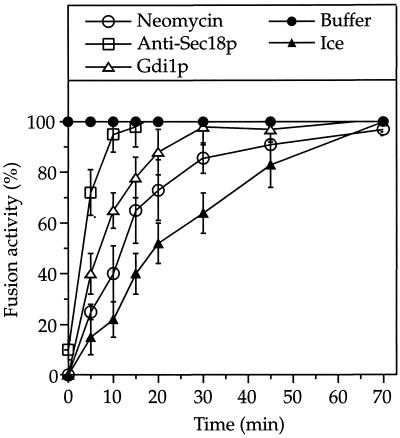
We determined the time course of the requirement for phosphoinositides in the in vitro reaction (Figure 5). The bulk of vacuoles completes distinct reaction steps within defined intervals and then becomes resistant to inhibitors of these steps (Conradt et al., 1994; Mayer et al., 1996). This facilitates an analysis of the sequence of events in vacuole fusion. Inhibitors were added into an ongoing fusion reaction at different times, and the samples were incubated further until the end of a standard fusion period (70 min). We asked how the inhibitors, when added at a particular time, influence the further course of the reaction. A standard sample that received only buffer served as control for maximal fusion (100%). A second aliquot was set on ice to stop fusion at the indicated time and measure the progression of the reaction. Other aliquots received inhibitors and were incubated further at 27°C. All inhibitors prevented fusion when added at the start of the reaction (Figure 5). However, the sensitivity of the reaction to the inhibitors differed if they were added at later time points. The reaction became resistant to antibodies to Sec18p, which inhibit priming, after 10–15 min and to Gdi1p within 30 min (Mayer et al., 1996). Gdi1p extracts the ras-like GTPase Ypt7p from the vacuolar membrane (Haas et al., 1995). Resistance of fusion to this protein indicates the completion of docking (Mayer and Wickner, 1997). Sensitivity to neomycin persisted longer than that to Gdi1p, indicating that phosphoinositides are required past the docking stage.
Figure 5.
Kinetic analysis of phosphoinositide requirement. Reaction mixtures of four times the standard volume were incubated at 27°C and divided into standard volume portions at different times. These aliquots were transferred to tubes containing inhibitors or only buffer, kept on ice for 10 min, and then incubated at 27°C for the rest of the 70-min reaction period. Another aliquot, which received only buffer, was set on ice. After 70 min of incubation at 27°C, fusion was measured. The graphs present the average of four independent experiments with SDs. To facilitate averaging, the data were normalized: The activity of the samples incubated at 27°C with buffer only was set to 100%. The activity of the sample set on ice at 0 min served as the 0% reference. The maximal fusion actvities in the samples with buffer only were 2.1 ± 0.2, 2.5 ± 0.3, 2.6 ± 0.3, and 2.9 ± 0.3 U. The fusion-independent background, given by samples set on ice at 0 min, varied from 0.18 to 0.27 U. Inhibitors were added as follows: Affinity-purified antibodies to Sec18p (2.5 μg), Gdi1p (3 μg), and neomycin (500 μM).
