Abstract
In the Madin-Darby canine kidney epithelial cell line, the proteins occludin and ZO-1 are structural components of the tight junctions that seal the paracellular spaces between the cells and contribute to the epithelial barrier function. In Ras-transformed Madin-Darby canine kidney cells, occludin, claudin-1, and ZO-1 were absent from cell–cell contacts but were present in the cytoplasm, and the adherens junction protein E-cadherin was weakly expressed. After treatment of the Ras-transformed cells with the mitogen-activated protein kinase kinase (MEK1) inhibitor PD98059, which blocks the activation of mitogen-activated protein kinase (MAPK), occludin, claudin-1, and ZO-1 were recruited to the cell membrane, tight junctions were assembled, and E-cadherin protein expression was induced. Although it is generally believed that E-cadherin–mediated cell–cell adhesion is required for tight junction assembly, the recruitment of occludin to the cell–cell contact area and the restoration of epithelial cell morphology preceded the appearance of E-cadherin at cell–cell contacts. Both electron microscopy and a fourfold increase in the transepithelial electrical resistance indicated the formation of functional tight junctions after MEK1 inhibition. Moreover, inhibition of MAPK activity stabilized occludin and ZO-1 by differentially increasing their half-lives. We also found that during the process of tight junction assembly after MEK1 inhibition, tyrosine phosphorylation of occludin and ZO-1, but not claudin-1, increased significantly. Our study demonstrates that down-regulation of the MAPK signaling pathway causes the restoration of epithelial cell morphology and the assembly of tight junctions in Ras-transformed epithelial cells and that tyrosine phosphorylation of occludin and ZO-1 may play a role in some aspects of tight junction formation.
INTRODUCTION
Tight junctions are the most apical structures of the junctional complex in epithelial and endothelial cells (Farquhar and Palade, 1963). Tight junctions serve as a permeability barrier regulating the passage of ions and small molecules through the paracellular pathway (barrier function) and restrict the lateral diffusion of membrane lipids and proteins between the apical and basolateral compartments to maintain cell polarity (fence function) (Claude and Goodenough, 1973; Cereijido et al., 1989; Schneeberger and Lynch, 1992; Gumbiner, 1993; Anderson and Van Itallie, 1995). Thin section electron microscopy reveals tight junctions as a series of membrane contacts between adjacent cells (Farquhar et al., 1963). In freeze fracture replicas, these membrane contacts are seen as branching and anastomosing intramembrane strands in the plane of the membrane (Goodenough and Revel, 1970; Staehelin, 1973).
A number of tight junction–associated proteins have been identified and cloned (Stevenson and Keon, 1998), including ZO-1 (Stevenson et al., 1986; Anderson et al., 1988), cingulin (Citi et al., 1988), 7H6 antigen (Zhong et al., 1993), ZO-2 (Gumbiner et al., 1991; Jesaitis and Goodenough, 1994), occludin (Furuse et al., 1993; Ando-Akatsuka et al., 1996), symplekin (Keon et al., 1996), and ZO-3 (Haskins et al., 1998). More recently, a new family of tight junction integral membrane proteins, called the claudin family, have been identified (Furuse et al., 1998). One of these claudins, called paracellin-1, has been shown to be critical for renal reabsorption of Mg2+ (Simon et al., 1999), which suggests that claudins may function as paracellular channels (Wong and Goodenough, 1999). Occludin is an integral membrane protein localized within tight junction strands that has been shown to serve as a functional component of the tight junction (Balda et al., 1996; McCarthy et al., 1996; Chen et al., 1997; Wong and Gumbiner, 1997; Bamforth et al., 1999). ZO-1 is a member of the membrane-associated guanylate kinase family, which contains PDZ, SH3, and GUK (guanylate kinase-like) domains (Woods and Bryant, 1993; Anderson, 1996). It has been shown that ZO-1 binds to occludin in vitro and is colocalized with F-actin in culture cells (Furuse et al., 1994; Fanning et al., 1998). Although the function of ZO-1 is still unknown, Dlg, the product of the Drosophila lethal(1)discs-large-1 tumor suppressor gene (a membrane-associated guanylate kinase family member), is essential for epithelial structure and growth control (Woods et al., 1996; Hough et al., 1997).
Tight junction assembly and function can be modulated by a number of signaling molecules, including cAMP, Ca2+, PKC, G proteins, phospholipase C, and diacylglycerol (Gonzalez-Mariscal et al., 1985; Balda et al., 1993; Mullin et al., 1998; Saha et al., 1998). More recently, the family of Ras-related small GTP-binding proteins, such as RhoA and Rac1, has been reported to regulate tight junction structure and function (Nusrat et al., 1995; Zhong et al., 1997; Gopalakrishnan et al., 1998; Jou et al., 1998; Potempa and Ridley, 1998). Using MCF10A breast epithelial cells transfected with oncogenically activated H-Ras as a model, Zhong et al. (1997) found that the fibroblastic phenotype of Ras-transformed epithelial cells is partially due to the activation of Rho, a downstream effector of Ras. Rho stimulates the assembly of focal adhesions and stress fibers by increasing the contractility of cells. Potempa and Ridley (1998) reported that in Madin-Darby canine kidney (MDCK) cells, both hepatocyte growth factor/scatter factor (HGF/SF) stimulation and microinjection of dominant active Ras (V12Ras) disrupted the adherens junctions, but not tight junctions and desmosomes, suggesting that these latter structures were regulated separately from adherens junctions. The loss of adherens junctions could be blocked by the mitogen-activated protein kinase kinase (MEK1) inhibitor PD98059 and the phosphatidylinositide 3-kinase (PI 3-kinase) inhibitor LY294002. These studies indicated that Ras and downstream signals regulated the breakdown of intercellular adhesions.
Lu et al. (1998) showed previously that inhibition of the MAPK pathway by the MEK1 inhibitor PD98059 up-regulated adherens junction proteins in MDCK cells transformed by ras oncogene. However, the tight junction organization in these Ras-transformed cells has not been fully investigated. In this study, we demonstrate that the tight junction proteins occludin, claudin-1, and ZO-1 were absent from cell–cell contacts but present in the cytoplasm in Ras-transformed MDCK cells. On the other hand, the adherens junction protein E-cadherin was hardly expressed. After inhibition of the MAPK pathway by MEK1 inhibitor, cells changed from their overlapping, fibroblast-like phenotype back to a cuboidal epithelial monolayer. Immunocytochemistry and Western blot analysis revealed that occludin, claudin-1, and ZO-1 were recruited to the cell membrane and that E-cadherin protein expression was induced. The PI 3-kinase inhibitor LY294002 did not have this effect, indicating that the down-regulation of the MAPK pathway, but not PI 3-kinase, is responsible for this phenotypic reversion. It is generally believed that E-cadherin–mediated cell–cell adhesion is required for tight junction assembly. However, we found that the recruitment of occludin to the cell–cell contact area and the restoration of epithelial cell morphology preceded the appearance of E-cadherin at cell–cell contacts. During the process of tight junction assembly, the transepithelial electrical resistance (TER) was increased almost fourfold, demonstrating the formation of functional tight junctions. MAPK activity also changed the stability of tight junction proteins. The half-life of occludin was increased >100% after MEK1 inhibitor treatment, whereas the half-life of ZO-1 was increased ∼50%. Moreover, we found that although claudin-1 was not tyrosine phosphorylated, tyrosine phosphorylation of occludin and ZO-1, which had been greatly reduced in Ras-transformed MDCK cells, recovered during the assembly of tight junctions after MEK1 inhibitor treatment, suggesting that tyrosine phosphorylation of occludin and ZO-1 may play an important role in tight junction assembly.
MATERIALS AND METHODS
Antibodies and Reagents
The rat monoclonal anti-E-cadherin antibody was purchased from Sigma (St. Louis, MO). The ZO-1 hybridoma (R40.76) was produced in this laboratory (Anderson et al., 1988). The rabbit polyclonal anti-occludin, anti-claudin-1, and anti-ZO-1 antibodies were from Zymed (South San Francisco, CA). The mouse anti-phosphotyrosine and mouse anti-c-myc (clone 9E10) mAbs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Boehringer Mannheim (Indianapolis, IN), respectively. It is known that MEK1 activates MAPK by dual phosphorylation on the MAPK activation domain (Seger and Krebs, 1995). An antibody specific for the activated (phosphorylated) form of MAPK has been used to detect the presence of activated (phosphorylated) MAPK (Warn-Cramer et al., 1998; Wilson et al., 1998). The polyclonal anti-phosphorylated MAPK (ERK1/2) antibody used was from Promega (Madison, WI). The constitutively active pUSE MEK1 construct (S218D/S222D) was purchased from Upstate Biotechnology (Lake Placid, NY). PD98059, a selective inhibitor for MEK1 (Alessi et al., 1995), was from New England Biolabs (Beverly, MA). LY294002, an inhibitor for PI 3-kinase, was purchased from Calbiochem (La Jolla, CA). FITC-conjugated goat anti-rat immunoglobulin G (IgG) was from Vector Laboratories (Burlingame, CA), and FITC-conjugated goat anti-rabbit IgG was from Boehringer Mannheim. Rhodamine-conjugated goat anti-mouse IgG and rhodamine-conjugated goat anti-rabbit IgG were obtained from Cappel (Malvern, PA) and Boehringer Mannheim, respectively.
Polycarbonate Transwell filters (0.4 μm pore size) were from Costar (Cambridge, MA). All chemicals and reagents were obtained from Sigma, unless indicated otherwise, and all tissue culture reagents were from Life Technologies (Gaithersburg, MD).
Cell Culture and Transfection
Ras-transformed MDCK cells (kindly provided by Dr. J. Collard, The Netherlands Cancer Institute, Amsterdam, The Netherlands) and normal MDCK II cells (a generous gift from Dr. Barry Gumbiner, Memorial Sloan-Kettering Cancer Center, New York, NY) were grown in DMEM containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified air-5% CO2 atmosphere at 37°C. Cells from subconfluent dishes were treated with 50 μM PD98059 (prepared as 50 mM stock in DMSO and stored at −20°C) for various times before being fixed for immunocytochemistry or lysed for Western blotting or immunoprecipitation.
MDCK II cells were transfected with a constitutively active MEK1 construct with the use of Lipofectamine Plus reagent according to the protocol provided by the manufacturer (Life Technologies). To examine the results of transient expression, cells were fixed with 100% methanol at −20°C for immunofluorescence light microscopy 24 h after transfection.
Immunoprecipitation
Ras-transformed MDCK cells with or without treatment with PD98059 were washed three times with ice-cold PBS containing 0.5 mM MgCl2 and 1 mM CaCl2 (PBS+) and then lysed in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 150 mM NaCl, 10 mM HEPES, pH 7.3, 2 mM EDTA, 10 μg/ml each of chymostatin, leupeptin, and pepstatin A, 20 μM PMSF, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, and 20 mM sodium fluoride). After 30 min of incubation at 4°C, the lysates were homogenized on ice by passing 20 times through a 22-gauge needle and centrifuged at 15,000 × g for 30 min at 4°C. The total protein concentration of each sample was measured with the BCA protein assay kit (Pierce, Rockford, IL) and adjusted to equal concentrations before the supernatants were incubated with polyclonal anti-occludin, anti-claudin-1, or anti-ZO-1 antibody at 4°C overnight. The supernatants were incubated with protein A–Sepharose for an additional 2 h. The beads were washed three times with RIPA buffer, once with high-salt buffer (0.5 M NaCl), and once with Tris buffer (10 mM Tris, pH 7.4). Bound protein was eluted from the beads in SDS sample buffer and boiled for 5 min.
To make soluble or insoluble pools, cells were first lysed in 1% Triton X-100 containing 100 mM NaCl, 10 mM HEPES, 2 mM EDTA, and the cocktail of protease and phosphatase inhibitors described above, then centrifuged at 15,000 × g for 30 min at 4°C. This supernatant was considered the Triton X-100–soluble pool. The pellet was solubilized in 1% SDS and referred to as the Triton X-100–insoluble pool.
Electrophoresis and Western Blotting
Cell lysates or immunoprecipitates in SDS sample buffer were analyzed by SDS-PAGE. Proteins were transferred onto an Immobilon membrane (Millipore, Bedford, MA). The membrane was blocked in 5% nonfat dried milk in Tris-buffered saline plus 0.1% Tween 20 and incubated with primary antibodies for 1 h followed by incubation with appropriate secondary antibodies for 1 h at room temperature. The dilutions for the primary antibodies were as follows: anti-phosphorylated MAPK, 1:10,000; E-cadherin, 1:2000; occludin, 1:2000; claudin-1, 1:1000; ZO-1 (R40.76), 1:50; anti-phosphotyrosine, 1:300. The immunoreactive bands were detected by ECL (Amersham, Arlington Heights, IL) according to the manufacturer's instructions.
Immunofluorescence
Cells grown on glass coverslips were fixed with 100% methanol at −20°C for 5 min or with 1% paraformaldehyde in PBS for 15 min at room temperature. For paraformaldehyde fixation, 0.2% Triton X-100 was used to permeabilize cells for 15 min at room temperature, and then 0.1 M glycine (pH 7.4) was used for 15 min to reduce the background signal. Then cells were blocked with 2% BSA for 30 min at room temperature and incubated with primary antibodies against E-cadherin (1:500 dilution), occludin (1:500 dilution), claudin-1 (1:50), ZO-1 (R40.76 culture supernatant, straight), and c-myc (1:200) for 1 h. After washing, cells were incubated with secondary antibody for 50 min at room temperature. The secondary antibody used for E-cadherin and ZO-1 (R40.76) was fluorescein-conjugated anti-rat IgG (1:200), and FITC-conjugated anti-rabbit IgG was used for occludin and claudin-1 (1:500). Anti-myc mAb was detected by rhodamine-conjugated anti-mouse IgG (1:300). Coverslips were mounted with Gel/Mount (Biomeda, Foster City, CA) medium. Samples were examined and photographed with the use of a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY).
Measurement of TER
For TER measurements, cells were plated on polycarbonate filters with a pore size of 0.4 μm. A Millicell-ERS volt–ohm meter (Millipore) was used to determine the TER value (McCarthy et al., 1996). All TER values were normalized for the area of the filter and were obtained after background subtraction (i.e., filter and bath solution).