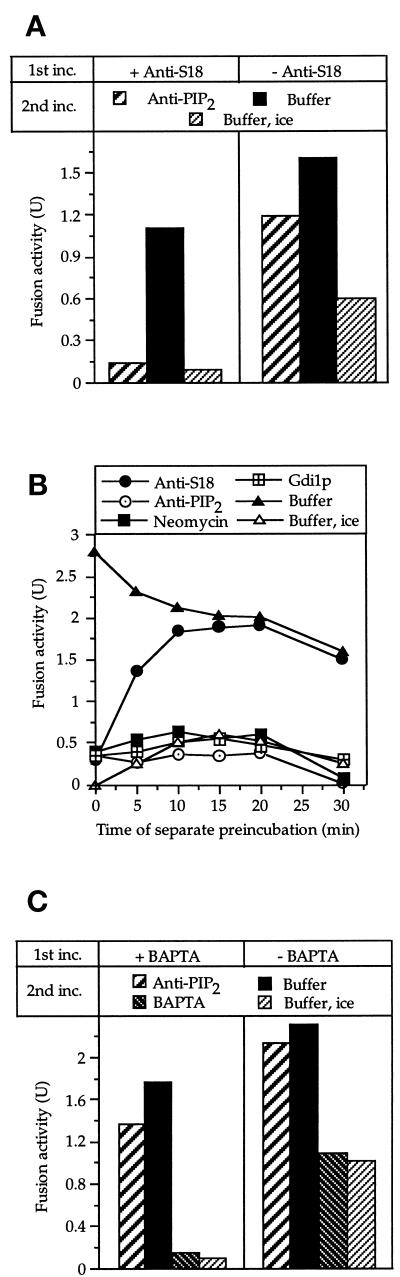
This could be confirmed by experiments aiming to determine the absolute order of requirements. Sec17p and Sec18p are involved in an early priming reaction that precedes the docking step (Mayer et al., 1996; Mayer and Wickner, 1997). First, we asked whether the phosphoinositide-requiring step must be preceded by priming. Alternatively, priming and the phosphoinositide-dependent processes might be part of parallel reaction pathways that converge at a later phase of the reaction. A two-stage reaction was performed (Figure 6A), and fusion reactions were run in the presence of antibodies to Sec18p that block priming (Mayer et al., 1996). After 20 min, a time that suffices to complete priming (Figure 5), the vacuoles were reisolated and resuspended in fresh buffer. Sec18p was added to overcome the block by the antibodies and the reaction was continued. Vacuoles that had been exposed to antibodies to Sec18p could still finish fusion after purified Sec18p had been added (Figure 6A, left panel). If anti-PI(4,5)P2 was added on top of Sec18p, the reaction could not progress, indicating that the requirement for PI(4,5)P2 could not be fulfilled without priming. If antibodies to Sec18p had not been present during the 20-min preincubation, some fusion had occurred in this phase (Figure 6A, right panel). However, the further fusion which occurred during the second incubation was partially resistant to anti-PI(4,5)P2. In contrast, if priming was blocked, the reaction remained fully sensitive. This indicates that priming is a prerequisite for the reaction to overcome the PI(4,5)P2 requirement.
Figure 6.
Absolute order of steps. (A) Sec18p action (priming) must precede the PI(4,5)P2-dependent step. Standard fusion reactions were started in the presence or absence of 0.5 μM affinity-purified antibody to Sec18p. After 20 min at 27°C, the samples received antibodies to PI(4,5)P2 (70 μM) or buffer. After 5 min at 27°C, dithiothreitol (75 μM) and Sec18p (0.2 μM) were added to reactivate the Sec18p pathway. A control sample received only buffer. After further incubation for 70 min at 27°C or on ice, fusion was measured. (B) The requirement for PI(4,5)P2 cannot be satisfied before docking. Two separate samples, each equivalent to six standard fusion reactions, were incubated at 27°C. One contained BJ3505 vacuoles carrying proalkaline phosphatase, whereas the other contained DKY6281 vacuoles harboring the maturation enzyme, proteinase A. At the indicated times, aliquots (30 μl) were withdrawn from the reactions and chilled. The aliquots received antibodies to Sec18p (1 μM), neomycin (500 μM), Gdi1p (3 μg), PI(4,5)P2 (70 μM), or buffer only and were left on ice for 10 min. The fusion partners were combined in one tube, centrifuged briefly (1 min, 8000 × g, 4°C), mixed by vortexing, and incubated for 70 min at 27°C or on ice. Then, fusion was assayed. (C) Relationship of the PI(4,5)P2 requirement to the BAPTA-sensitive step. Reaction mixtures of four times the standard volume were incubated (45 min, 27°C) in the presence or absence of 2 mM BAPTA. The samples were diluted 10-fold with 150 mM KCl, 10 mM PIPES/KOH, pH 6.8, and 200 mM sorbitol and centrifuged (8 min, 8000 × g, 4°C). The vacuoles were resuspended in fresh reaction buffer and split into four aliquots. Three aliquots received anti-PI(4,5)P2 (70 μM) or BAPTA (2 mM) or only buffer and were incubated further at 27°C. The fourth aliquot received buffer and was kept on ice to monitor the degree of fusion that had occurred during the first incubation. After 60 min, fusion was assayed.