Metabolic Labeling and Autoradiography
To study the half-lives of occludin and ZO-1, cells were metabolically labeled for 18 h with 250 μCi of [35S]methionine per plate (ICN Pharmaceuticals, Irvine, CA) and then chased with 10× unlabeled methionine for various times. At the end of the chase period, cells were washed three times with ice-cold PBS+, and the immunoprecipitation experiments were performed as described above. Proteins were separated by SDS-PAGE on 7.5% gel and fixed for at least 30 min in solution containing 20% methanol and 10% acetic acid and for 30 min in 1 M salicylate to enhance the 35S signal. The resulting fluorographs were analyzed by densitometry (alphaImager 2000 documentation and analysis system, alpha Innotech, San Leandro, CA) to determine the relative level of labeling intensity in each band. The half-life was calculated as described previously (Fallon and Goodenough, 1981). The labeling experiments were repeated three times in each condition (Ras-transformed MDCK cells with or without MEK1 inhibitor treatment).
Electron Microscopy
For freeze fracture studies, monolayers were fixed in 2.5% glutaraldehyde in PBS for 30 min at room temperature. The sheets of cells were infiltrated with 25% glycerol, frozen in liquid nitrogen slush, and freeze fractured in a Balzers 400 freeze fracture unit (Balzers, Liechtenstein). Replicas were cleaned with sodium hypochlorite, washed in distilled water, placed on Formvar-coated grids, and examined in a Philips (Eindhoven, The Netherlands) 301 electron microscope.
For thin section electron microscopy, cells were fixed in 2.5% glutaraldehyde and 1% tannic acid in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at room temperature, washed in 0.1 M cacodylate buffer, and then postfixed with 1% OsO4 in the same buffer. Samples were stained en bloc with 1% uranyl acetate, dehydrated in ethanols, and embedded in Epon 812 (Electron Microscopy Sciences, Fort Washington, PA). Sections were stained with lead citrate and examined with a JEOL (Tokyo, Japan) 1200EX electron microscope.
RESULTS
Inhibition of the MAPK Pathway Leads to the Assembly of Tight Junctions
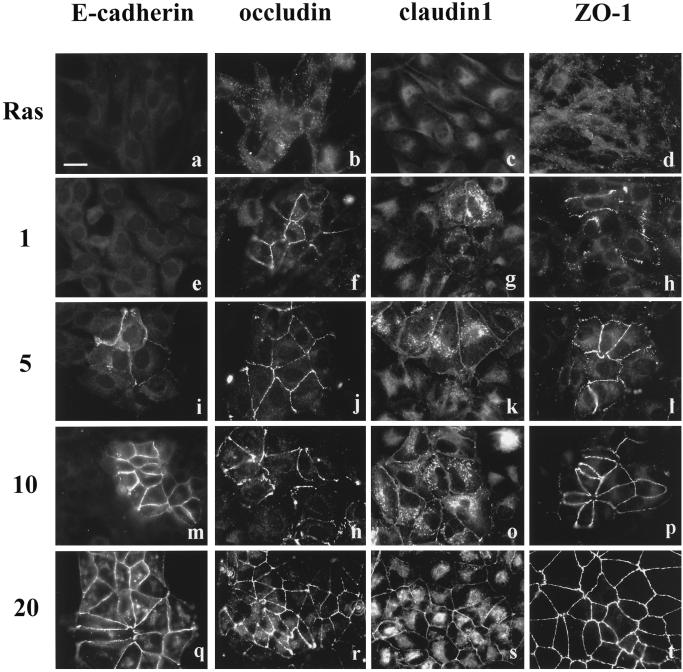
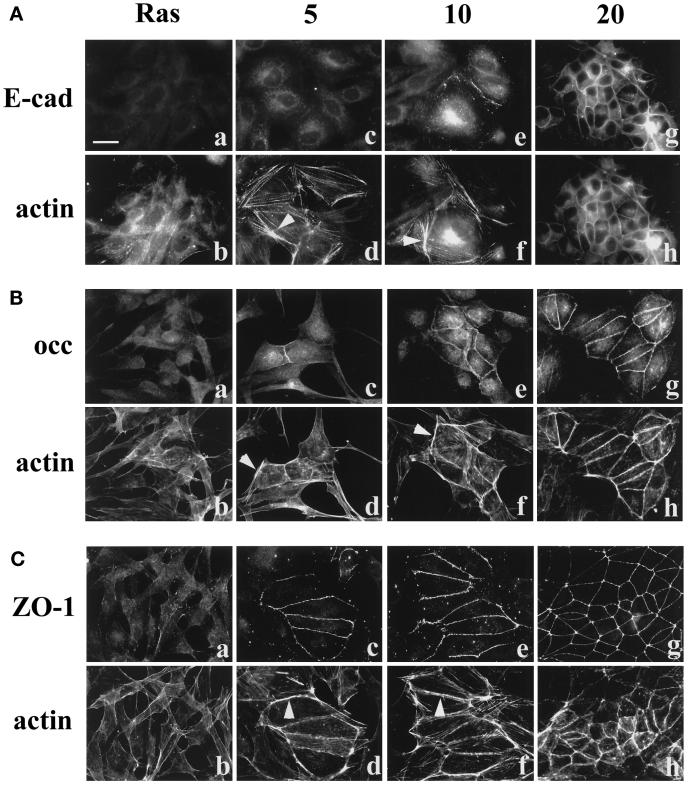
The MDCK II cells transformed by Ha-ras oncogene display a fibroblastic phenotype and hardly express E-cadherin (Vleminckx et al., 1991; Hordijk et al., 1997). In these Ras-transformed MDCK cells, we found that E-cadherin was undetectable by immunocytochemistry (Figure 1a), whereas the tight junction proteins occludin, claudin-1, and ZO-1 were present in the cytoplasm (Figure 1, b–d). After PD98059 treatment for 1 h, we found a low number of cells (<1%) that had recruited occludin, claudin-1, and ZO-1 to the cell membrane (Figure 1, f–h). In contrast, E-cadherin could not be detected at the cell interfaces until 5 h of PD98059 treatment (Figure 1i). At 5 h, ∼10% of the cells had occludin, claudin-1, and ZO-1 at cell–cell contact sites (Figure 1, j–l). By 10 h of PD98059 treatment, almost half of the cells showed apical staining of occludin, claudin-1, and ZO-1, whereas only ∼10–20% of cells showed E-cadherin staining (Figure 1, m–p). After 20 h of PD treatment, all cells showed positive signals at their cell–cell contact areas for occludin, claudin-1, and ZO-1, and >90% of cells had positive signal for E-cadherin (Figure 1, q–t).
Figure 1.
The time sequence of tight junction and adherens junction assembly in Ras-transformed MDCK cells after addition of PD98059 for 1 (e–h), 5 (i–l), 10 (m–p), and 20 (q–t) h. Immunofluorescence staining of E-cadherin, occludin, claudin-1, and ZO-1 showed that E-cadherin was hardly expressed in Ras-transformed cells (a), whereas tight junction proteins (b–d) were present in the cytoplasm. After addition of PD98059 for 1 h, occludin, claudin-1, and ZO-1 started to be recruited to the cell–cell contact area (f–h), whereas E-cadherin was not (e). E-cadherin, occludin, claudin-1, and ZO-1 were all gradually localized to the sites of cell–cell contact (i–p). By 20 h of PD98059 treatment, the immunostaining of E-cadherin (q), occludin (r), claudin-1 (s), and ZO-1 (t) at the intercellular junctions was well developed. Bar, 20 μm.
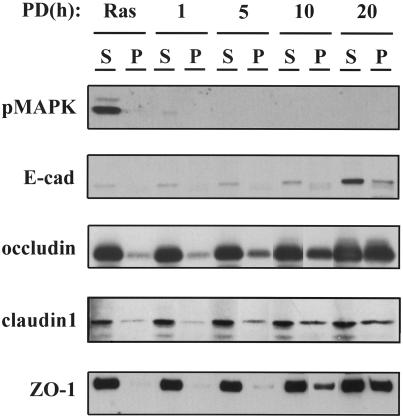
The redistribution of tight junction proteins was accompanied by a change in their solubility in Triton X-100. As shown in Figure 2, Ras-transformed MDCK cells untreated (labeled as Ras) or treated with PD98059 for 1, 5, 10, and 20 h were fractionated in 1% Triton X-100 and divided into soluble (S) and insoluble (P) pools. Figure 2 shows that MAPK activity was almost completely inhibited after 1 h of PD98059 treatment. E-cadherin was only weakly detectable in the soluble fraction at the 1- and 5-h time points and then increased at the 10- and 20-h time points. Occludin, claudin-1, and ZO-1, on the other hand, were robustly present in the Triton-soluble fraction in untreated cells and increased in concentration in the insoluble fraction beginning at the 5-h time point, consistent with the relocation of these tight junction proteins from the cytoplasm to cell–cell junctions (Sakakibara et al., 1997).
Figure 2.
Western blot of 1% Triton X-100–soluble (S) and Triton X-100–insoluble (P) fractions from Ras-transformed MDCK cells untreated (Ras) or treated with 50 μM PD98059 for 1, 5, 10, and 20 h. The blot was probed with antibodies specific for the activated (phosphorylated) MAPK (pMAPK), E-cadherin, occludin, claudin-1, and ZO-1. Inhibition of MAPK activity by the MEK1 inhibitor PD98059 induced E-cadherin expression and changed the solubility of occludin, claudin-1, and ZO-1.
Both Figures 1 and 2 reveal that the expression of E-cadherin is very low in the Ras-transformed cells and increases significantly after MEK1 inhibitor treatment for 20 h. To understand whether E-cadherin and tight junction proteins are regulated at the transcriptional or translational level by the MAPK pathway, we performed reverse transcriptase-PCR experiments. Total RNA was isolated from Ras-transformed MDCK cells with or without 20 h of PD98059 treatment. Specific primers for E-cadherin, occludin, claudin-1, ZO-1, and GAPDH were selected. GAPDH was used as an internal control. Reverse transcriptase-PCR experiments show that there were no significant differences at the mRNA level for E-cadherin, occludin, claudin-1, and ZO-1 in Ras-transformed MDCK cells with or without PD98059 treatment (our unpublished results). Thus, the reverse transcriptase-PCR results and data shown in Figure 2 indicate that the MAPK pathway regulated the expression of E-cadherin at the translational level and the localization of tight junction proteins at the posttranslational level.
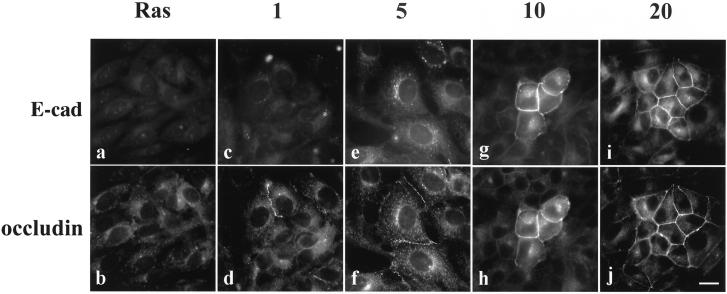
The assembly of tight junction proteins at the cell membrane preceded the stabilization of E-cadherin at cell–cell contacts, as shown in Figures 1 and 2. To verify the observation that occludin preceded E-cadherin localization at the cell surface, we performed double immunofluorescence staining for E-cadherin and occludin, as shown in Figure 3. In untreated cells (Ras), there was no junctional staining for E-cadherin or occludin (Figure 3, a and b, respectively). After 1 h of treatment with 50 μM PD98059, occludin could be found at some cell–cell interfaces (Figure 3d), but it was unaccompanied by E-cadherin (Figure 3c). By 5 h of treatment, E-cadherin immunoreactivity increased in the cytoplasm and occludin was already distributed at the cell–cell junctions of the same cells (Figure 3, e and f). E-cadherin and occludin eventually colocalized at cell–cell contact areas after 10 or 20 h of PD98059 treatment (Figure 3, g–j).
Figure 3.
In double-immunostained cells, the localization of occludin to the cell–cell contact area after PD98059 treatment preceded E-cadherin in Ras-transformed MDCK cells. Cells were either untreated (Ras) or treated with 50 μM PD98059 for 1, 5, 10, and 20 h and then double immunostained with anti-E-cadherin antibody (a, c, e, g, and i) or anti-occludin antibody (b, d, f, h, and j). Bar, 20 μm.
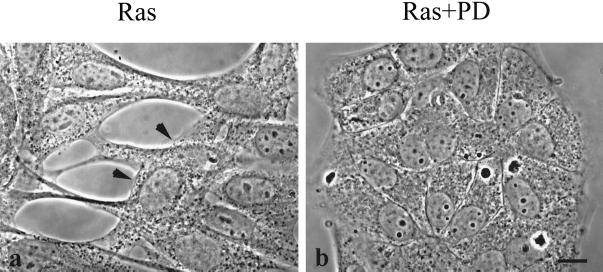
Inhibition of the MAPK Pathway Restores Epithelial Cell Morphology and Causes Cytoskeletal Reorganization
Ras-transformed MDCK cells exhibited a fibroblastic morphology and often overlapped one another, as shown in Figure 4a (arrowheads). However, when these cells were treated with PD98059 for 20 h, they reacquired their cuboidal epithelial cell morphology (Figure 4b) and formed a nonoverlapping monolayer. To examine cytoskeletal changes associated with phenotype reversion after PD98059 treatment, we stained actin filaments with fluorescence-labeled phalloidin. Figure 5 shows the colocalization of actin filaments with E-cadherin (A), occludin (B), and ZO-1 (C). The actin filaments in Ras-transformed MDCK cells possessed stress fibers similar to those seen in fibroblasts (Figure 5, Ab, Bb, and Cb). However, actin bundles became much more condensed and began to form circumferential rings in those cells initiating junction formation after 5 and 10 h of PD98059 treatment (columns 5 and 10). After 20 h of PD98059 treatment, the actin filaments were reorganized and formed cortical rings that colocalized with E-cadherin (A, g and h), occludin (B, g and h), and ZO-1 (C, g and h). It has been shown that Ras is involved in the regulation of the organization of the actin cytoskeleton, with Rho and Rac as downstream effectors (Hall, 1994). However, the expression of RhoA was significantly reduced after 10 h of PD98059 treatment (our unpublished results). Because Ras was still activated in our cells after PD98059 treatment, the factor(s) causing the reorganization of actin filaments must be downstream of the MEK1. This observation suggests that there is an unknown linkage between the MAPK pathway and Rho/Rac that can influence actin cytoskeletal organization.
Figure 4.
Ras-transformed MDCK cells revert to normal epithelial cell morphology after a 20-h PD98059 treatment. (a) Phase-contrast image shows the fibroblastic morphology of Ras-transformed MDCK cells. The arrowheads point to a cell growing on top of other cells. (b) After treatment with 50 μM of the MEK1 inhibitor PD98059 for 20 h, these Ras-transformed cells resume their normal epithelial phenotype. Bar, 10 μm.