To determine whether the PI(4,5)P2 requirement could be fulfilled before docking, we incubated the vacuoles bearing proalkaline phosphatase and the vacuoles with the activating proteases in separate tubes but otherwise under complete reaction conditions. This only allows priming. Docking and fusion of the two vacuole partners, which lead to the production of alkaline phosphatase activity, can only occur after aliquots from these separate tubes have been mixed. When vacuoles were incubated separately for 30 min and then mixed in the presence of anti-PI(4,5)P2 or neomycin, fusion did not occur between the two vacuole types (Figure 6B). Only the requirement for Sec18p (priming) could be fulfilled before the vacuoles from the separate tubes met each other, that is, in a predocking step of fusion (Mayer et al., 1996). Hence, the requirement for PI(4,5)P2 can only be fulfilled after docking has occurred.
The late, postdocking phase of vacuole fusion is sensitive to the calcium chelator BAPTA (Peters and Mayer, 1998). We investigated the relationship of the PI(4,5)P2 requirement to the BAPTA-sensitive step by starting a fusion reaction with BAPTA (Figure 6C). After 45 min, a time that is sufficient to largely overcome the PI(4,5)P2 requirement under standard conditions (Figure 5), the vacuoles were reisolated, resuspended in fresh reaction mixture and incubated for further 60 min. During this second incubation, the vacuoles could fuse if BAPTA was omitted but not if BAPTA was added to the mixture. Thus, the BAPTA block was reversible. The rescue of fusion activity after removal of BAPTA also was observed if antibodies to PI(4,5)P2 were added to the second incubation at a concentration that prevents fusion in standard reactions (50 μM; cf. Figure 1A). The reaction had become resistant to anti-PI(4,5)P2, despite the presence of BAPTA. Equivalent results were obtained with neomycin (our unpublished results). Hence, the PI(4,5)P2 requiring step that occurs after docking must precede the BAPTA-sensitive step or lie on a parallel reaction pathway that converges with the BAPTA-sensitive branch at a later point.
Phosphoinositide Requirement for Priming
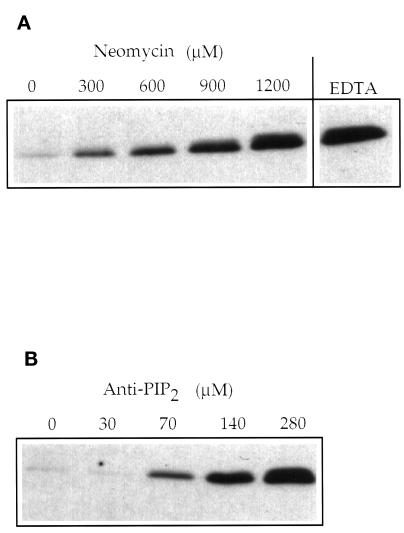
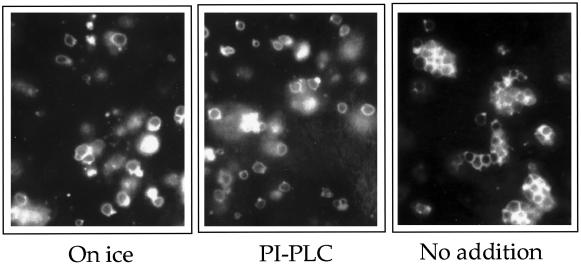
The results described above suggest a postdocking role for phosphoinositides but do not exclude an additional involvement of phosphoinositides in earlier steps of the reaction. To test this, we monitored the early reactions of priming and docking directly. Priming includes the Sec18p- and ATP-mediated release of Sec17p from the vacuolar surface and activates the vacuoles for subsequent tethering and docking (Mayer et al., 1996; Mayer and Wickner, 1997; Ungermann et al., 1998). We performed fusion reactions in the presence of neomycin or anti-PI(4,5)P2 and assayed vacuole-bound Sec17p (Figure 7). Both reagents completely blocked release of Sec17p from the vacuoles, whereas in their absence, Sec17p release occurred normally. We note that the inhibition of Sec17p release required approximately four times higher concentrations of PI(4,5)P2-binding