Figure 5.
Reorganization of the actin cytoskeleton in Ras-transformed MDCK cells after treatment with PD98059. Cells were either untreated (Ras) or treated with 50 μM PD98059 for 5, 10, and 20 h and double immunostained with E-cadherin and rhodamine-conjugated phalloidin (A), occludin and phalloidin (B), or ZO-1 and phalloidin (C). The arrowheads in panels d and f indicate that the actin filaments became much more condensed and formed bundles after 5 and 10 h of PD98059 treatment. After 20 h of treatment, the actin filaments were reorganized into cortical rings that were colocalized with E-cadherin (A), occludin (B), and ZO-1 (C). Bar, 20 μm.
MEK1 Activation Caused MDCK Cells to Become Migratory and Disrupted E-Cadherin and Occludin Distribution
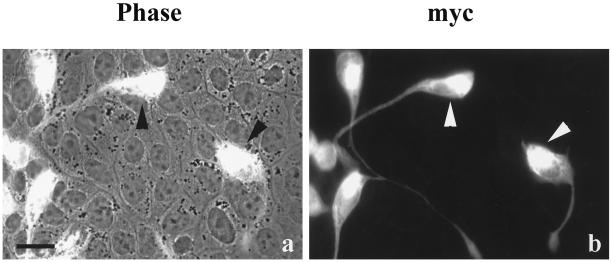
To determine whether the activation of MEK1 alone was sufficient to transform normal MDCK cells and disrupt tight junction protein distribution, normal MDCK II cells were transfected with a constitutively active MEK1 cDNA tagged with the myc epitope. Transfected cells lost the typical morphology of epithelial cells (Figure 6). The myc+ cells formed long processes (Figure 6b) that extended over neighboring cells, as seen in a double-exposed phase-contrast and fluorescence image (Figure 6a) in which the transfected cells could be seen overlapping the monolayer of untransfected cells.
Figure 6.
A constitutively active MEK1 construct caused normal MDCK cells to lose epithelial cell morphology. Normal MDCK cells were transiently transfected with constitutively active MEK1 cDNA epitope tagged with myc and fixed 24 h after transfection. The cells were immunostained with mouse anti-myc mAb. The myc-positive cells grew on top of untransfected cells, as shown in the combined phase-contrast and fluorescence image (a). These transfected cells formed long processes and were probably migrating (b). The arrowheads indicate the same myc-positive cells in both a and b. Bar, 15 μm.
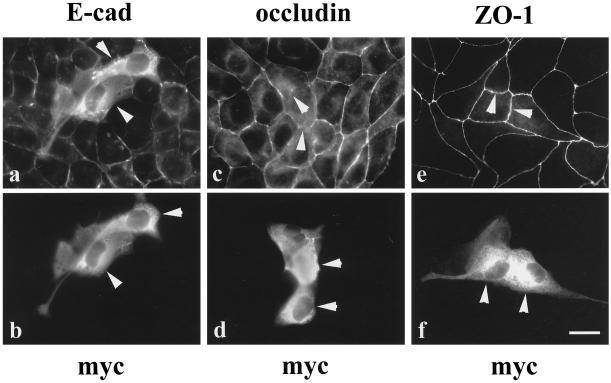
The distribution of E-cadherin, occludin, and ZO-1 in the cells expressing constitutively active MEK1 was examined by immunocytochemistry (Figure 7). E-cadherin and occludin localization were both disrupted in the transfected cells (Figure 7, a–d, arrowheads) compared with the surrounding nontransfected cells, which retained their normal epithelial cell shapes and E-cadherin and occludin distributions. The transfected cells exhibited a weak or undetectable occludin signal between two transfected cells (Figure 7c, arrowheads). In contrast, ZO-1 localization was normal in cells transfected with constitutively active MEK1 (Figure 7, e and f, arrowheads). Interestingly, even in those transfected cells that extended processes over the nontransfected cells, ZO-1 immunostaining could still be seen at the cell membranes (Figure 7, e and f). These results suggest that active MEK1 was sufficient to initiate some changes in MDCK cell morphology and to disrupt E-cadherin and occludin localization but did not change the distribution of ZO-1.
Figure 7.
Transfection of MDCK II cells with a constitutively active MEK1 cDNA disrupted E-cadherin and occludin distribution. The transfected MDCK cells were double immunolabeled with antibodies against E-cadherin (a) and myc (b), occludin (c) and myc (d), or ZO-1 (e) and myc (f). The distribution of E-cadherin and occludin in myc-positive cells (a and c, arrowheads) was disrupted compared with that in untransfected cells. In contrast, the localization of ZO-1 in myc-positive cells (e, arrowheads) was intact, although these cells clearly extended processes beneath adjacent cells (f). Arrowheads in b, d, and f locate the same cells indicated in a, c, and e. Bar, 15 μm.
Ras-transformed MDCK Cells Form Functional Tight Junctions after MEK1 Inhibition
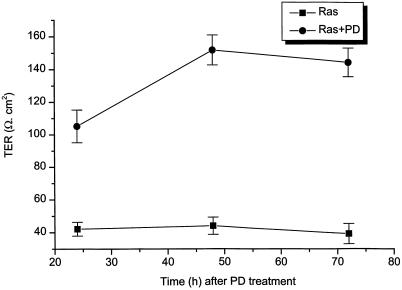
To determine whether Ras-transformed MDCK cells formed functional tight junctions between neighboring cells after treatment with MEK1 inhibitor, we measured TER of the cells with or without treatment. Ras-transformed MDCK cells were grown on filters for 3 d before the addition of MEK1 inhibitor. Figure 8 shows the results of the TER measurements from eight experiments. After treatment of cells with PD98059 for 24 h, TER increased more than twofold in PD98059-treated cells compared with cells without PD98059 treatment. Ras-transformed MDCK cells had an average TER value of 42 Ω/cm2, which did not change significantly with time. However, after these Ras-transformed MDCK cells were treated with 50 μM PD98059 for 2 d, TER reached 150 Ω/cm2, a value within the range of normal MDCK II cells, suggesting that a tight seal was formed between the neighboring cells.
Figure 8.
TER increased almost fourfold after Ras-transformed MDCK cells were treated with 50 μM PD98059 for 48 h. TER was measured 24, 48, and 72 h after PD98059 (PD) treatment. TER values were normalized for the area of the filter and were obtained after background subtraction (filter and bath solution). The data represent the average ± SE from eight experiments.
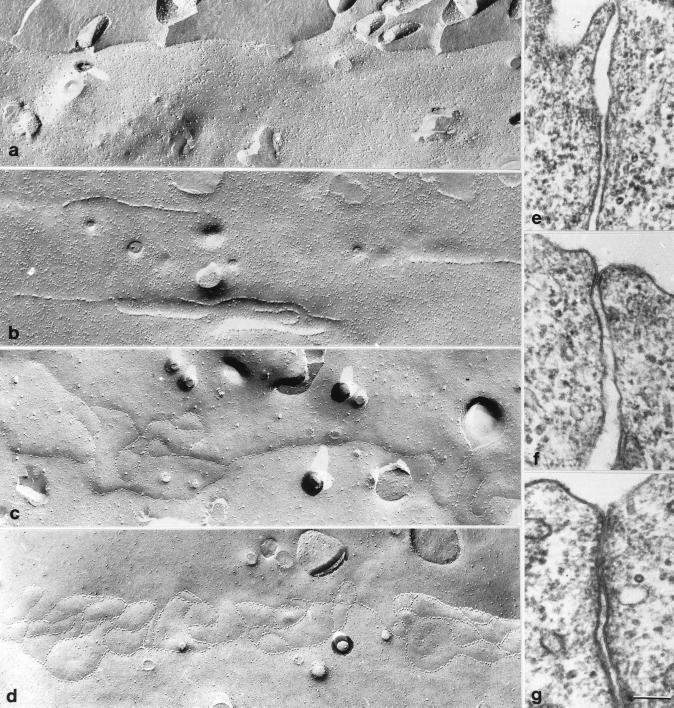
To further examine the assembly of tight junctions in Ras-transformed MDCK cells after MEK1 inhibition, we studied tight junction ultrastructure by freeze fracture and thin section electron microscopy. Figure 9 shows the comparison of freeze fracture and thin section electron microscopic images of tight junctions in Ras-transformed MDCK cells untreated (a and e) or treated with PD98059 for 10 h (b) or 20 h (c, d, f, and g). Tight junction strands or membrane contacts could not be found at the apical membranes in untreated cells (Figure 9, a and e), which correlated well with immunocytochemistry data and TER data. After the cells were treated for 10 h with PD98059, discontinuous segments of tight junction strands were readily observed (Figure 9b). By 20 h of PD98059 treatment, mixtures of single and multiple tight junction strands were observed in freeze fracture (Figure 9, c and d) together with corresponding single (Figure 9f) and multiple (Figure 9g) membrane contacts in thin sections. These images were consistent with an intermediate level of TER at this time.
Figure 9.
Freeze fracture replicas of Ras-transformed MDCK cells untreated (a) or treated with PD98059 for 10 (b) or 20 h (c and d). (e–g) Electron microscopic images of thin sections corresponding to Ras-transformed MDCK cells untreated (a) or treated with PD98059 for 20 h (c and d). Bar, 100 nm.
Inhibition of the MAPK Pathway Increases Both Protein Half-Life and Tyrosine Phosphorylation of Occludin and ZO-1
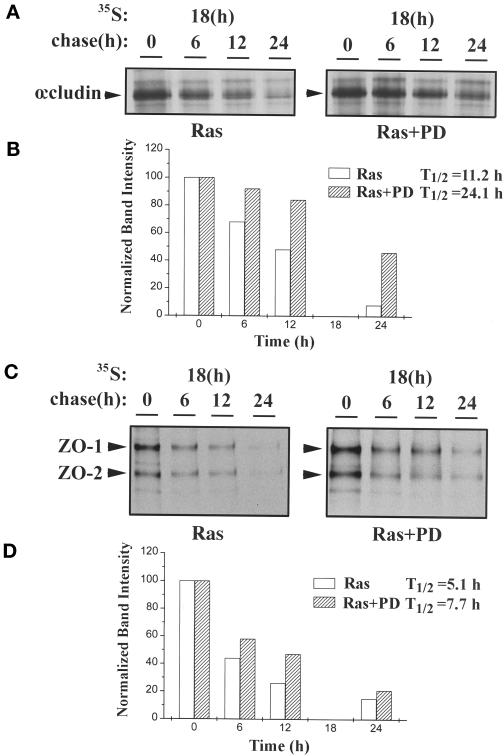
To determine the stability of occludin and ZO-1 in Ras-transformed MDCK cells untreated or treated with MEK1 inhibitor, metabolic labeling experiments were performed. After 18 h of labeling with [35S]methionine, cells were chased in cold medium for various times. The half-lives of occludin and ZO-1 in Ras-transformed MDCK cells with or without MEK1 inhibitor treatment were then compared. As indicated in Figure 10A (and shown quantitatively in Figure 10B), the half-life of occludin was 11.2 h in untreated cells and increased to 24.1 h after treatment with 50 μM PD98059 for 20 h. Thus, the turnover rate of occludin decreased >100% after MEK1 inhibition. The change of half-life of ZO-1 was more moderate, increasing from 5.1 to 7.7 h (Figure 10, C and D), indicating a 50% reduction in the turnover rate for ZO-1. The band below ZO-1 with similar turnover kinetics was most likely ZO-2 (Gumbiner et al., 1991).
Figure 10.
Half-lives of occludin and ZO-1 in Ras-transformed MDCK cells with or without treatment with 50 μM PD98059 for 20 h. Cells were metabolically labeled for 18 h with 250 μCi of [35S]methionine per plate and then chased with unlabeled methionine for 0, 6, 12, and 24 h. At the end of the chase, cell lysates were immunoprecipitated with anti-occludin (A) or anti-ZO-1 (C) antibodies. The data in A and C are quantitated by densitometry in B and D, respectively. The half-life (T1/2) was 11.2 h for occludin without PD98059 treatment (Ras) and 24.1 h for occludin with PD98059 treatment (Ras+PD), whereas the half-life was 5.1 h for ZO-1 without PD98059 treatment (Ras) and 7.7 h for ZO-1 with PD98059 treatment (Ras+PD). These experiments were repeated three times. From all three experiments, the average half-life (±SE) for occludin was 10.5 ± 0.7 h (Ras) and 27 ± 2.9 h (Ras+PD), and the average half-life for ZO-1 was 5.6 ± 0.8 h (Ras) and 8.0 ± 1.4 h (Ras+PD).
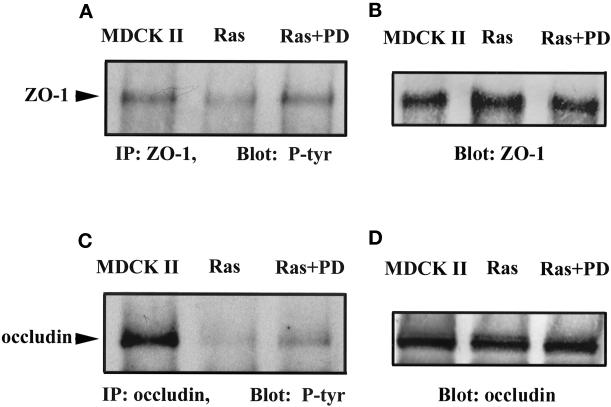
We also observed that tyrosine phosphorylation of occludin and ZO-1 showed changes when Ras-transformed MDCK cells were treated with MEK1 inhibitor (Figure 11). In MDCK II cells, ZO-1 was tyrosine phosphorylated (Figure 11, A and B, MDCK II). However in Ras-transformed MDCK cells, ZO-1 tyrosine phosphorylation was decreased significantly (Figure 11, A and B, Ras). After Ras-transformed MDCK cells were treated with PD98059 for 20 h, ZO-1 tyrosine phosphorylation returned to a level similar to that seen in MDCK II cells (Figure 11, A and B, Ras+PD). Occludin was also tyrosine phosphorylated in MDCK II cells (Figure 11, C and D, MDCK II), and this level of phosphorylation was barely detectable in Ras-transformed MDCK cells (Figure 11, C and D, Ras). Occludin tyrosine phosphorylation increased after these cells were treated with PD98059 for 20 h but to a lesser extent than that seen in normal MDCK cells (Figure 11, C and D, Ras+PD).
Figure 11.