reagents than the complete inhibition of vacuolar fusion. This lower sensitivity to the PI(4,5)P2-binding reagents distinguishes the early phosphoinositide requirement at the priming step from that after the docking step. Because priming is a prerequisite for docking, phosphoinositides should also affect docking. Using a microscopic assay of docking (Mayer and Wickner, 1997), this was indeed observed (Figure 8): PI-PLC interfered with the formation of vacuole clusters, which normally occurs within the first 15 min of the reaction. We conclude that synthesis of PI(4,5)P2 is required for two steps of vacuole fusion. The earlier one, associated with priming, is less sensitive to phosphoinositide-binding agents and can be observed if priming is monitored directly via Sec17p release. The second phosphoinositide-dependent event occurs during or after docking (Figures 5 and 6) and is more sensitive to the inhibitors. In the overall fusion assay, only this later requirement is apparent.
Figure 7.
Sec17p release depends on PI(4,5)P2. Vacuoles equivalent to four fusion reactions were preincubated with the indicated amounts of (A) neomycin or (B) antibodies to PI(4,5)P2 as in Figure 1 (without cytosol). A control sample received no phosphoinositide ligands, but EDTA was added to prevent ATP hydrolysis and thereby block Sec17p release. The samples were supplemented to yield standard fusion conditions (minus cytosol) and incubated at 27°C for 15 min. Aliquots equivalent to three standard reactions (18 μg of vacuoles) were withdrawn, and the vacuoles were reisolated by centrifugation and assayed for bound Sec17p by SDS-PAGE and Western blotting. The remaining aliquots were incubated for further 55 min and used to assay fusion. Complete inhibition of fusion occurred at the same concentrations as in Figure 1 ( i.e., with 60 μM antibody and 300 μM neomycin).
Figure 8.
Phosphoinositides are required for docking. Vacuoles were prepared from the strain RSY249 and used in 30-μl fusion reactions for microscopic analysis as described in MATERIALS AND METHODS. After adding PI-PLC (10 U/ml) or buffer only, the samples were preincubated for 5 min on ice. They were then transferred to 27°C for 20 min or left on ice. The samples were chilled and mixed with low melting agarose containing 20 μM FM4–64 stain. The suspension was transferred onto a prechilled microscopy slide, left at 4°C for 5 min, and analyzed by fluorescence microscopy.
DISCUSSION
High-affinity monoclonal antibodies to PI(4,5)P2 (Greenberg et al., 1979; Fukami et al., 1988) have been useful, for example, in investigations of the asymmetric distribution of phosphoinositides in erythrocytes (Gascard et al., 1991), of the roles of PI(4,5)P2 in the proliferation of yeast cells (Uno et al., 1988) and of higher eukaryotic cells (Matuoka et al., 1988), and in Ca2+-activated secretion from PC12 cells (Hay et al., 1995). In our study, these antibodies inhibited the fusion reaction almost completely and did so at concentrations similar to those necessary for maximal biological effect in other systems (Matuoka et al., 1988).
The other reagent used in this study, neomycin, is a very-high-affinity ligand for polyphosphorylated phosphoinositides and indeed can be used to create an affinity matrix for their purification (Schacht, 1978; Lodhi et al., 1979). Its interaction with phosphoinositides is similar to the tight binding of PI(4,5)P2 to profilin, which inhibits hydrolysis of the phospholipid by phospholipase C (Goldschmidt-Clermont et al., 1990). The concentrations of neomycin needed to affect PI(4,5)P2 turnover in different systems are quite variable, ranging from 10 μM to more than 1 mM (Gabev et al., 1989). These findings have led to the hypothesis that much of PI(4,5)P2 may not be readily accessible in some membranes because it is tightly bound to proteins (Gabev et al., 1989). In the case of γ-actinin (Fukami et al., 1992), this association is so strong that even boiling in SDS sample buffer and subsequent denaturing electrophoresis do not separate the protein from its bound PI(4,5)P2.
Neomycin inhibited vacuole fusion at low concentrations (De Andres et al., 1991; Khouja and Jones, 1993), similar to those that inhibit endosome fusion (Jones and Wessling-Resnick, 1998). At higher concentrations, neomycin also inhibited the release of Sec17p from vacuole membranes. Comparably high concentrations of neomycin (1–2 mM) are also required to inhibit protein kinase C, which strongly binds PI(4,5)P2 as a physiological activator (Chauhan, 1990). Remarkably, the concentrations both of neomycin and of anti-PI(4,5)P2, which were necessary to inhibit the priming step (Sec17p release) were fourfold higher than those needed to prevent the overall reaction. These data suggest that both phosphatidylinositol 4,5-diphosphate ligands act on the same targets in the vacuole membranes. They also suggest that a lower concentration of free phosphoinositides may suffice for the priming reaction. Alternatively, the PI(4,5)P2 needed for Sec17p release may not be as readily accessible as the PI(4,5)P2 that operates after docking. This may be a consequence of a tight binding of PI(4,5)P2 to vacuolar membrane proteins.
Phosphoinositides have been implicated in regulated secretion and in various intracellular membrane trafficking reactions. So far, the available data for intracellular trafficking reactions indicated a requirement for (3)-phosphorylated phosphoinositides but not for the (4)-phosphorylated species; for example, PI3-kinases are involved in endosome fusion (Jones and Clague, 1995; Li et al., 1995). Furthermore, the interaction of EEA1, an effector of the GTPase Rab5, with the endosomal membrane is PI(3)P dependent . (Simonsen et al., 1998; Christoforidis et al., 1999). PI(3)-kinase activity is also essential for vesicular traffic between the Golgi, endosomes, and the vacuole (for review, see Burd et al., 1998). The yeast PI(3) kinase, Vps34p, is completely inhibited by 10 μM wortmannin (Stack and Emr, 1994). In contrast, vacuole fusion was not inhibited by up to 300 μM wortmannin, although wortmannin suppressed the synthesis of PI(3)P and PI(3,5)P2 in the reaction. In addition, vps34 mutants do not show aberrations of vacuolar structure, which are often associated with mutations in genes relevant for vacuole fusion (Herman and Emr, 1990; Wada et al., 1992). Therefore, (3)-phosphorylated phosphoinositides, which are required for endosome fusion, do not seem to have an essential role in vacuole fusion. It should be kept in mind, however, that the targets of wortmannin include not only PI(3) kinases but also PI(4) kinases (Cutler et al., 1997; Meyers and Cantley, 1997). Some wortmannin effects on endosome fusion might therefore be related to PI(4) phosphates.