Increased tyrosine phosphorylation of ZO-1 and occludin in Ras-transformed MDCK cells treated with PD98059 compared with the same cells without treatment and with normal MDCK II cells. (A and C) Anti-phosphotyrosine immunoblots of anti-ZO-1 (A) and anti-occludin (C) immunoprecipitates (IP) from normal MDCK II cells, untreated cells (Ras), and PD98059-treated cells (Ras+PD). (B and D) The same blots as in A and B stripped and reprobed with anti-ZO-1 and anti-occludin immunoprecipitates to demonstrate equivalent protein loading.
DISCUSSION
In this study, we demonstrate that after inhibition of the MAPK pathway, Ras-transformed MDCK cells changed their morphology from an overlapping, fibroblastic-like phenotype back to an epithelial cell monolayer. During the course of this phenotypic reversal, the tight junction proteins occludin, claudin-1, and ZO-1 relocated from cytoplasm to the cell–cell interfaces, which preceded the stabilization of E-cadherin at cell–cell contact sites. These changes were accompanied by both the appearance of freeze fractured tight junction strands and an almost fourfold increase in the TER. The MAPK pathway also influenced the stability of tight junction proteins, because after MEK1 inhibition the occludin half-life increased >100% and the half-life of ZO-1 was increased by 50%. The assembly of tight junctions was correlated with changes in the tyrosine phosphorylation of occludin and ZO-1, suggesting that this modification of occludin and ZO-1 may play a role in the process of tight junction formation.
Ras is a 21-kDa GTP-binding protein and is known to be activated or mutated in many human cancers (Thor et al., 1986; Bos, 1989; Bourne et al., 1990; Clark and Der, 1995). The ras oncogene has been virally transformed into several mammary epithelial cell lines, and these Ras-transformed epithelial cells either stratify or acquire invasive properties and become migratory (Mareel and Van Roy, 1986; Schoenenberger et al., 1991; Hordijk et al., 1997). In particular, stably transformed MDCK cell lines expressing the K-ras oncogene (viral Kirsten ras oncogene) form a continuous monolayer with epithelium-like morphology when grown on plastic substrata. However, these transformed cells detach from the substratum and become multilayered on permeable filter supports (Schoenenberger et al., 1991). On the other hand, Vleminckx et al. (1991) showed that both v-ras–transformed MDCK cells and NM-f-ras-TD cells (murine mammary gland cells expressing the activated human ras oncogene) exhibited fibroblastic morphology and lacked E-cadherin expression. As in the latter study, the cell line used in the present work was stably transformed by Ha-ras oncogene (viral Harvey ras oncogene), which displayed fibroblastic phenotype and lacked cell junctional structure.
Recently, Potempa and Ridley (1998) reported that in MDCK cells both HGF/SF and V12Ras disrupt the adherens junctions. This loss of adherens junctions is blocked by the MEK1 inhibitor PD98059 and the PI 3-kinase inhibitor LY294002. However, in normal MDCK cells, we found that MAPK had only basal activity both in confluent monolayers and after treatment with low Ca2+. Under these conditions, MAPK activity was barely detectable (our unpublished data). In addition, there were no TER or morphological changes when PD98059 was applied to normal MDCK cells (our unpublished results). Therefore, it is unlikely that the MAPK pathway plays a major role in the regulation of tight junction assembly in normal MDCK cells.
Previous studies have implicated E-cadherin–mediated cell–cell adhesion as a first step in the assembly of both tight and gap junctions (Gumbiner and Simons, 1986; Mege et al., 1988). Surprisingly, the targeting of tight junction proteins to the cell surface in Ras-transformed MDCK cells after MEK1 inhibition appeared to precede the stabilization of E-cadherin at cell–cell contacts, although the formation of physiologically functional tight junctions could not be measured at these early time points. Van Itallie and Anderson (1997) showed that the ectopic expression of human occludin in some fibroblast cells induced cell–cell adhesion in the absence of calcium, suggesting that occludin may have an adhesive function. Although E-cadherin was absent from the membrane in Ras-transformed MDCK cells, K-cadherin was present with or without treatment of PD98059 (our unpublished results). Therefore, it remains to be tested whether K-cadherin can replace E-cadherin in initiating tight junction assembly.
Potempa and Ridley (1998) showed that both HGF/SF and V12Ras induced the loss of the adherens junction proteins E-cadherin and β-catenin from intercellular junctions during cell spreading. Desmosomes and the tight junction protein ZO-1 were regulated separately from adherens junctions because they were not disrupted by V12Ras. Our data showed that in Ras-transformed MDCK cells, ZO-1 was located in the cytoplasm. However, when we transiently transfected constitutively active MEK1 construct to the normal MDCK cells, ZO-1 remained at the cell surface, similar to the results seen by Potempa and Ridley (1998). This suggests that there are different regulatory mechanisms involved in the cell system by which a protein is transiently expressed or stably expressed.
Inhibition of the MAPK pathway alone was sufficient to induce both tight junction assembly and redistribution of occludin, claudin-1, and ZO-1 from the cytoplasm to the cell surface. Inhibition of PI 3-kinase with LY294002 did not have the same effect (our unpublished results). Recently, several groups have reported that Rho GTPase signaling regulates tight junction and adherens junction assembly in epithelial cells (Braga et al., 1997; Takaishi et al., 1997; Gopalakrishnan et al., 1998; Jou et al., 1998). In Ras-transformed MDCK cells, we found that inhibition of the MAPK pathway down-regulated RhoA protein expression even in the presence of Ras activity (our unpublished results), suggesting that RhoA may function downstream of MEK1. Inhibition of the MAPK pathway also resulted in the reorganization of actin filaments from stress fibers into cortical rings that colocalized with tight junction proteins and E-cadherin. These results suggest the involvement of the small GTPases Rho and Rac in tight junction assembly, although further experiments need to be performed to elucidate the underlying mechanisms.
The process of tight junction assembly has been studied with the use of a Ca2+-switch model. In Ca2+-switch experiments, the freeze fracture fibrils of the tight junction disappeared at low calcium concentrations, although occasionally a knot of strands or a short segment could be observed (Gonzalez-Mariscal et al., 1985; Balda et al., 1993). Restoration of Ca2+ produced a rapid (<15 min) development of tight junction strands (Gonzalez-Mariscal et al., 1985). Similarly, our freeze fracture data revealed that the network of tight junction strands was completely absent in the Ras-transformed MDCK cells. After 10 h of PD98059 treatment, short, discontinuous tight junction strands were readily observed. This time course was much longer than in the Ca2+-switch model. By 20 h of PD98059 treatment, continuous, parallel tight junction strands appeared, although single strands were observed more frequently, consistent with the TER data. Our data indicated that the formation of the tight junction network was a gradual process starting with segments of small strands that were then interconnected to form a continuous seal around the apical region of the cells.
We have shown that the half-life of occludin increased from 11.2 to 24.1 h in Ras-transformed MDCK cells after MEK1 inhibition, whereas the half-life of ZO-1 increased from 5.1 to 7.7 h. This latter value is close to the 9.4-h half-life measured for ZO-1 in normal subconfluent MDCK cells (Gumbiner et al., 1991). These increases in the stability of tight junction proteins could contribute to the stabilization of tight junction structure visualized by freeze fracture after MEK1 inhibition.