Could PI(4,5)P2 be needed as a cofactor for specific PI(4,5)P2 binding proteins? The fact that several PI(4,5)P2 binding proteins are involved in regulated exocytosis argues in favor of this hypothesis (for review, see Martin, 1998). PI(4,5)P2 is synthesized in the ATP-dependent priming phase of exocytosis (Hay et al., 1995; Martin et al., 1997). It binds, for example, to synaptotagmin, CAPS, and Mint proteins. CAPS is a peripheral membrane protein found on the plasma membrane as well as on secretory vesicles (Berwin et al., 1998). CAPS and PI(4,5)P2 are required at the Ca2+-dependent triggering step that follows docking and priming (Walent et al., 1992; Ann et al., 1997). CAPS preferentially binds to PI(4,5)P2 and could be a means to sequester PI(4,5)P2 in defined regions of the membrane (Loyet et al., 1998). Synaptotagmin undergoes a Ca2+-dependent switch in its ability to bind PI(4,5)P2 (Schiavo et al., 1996). It was suggested that this switching may be part of a docking mechanism that operates at Ca2+ concentrations below those necessary to complete fusion (Schiavo et al., 1996). A role in docking was also proposed for the Munc-18–interacting (Mint) proteins. This activity could result from Mint proteins binding to PI(4,5)P2 on the vesicle membrane and to Munc18 on the plasma membrane (Okamoto and Südhof, 1997). Taken together, these data suggest that PI(4,5)P2, via different binding factors, may act in exocytosis in the priming and in the final triggering stage. This would match the situation we found for vacuole fusion, during which PI(4,5)P2 is required for ATP-dependent priming and in a later phase, which can only occur after vacuole contact. Further studies are needed to identify potential binding partners for PI(4,5)P2 and explore its metabolism on the vacuoles in detail.
ACKNOWLEDGMENTS
We thank Dr. Masato Umeda for the generous gift of monoclonal antibodies to phosphatidylserine and phosphatidylcholine and Drs. K. Fukami and F. Schuurmanns-Stekhoven for advice. A.M. is grateful to Drs. Peter Overath and Ulf Henning for their support and the opportunity to work in their laboratories. This work was supported by grants from the National Institute of General Medical Science (to W.W.), from the Deutsche Forschungsgemeinschaft (to A.M. and A.H.), and the Boehringer Ingelheim foundation (to A.M.).
Abbreviations used:
- PI
phosphatidyl inositol
- PI-PLC
phosphatidylinositol-specific phospholipase C
- PI(3)P
phosphatidylinositol 3-phosphate
- PI(3,5)P2
phosphatidylinositol 3,5-bisphosphate
- PI(4)P
phosphatidylinositol 4-phosphate
- PI(4,5)P2 phosphatidylinositol 4,5-bisphosphate.
REFERENCES
- Ann K, Kowalchyk JA, Loyet KM, Martin TFJ. Novel Ca2+ binding protein (CAPS) related to UNC-31 required for Ca2+ activated exocytosis. J Biol Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- Berwin B, Floor E, Martin TF. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:37–45. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Bone N, Millar JBA, Toda T, Armstrong J. Regulated vacuole fusion and fission in Schizosaccaromyces pombe: an osmotic response dependent on MAP kinases. Curr Biol. 1998;8:135–144. doi: 10.1016/s0960-9822(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Burd CG, Babst M, Emr SD. Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking. Semin Cell Dev Biol. 1998;9:527–533. doi: 10.1006/scdb.1998.0255. [DOI] [PubMed] [Google Scholar]
- Cardenas ME, Heitman J. FKBP12-rapamcyin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J. 1995;14:5892–907. doi: 10.1002/j.1460-2075.1995.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VPS. Phosphatidylinositol 4,5-bisphosphate stimulates protein kinase C-mediated phosphorylation of soluble brain proteins: inhibition by neomycin. FEBS Lett. 1990;272:99–102. doi: 10.1016/0014-5793(90)80457-t. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride H, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;347:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol, 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B, Haas A, Wickner W. Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J Cell Biol. 1994;126:99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke FT, Dove SK, McEwen RK, Painter G, Holmes AB, Hall MN, Michell RH, Parker PJ. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol. 1998;8:1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- Cutler NS, Heitman J, Cardenas ME. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J Biol Chem. 1997;272:27671–27677. doi: 10.1074/jbc.272.44.27671. [DOI] [PubMed] [Google Scholar]