A number of studies have shown that changes in tyrosine phosphorylation may accompany tight junction biogenesis (Van Itallie et al., 1995). However, the available data demonstrating a role of tyrosine phosphorylation in tight junction formation are controversial. For example, the protein tyrosine phosphatase inhibitors vanadate/H2O2 resulted in a rapid increase in paracellular permeability and the redistribution of E-cadherin and ZO-1 in MDCK cells (Collares-Buzato et al., 1994, 1998). Staddon et al. (1995) also reported that pervanadate, an inhibitor of tyrosine phosphatases, produced a decrease in TER of both MDCK cells and brain endothelial cells. Tyrosine phosphorylation of both ZO-1 and ZO-2 induced by pp60v-src, however, did not change either tight junction structure or TER (Takeda and Tsukita, 1995), and tyrosine phosphorylation of ZO-1 and other proteins occurred during the formation of podocyte junctions in the glomerulus (Kurihara et al., 1995). Van Itallie et al. (1995) noted a transient increase in tyrosine phosphorylation of both ZO-1 and ZO-2 in A431 cells after EGF stimulation, although it was unclear whether functional tight junctions formed under these conditions. More recently, Tsukamoto and Nigam (1999) reported that tyrosine phosphorylation may play an important role in the reassembly of occludin and other tight junction proteins during ATP repletion. We examined the tyrosine phosphorylation of occludin and ZO-1 in normal MDCK cells and in Ras-transformed MDCK cells untreated or treated with PD98059. Tyrosine phosphorylation of occludin and ZO-1 was significantly decreased in the Ras-transformed MDCK cells that did not have assembled tight junctions compared with both wild-type MDCK cells and transformed cells “rescued” by PD98059 treatment. Occludin tyrosine phosphorylation in transformed cells treated with MEK1 inhibitor was modest compared with that in normal MDCK cells. It is possible that the level of occludin tyrosine phosphorylation is correlated with the TER value, because the tyrosine phosphorylation was assayed after 20 h of MEK1 inhibition but its maximum value was not reached until 48 h of MEK1 inhibition. In contrast, tyrosine phosphorylation of claudin-1 was undetectable in Ras-transformed MDCK cells treated or untreated with PD98059 (our unpublished results). Our results suggest that tyrosine phosphorylation of occludin and ZO-1 may play a role in some aspects of tight junction formation.
In summary, our studies suggest that in certain cancer cells, when MAPK is activated by an oncogene, inhibition of the MAPK pathway could restore cell morphology and junctional structure even in the presence of the activated oncogene. This finding may not only provide useful information relevant to cancer biology but may also suggest avenues for the development of therapeutic reagents.
ACKNOWLEDGMENTS
We thank Dr. David L. Paul for his advice and helpful discussion and the members of the Goodenough/Paul laboratory for critical reading of the manuscript. We acknowledge Joanne M. McCormack for her technical assistance. Y.-h.C. and Q.L. contributed equally to this work. This work was supported by National Institutes of Health grants GM18974 to D.A.G. and HL25822 to E.E.S.
Abbreviations used:
- HGF/SF
hepatocyte growth factor/scatter factor
- MDCK
Madin-Darby canine kidney
- MEK1
mitogen-activated protein kinase kinase
- PI 3-kinase
phosphatidylinositide 3-kinase
- TER
transepithelial electrical resistance
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Anderson JM. Cell signaling: MAGUK magic. Curr Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112:1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Bourne HR, Wrischnik L, Kenyon C. Ras proteins: some signal developments. Nature. 1990;348:678–679. doi: 10.1038/348678a0. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Gonzalez-Mariscal L. Development and alteration of polarity. Annu Rev Physiol. 1989;51:785–795. doi: 10.1146/annurev.ph.51.030189.004033. [DOI] [PubMed] [Google Scholar]
- Chen Y-H, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Clark GJ, Der CJ. Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat. 1995;35:133–144. doi: 10.1007/BF00694753. [DOI] [PubMed] [Google Scholar]
- Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collares-Buzato CB, Jepson MA, McEwan GT, Simmons NL, Hirst BH. Junctional uvomorulin/E-cadherin and phosphotyrosine-modified protein content are correlated with paracellular permeability in Madin-Darby canine kidney (MDCK) epithelia. Histochemistry. 1994;101:185–194. doi: 10.1007/BF00269543. [DOI] [PubMed] [Google Scholar]
- Collares-Buzato CB, Jepson MA, Simmons NL, Hirst BH. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur J Cell Biol. 1998;76:85–92. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- Fallon RF, Goodenough DA. Five hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981;90:521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Membr Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signaling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol. 1998;275:C798–C809. doi: 10.1152/ajpcell.1998.275.3.C798. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu LJ, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Increased tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol. 1995;37:F514–F524. doi: 10.1152/ajprenal.1995.268.3.F514. [DOI] [PubMed] [Google Scholar]
- Lu Q, Paredes M, Zhang J, Kosik KS. Basal extracellular signal-regulated kinase activity modulates cell-cell and cell-matrix interactions. Mol Cell Biol. 1998;18:3257–3265. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareel MM, Van Roy FM. Are oncogenes involved in invasion and metastasis? Anticancer Res. 1986;6:419–435. [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Mege RM, Matsuzaki F, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci USA. 1988;85:7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Kampherstein JA, Laughlin KV, Clarkin CEK, Miller RD, Szallasi Z, Kachar B, Soler AP, Rosson D. Overexpression of protein kinase C-delta increases tight junction permeability in LLC-PK1 epithelia. Am J Physiol. 1998;275:C544–C554. doi: 10.1152/ajpcell.1998.275.2.C544. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha C, Nigam SK, Denker BM. Involvement of Gα2 in the maintenance and biogenesis of epithelial cell tight junctions. J Biol Chem. 1998;273:21629–21633. doi: 10.1074/jbc.273.34.21629. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. (Review). [DOI] [PubMed] [Google Scholar]
- Schoenenberger C-A, Zuk A, Kendall D, Matlin KS. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol. 1991;112:873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Simon DB, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsukita S. Effects of tyrosine phosphorylation on tight junctions in temperature-sensitive v-src-transfected MDCK cells. Cell Struct Funct. 1995;20:387–393. doi: 10.1247/csf.20.387. [DOI] [PubMed] [Google Scholar]
- Thor A, Ohuchi N, Hand PH, Callahan R, Weeks MO, Theillet C, Lidereau R, Escot C, Page DL, Vilasi V. ras gene alterations and enhanced levels of ras p21 expression in a spectrum of benign and malignant human mammary tissues. Lab Invest. 1986;55:603–615. [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Balda MS, Anderson JM. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci. 1995;108:1735–1742. doi: 10.1242/jcs.108.4.1735. [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet LJ, Mareel M, Fiers W, Van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Umeh I, Kang K-S, Trosko JE. A role for mitogen activated protein kinase (MAPK) during RAS-induced down-regulation of gap junctional intercellular communication. In: Werner R, editor. Gap Junctions. Amsterdam: IOS Press; 1998. pp. 239–243. [Google Scholar]
- Wong V, Goodenough DA. Paracellular channels! Science. 1999;285:62. doi: 10.1126/science.285.5424.62. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. ZO-1, DLGA and PSD-95/SAP90: homologous proteins in tight, septate and synaptic cell junctions. Mech Dev. 1993;44:85–89. doi: 10.1016/0925-4773(93)90059-7. (Review). [DOI] [PubMed] [Google Scholar]
- Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong YT, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]