- De Andres B, Del Pozo V, Cardaba B, Martin E, Tramon P, Lopez-Rivas A, Palomino P, Lahoz C. Phosphoinositide breakdown is associated with Fc-gamma RII-mediated activation of 5′-lipoxygenase in murine eosinophils. J Immunol. 1991;146:1566–1570. [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators of membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Fukami K, Matsuoka K, Nakanishi O, Yamakawa A, Kawai S, Takenawa T. Antibody to phosphatidylinositol 4,5-bisphosphate inhibits oncogene-induced mitogenesis. Proc Natl Acad Sci USA. 1988;85:9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenaea T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992;359:150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2) Biochim Biophys Acta. 1989;978:105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Gascard P, Tran D, Sauvage M, Sulpice J-C, Fukami K, Takenawa T, Claret M, Giraud F. Asymmetric distribution of phosphoinositides and phosphatidic acid in the human erythrocyte membrane. Biochim Biophys Acta. 1991;1069:27–36. doi: 10.1016/0005-2736(91)90100-m. [DOI] [PubMed] [Google Scholar]
- Gary JD, Wurmser AD, Bonangelino CJ, Weisman LS, Emr SD. Fab1 is essential for PtdIns(3)P5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990;247:1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Greenberg AJ, Trevor AJ, Johnson DA, Loh HH. Immunchemical studies of phospholipids: production of antibodies to triphosphoinositides. Mol Immunol. 1979;16:193–196. doi: 10.1016/0161-5890(79)90145-7. [DOI] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol, 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Schleglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7 of Saccharomyces cerevisiae is required on both parner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Martin TFJ. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TFJ. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Catlett NL, Weisman LS. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz RW, Bittner MA, Peppers SC, Senter RA, Eberhard DA. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989;264:5412–5419. [PubMed] [Google Scholar]
- Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995;311:31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AT, Wessling-Resnick M. Inhibition of in vitro endosomal vesicle fusion activity by aminoglycoside antibiotics. J Biol Chem. 1998;273:25301–9. doi: 10.1074/jbc.273.39.25301. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Khouja A, Jones CT. Phospholipase A2 and arachidonic acid release from permeabilized myometrial cells from guinea pig uterus. J Dev Physiol. 1993;19:61–6. [PubMed] [Google Scholar]
- Li G, DíSouza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi S, Weiner ND, Schacht J. Interactions of neomycin with monomolecular films of polyphosphoinositides and other lipids. Biochim Biophys Acta. 1979;557:1–8. doi: 10.1016/0005-2736(79)90084-1. [DOI] [PubMed] [Google Scholar]
- Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwich G, Martin TFJ. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J Biol Chem. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- Martin TFJ, Loyet KM, Barry VA, Kowalchyk JA. The role of Ptd(4,5)-bisphosphate in exocytic membrane fusion. Biochem Soc Trans. 1997;25:1137–1141. doi: 10.1042/bst0251137. [DOI] [PubMed] [Google Scholar]
- Martin TFJ. Phosphoinositide lipids as signaling molecules: Common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- Matuoka K, Fukami K, Nakanishi O, Kawai S, Takenawa T. Mitogenesis in response to PDGF and bombesin abolished by microinjection of antibody to PIP2. Science. 1988;239:640–643. doi: 10.1126/science.2829356. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (a-SNAP) precedes docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles if catalyzed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers R, Cantley LC. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:4384–90. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- Nam KS, Igarashi K, Umeda M, Inoue K. Production and characterization of monoclonal antibodies that specifically bind to phosphatidylcholine. Biochim Biophys Acta. 1990;1046:89–96. doi: 10.1016/0005-2760(90)90098-i. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. . Okamoto, M., and Südhof, T.C. (1997). Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J. Biol. Chem. 272, 31459–31464. [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/Calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA. GTP hydrolysis by ADP-ribosylation factor is dependent on both an ADP-ribosylation factor GTPase-activating protein and acid phospholipids. J Biol Chem. 1994;269:10758–10763. [PubMed] [Google Scholar]
- Reza F, Igarashi K, Tokita S, Asai K, Aoki J, Asaoka Y, Umeda M, Inoue K. Anti-idiotypic monoclonal antibody recognizes a consensus recognition site for phosphatidylserine in phosphatidylserine-specific monoclonal antibody and protein kinase C. FEBS Lett. 1994;339:229–233. doi: 10.1016/0014-5793(94)80421-4. [DOI] [PubMed] [Google Scholar]
- Roth MG. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 1999;9:174–179. doi: 10.1016/s0962-8924(99)01535-4. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978;19:1063–1067. [PubMed] [Google Scholar]
- Schiavo G, Gu Q-M, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci USA. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Schuurmanns Stekhoven FMAH, Tijimes J, Umeda M, Inoue K, DePont JJHHM. Monoclonal antibody to phosphatidylserine inhibits Na+/K+-ATPase activity. Biochim Biophys Acta. 1994;1194:155–165. doi: 10.1016/0005-2736(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Reaves BJ, Davidson HW. Phosphoinositide 3-kinases and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B, Nemoto Y, Daniell L, Ferro-Novick S, De Camilli P. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J Cell Sci. 1998;111:3347–3356. doi: 10.1242/jcs.111.22.3347. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Seaman M, Nemoto Y, Daniell L, Suchy SF, Emr SD, De Camilli P, Nussbaum R. Disruption of three phosphatidylinositol-polyphosphate 5-phosphatase genes from S. cerevisiae results in pleiotropic abnormalities of vacuole morphology, cell shape, and osmohomeostasis. Eur J Cell Biol. 1997;74:350–360. [PubMed] [Google Scholar]
- Stack JH, Emr SD. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits borh protein kinase and phosphatidylinositol-specific PI3-kinase activity. J Biol Chem. 1994;269:31552–31562. [PubMed] [Google Scholar]
- Stolz LE, Huynh CV, Thorner J, York JD. Identification and characterization of an essential family of inositol polyphosphate 5-phosphatases (INP51, INP52 and INP53 gene products) in the yeast cerevisiae. Genetics. 1998;148:1715–29. doi: 10.1093/genetics/148.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R, Alberts AW, Vagelos PR. Enzymatic properties of phosphatidylinositol inositolphosphohydrolase from Bacillus cereus. J Biol Chem. 1978;253:4175–4179. [PubMed] [Google Scholar]
- Terui T, Kahn RA, Randazzo PA. Effects of acid phospholipids on nucleotide exchange properties of ADP-ribosylation factor1. Evidence for specific interaction with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1994;269:28130–28135. [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Uno I, Fukami K, Kato H, Takenawa T, Ishikawa T. Essential role for phosphatidylinositol 4,5-bisphosphate in yeast cell proliferation. Nature. 1988;333:188–190. doi: 10.1038/333188a0. [DOI] [PubMed] [Google Scholar]
- Wada Y, Ohsumi Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. J Biol Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Walent JH, Porter BW, Martin TFJ. A novel 145 kd brain cytosolic protein reconstitutes Ca2+-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Weisman LS, Wickner WT. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988;241:289–291. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A, Matile P, Moor H. Vacuolar dynamics in synchronously budding yeast. Archiv Mikrobiol. 1970;70:89–103. doi: 10.1007/BF00412200. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in S. cerevisiae. J Cell Biol. 1995;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Mayer A, Muller E, Wickner W. A heterodimer of thioredoxin and IB2 cooperates with Sec18p to promote yeast vacuole inheritance. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sato K, Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–34. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]